Abstract
The effects of salt stress on growth parameters, free proline content, ion accumulation, lipid peroxidation, and several antioxidative enzymes activities were investigated in S. persica and S. europaea. The seedlings were grown for 2 months in half-strength Hoagland solution and treated with different concentrations of NaCl (0, 85, 170, 340, and 510 mM) for 21 days. The fresh and dry weights of both species increased significantly at 85 and 170 mM NaCl and decreased at higher concentrations. Salinity increased proline content in both the species as compared to that of control. Sodium (Na+) content in roots and shoots increased, whereas K+ and Pi content in both organs decreased. At all NaCl concentrations, the total amounts of Na+ and K+ were higher in shoots than in roots. Malondialdehyde (MDA) content declined at moderate NaCl concentrations (85 and 170 mM) and increased at higher levels. With increased salinity, superoxide dismutase (SOD), catalase (CAT), and guaiacol peroxidase (GPX) activities also increased gradually in both species. In addition, it seems that GPX, CAT, and SOD activities play an essential protective role in the scavenging reactive oxygen species (ROS) in both species. Native polyacrylamide gel electrophoresis (PAGE) indicated different isoform profiles between S. persica and S. europaea concerning antioxidant enzymes. These results showed that S. persica exhibits a better protection mechanism against oxidative damage and it is more salt-tolerant than S. europaea possibly by maintaining and/or increasing growth parameters, ion accumulation, and antioxidant enzyme activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In arid and semiarid regions of the world, limited rainfall, high evaporation, and high temperature contribute to increase soil salinity. In these regions, planting salt-tolerant species, particularly N2-fixing species, is the most useful approach in rehabilitating salt-affected degraded lands (Oba et al. 2001). There are a great number of plant species, which are regarded as halophytes. They are known for their ability to live in salty environments by alerting their energy metabolism (Winicov and Bastol 1997).
Mechanisms of salt tolerance are not yet completely clear but can be explained to some extent by stress adaptation effectors that mediated ion homeostasis, osmolyte biosynthesis, toxic radical scavenging, water transport, and long distance response co-ordination (Hasegawa et al. 2000). Most halophytes utilize the controlled accumulation and sequestration of inorganic ions as the basic mechanism by which they adjust the osmotic potential of their internal tissues to external salinity (Flowers and Yeo 1986; Cheesman 1988; Aghaleh et al. 2009).
One of the biochemical changes occurring when plants are subjected to biotic or abiotic stresses is the production of reactive oxygen species (ROS) (Allen 1995). ROS generation can be enhanced under environmental stresses as salinity, and the free radicals disrupt normal metabolism through lipid peroxidation, denaturing proteins, and nucleic acids (Jiang and Zhang 2001; Bor et al. 2003). Lipid peroxidation causes degradation and impairment of structural components. This leads to change in selective permeability of bio-membranes (Weckx and Clijsters 1997) and thereby membrane leakage and change in activities of enzymes bound to membrane. Therefore, the cell membrane stability has been widely used to differentiate stress tolerance and susceptible cultivars of crops (Aziz and Larher 1998; Liang et al. 2003). In order to survive under stress conditions, plants are equipped with oxygen radical detoxifying enzymes such as superoxide dismutase, ascorbate peroxidase; catalase and antioxidant molecules like ascorbic acid, α-tocopherol and reduced glutathione. Antioxidant mechanisms may provide a strategy to enhance salt tolerance in plants (Jaleel et al. 2008).
Salicornia persica Akhani and S. europaea L. are annual and succulent euhalophytes belonging to Chenopodiaceae. S. persica and are distinguished from S. europaea by having ascending habit, verticillate inflorescence branches and reversed pentagonal central flowers that are truncated at the apex and reach to the upper segment and the species is tetraploid (2n = 36) (Akhani 2003). Previous study on Salicornia species involved some responses of seedling of two Salicornia species to NaCl salinity in vitro (Aghaleh et al.2009) but so far none have focused on comparative antioxidative responses of S. persica and S. europaea to salt stress. In order to look into special manner of salinity-induced alternations and to elucidate adaptive mechanisms, we investigated the status of growth, free proline content, ion accumulation, and lipid peroxidation level and antioxidant enzymes activities in two species of Salicornia under salt stress.
Materials and methods
Plant materials and salt treatment
Seeds of S. persica Akhani and S. europaea L. were collected from inland salt marshes of Iran in Fars and Azerbaijan provinces, respectively. Seeds were sown in 30 plastic pots (10 × 10 cm) filled with perlite and maintained under greenhouse conditions. The pots were irrigated daily with ca. 75 ml half-strength Hoagland solution for 2 months. The pots were then divided into five groups of six pots each. Each group started to receive half-strength Hoagland solution containing different concentrations of NaCl (0, 85, 170, 340, and 510 mM). Three weeks after NaCl treatment, three or five plants per treatment were collected for analyses.
Plant water relations
Shoot or root water content (WC) was calculated based on the equation (Molassiotis et al. 2006):
Leaf relative water content (RWC) was estimated according to Wheatherley (1973) and calculated as follows:
Saturated mass of fresh seedlings was determined by keeping them in water for 24 h, followed by drying in a hot air oven (60°C for 48 h) until constant weight was achieved.
The osmotic potential (ψs) of plants was determined according to Ball and Oosterhuis (2005). Specifically, plants were sampled in the early morning at the end of the experiment, sealed in a plastic bag, and immediately stored in a freezer until analysis. Frozen plants were thawed in a plastic bag at room temperature before sap was pressed with a Markhart leaf press (LP-27, Wescor, Logan, UT, USA) and analyzed using a vapor pressure osmometer (VAPOR 5520, Wescor, Logan, UT, USA). Osmometer readings (mmol kg−1) were converted to MPa using the van’t Hoff equation at 25°C (Nobel 1991).
Proline content
Free proline content was determined according to Bates et al. (1973) using l-proline as a standard. High-speed centrifuge (Beckman J2-21M, Palo Alto, USA) and UV–visible spectrophotometer (Shimadzu UV-160, Tokyo, Japan) with 10 mm matched quartz cells were used for centrifugation of the extracts and determination of the absorbance, respectively.
Malondialdehyde content
The level of lipid peroxidation was measured in terms of thiobarbituric acid reactive substances (TBARS), following the method of Heath and Packer (1968). The plant materials (0.5 g) were homogenized in 5 ml of 0.1% (w/v) trichloroacetic acid (TCA) and centrifuged at 10,000×g for 20 min. To 1 ml aliquot of the supernatant, 4 ml of 0.5% thiobarbituric acid (TBA) in 20% TCA was added. The mixture was heated at 95°C for 30 min and quickly cooled in an ice bath. After centrifugation at 10,000×g for 15 min, the absorbance of the supernatant was recorded at 532 and 600 nm. The value for nonspecific absorption at 600 nm was subtracted. The concentration of MDA was calculated using extinction coefficient of 155 mM−1 cm−1.
Ions content
The K+ and Na+ contents were determined by flame photometer (Model Jenway PFP7, UK). Before measurement, 20 mg of dried sample was digested by using 8 ml of 70% (v/v) perchloric acid.
Inorganic phosphate was determined spectrophotometrically following Jackson (1962). The extract (2 ml) was dissolved in 2 ml of Barton’s reagent and total volume was made to 50 ml. The samples were vortexed and kept for 20 min at room temperature. The absorbance of samples was measured at 430 nm with spectrophotometer (Shimadzu UV-160, Tokyo, Japan), using water as blank and values of phosphate were calculated using standard curve. A graded series of standards ranging from 2.5 to 15 mg l−1 of PO4−2 was prepared to determine the quantity of phosphate in the samples. The Barton’s reagent was prepared as described below:
Solution A: 25 g of ammonium molybdate was dissolved in 400 ml of distilled water. Solution B: Ammonium metavandate (1.25 g) was dissolved in 300 ml of boiling water, cooled and 250 ml of concentrated HNO3 was added to it. The solution was again cooled at room temperature. The solutions, A and B, were mixed and the volume was maintained up to one liter and stored at room temperature.
Antioxidant enzymes assay
Shoots of plants were homogenized in a chilled (4°C) mortar using a 50 mM Tris–HCl buffer (pH 7.0) containing 10 mM EDTA, 2 mM MgSO4, 20 mM DTT, 10% (v/v) glycerol, and 2% (w/v) PVP. The homogenate was centrifuged at 13,000×g for 25 min at 4°C. The supernatant was re-centrifuged at 13,000×g for 25 min at 4°C, transferred to Eppendorf tubes, and kept on ice at 4°C. The enzyme extract was stored at −70°C for enzyme activity assay. Total protein content was measured by the Bradford method (1976) using bovine serum albumin (BSA) as the standard.
Superoxide dismutase (EC 1.15.1.1) activities
Total SOD activities were determined by monitoring the inhibition of the photochemical reduction of nitroblue tetrazolium (NBT) according to the protocol of Yu and Rengel (1999). One unit of SOD activities was defined as the amount of enzyme required to cause 50% inhibition of reduction of NBT as monitored at 560 nm and the activities were expressed as units per milligram of protein.
SOD isoform was examined in 10% acrylamide gel using the procedures of Laemmli (1970). For SOD isoform identification, assays were performed in the presence of selective inhibitors. KCN (3 mM) inhibited only Cu/Zn-SOD. H2O2 (5 mM) inhibited both Cu/Zn-SOD and Fe-SOD. Mn-SOD was not inhibited by KCN or H2O2 (Lee et al. 2001).
Catalase (EC 1.11.1.11) activities
Total CAT activities were assayed by measuring the initial rate of disappearance of H2O2 according to the Aebi method (1984). The reaction mixture contained 0.05 M phosphate buffer (pH 7.0), H2O2 (3%) and 40 μl enzyme extract. The decrease in absorption was followed for 30 s and CAT activities were expressed as units (μmol of H2O2 decomposed per minute) per milligram of protein.
CAT isoforms separated on 7% nondenatured acrylamide gels at 120 V for 2.30 h at 4°C. Gels were soaked in 3.27 mM H2O2, for 15 min, rinsed in water, and stained in potassium ferricyanide and 2% ferric chloride (Anderson et al. 1995).
Guaiacol peroxidase (EC 1.11.1.7) activities
Guaiacol peroxidase activities were measured in the reaction medium containing 50 mM phosphate buffer (pH 7.0), 9 mM guaiacol, and 19 mM H2O2 according to the Lin and Kao method (1999). The kinetic evolution of the absorbency at 470 nm was measured during 1 min. The enzyme activities were calculated using the extinction coefficient (26.6 mM−1 cm−1 at 470 nm). One unit of guaiacol was defined as the amount of enzyme needed to produce 1 μM tetraguaiacol per min.
The electrophoretic separation of GPX was performed on a native 10% acrylamide gel using the procedures of Fielding and Hall (1978). Gel was incubated in 25 mM potassium buffer (pH 7.0) for 15 min to lower the pH and then it was submerged in a freshly prepared solution containing 18 mM guaiacol and 25 mM H2O2 in 25 mM K-phosphate buffer (pH 7.0) until the GPX activity-containing band was visualized clearly.
Statistical analysis
All analyses were performed based on a completely randomized design. The data were analyzed by using either one- or two-way analysis of variance (ANOVA) and the mean differences were compared by the lowest standard deviations (LSD) test. Each data point was the mean of three replicates (n = 3), except for dry and fresh weights, osmotic potential, water content and ion content of Salicornia plants (n = 5). Comparisons with P values <0.05 were considered significantly different. In all the figures, the spread of values is shown as error bars representing standard errors of the means.
Results
Growth and tissue water content
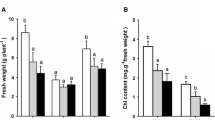
In contrast to glycophytes, Salicornia was able to complete its life cycle at high NaCl concentrations (Fig. 1a, b). There were significant differences in status of growth between the two species with increasing NaCl concentrations (Table 1). Both species showed an increase in growth (FW and DW) at moderate salinities (85 and 170 mM NaCl) and a decrease at higher salinities. Moreover, biomass of S. persica plants was higher than that of S. europaea in all treatments. The RWC in both species did not decrease significantly at NaCl concentrations up to 170 mM and maximum RWC of 92 and 93% was obtained for control plants of S. persica and S. europaea, respectively. The NaCl in WC in both species increased by up to 170 mM and then decreased at higher concentrations. The osmotic potential (ψs) of plants became more negative with an increase in salinity (Fig. 2; Table 2). The osmotic potential in S. persica and S. europaea in control and salt stress treatments ranged from −2.43 to −6.35 MPa and from −2.95 to −6.47 MPa, respectively.
Proline content
Free proline contents were higher in S. persica than in S. europaea under NaCl salinity. However, at 340 mM NaCl, no significant difference in proline content was observed between the two species. An increase was seen in proline content of both the species under salinity as compared to control (Fig. 2). Overall, proline content change under NaCl treatments was significant in both species (Fig. 2; Table 1).
Ion concentrations
In both species of Salicornia the Na+ accumulation was proportional to the exogenous NaCl concentration. Salicornia plants cultivated under NaCl concentrations showed considerably higher sodium content in the shoots, as compared to the roots (Table 2). Shoot Na+ concentration ranged from ca. 1,536 to 8,469 μmol g−1 DW in S. persica and 1,362 to 8,161 μmol g−1 DW in S. europaea, whereas those numbers in roots varied from ca. 739 to 3,120 μmol g−1 DW and 1,000 to 4,341 μmol g−1 DW in S. persica and S. europaea, respectively. Salt treatment reduced K+ and Pi concentrations in shoots and roots of both species (Tables 1, 2). Moreover, K+ content in roots and shoots of S. persica was higher than that of S. europaea under all NaCl treatments.
Membrane lipid peroxidation
In both species, moderate salinities had no significant effect on MDA content as compared to that of control (Fig. 3; Table 1), whereas high NaCl concentrations (340 and 510 mM) caused a significant increase in MDA content.
Activities of antioxidative enzymes
The activities of SOD, CAT, and GPX in two species of Salicornia under the effect of different NaCl concentrations are given in Figs. 4, 5, and 6. Antioxidant enzyme activities at 510 mM NaCl were not determined due to lack of sufficient fresh plant materials. The total SOD activities in both species gradually increased with increasing NaCl concentrations, and the highest SOD activity in both species was determined at 340 mM NaCl, which was ca. 220 and 330% of the control values in S. persica and S. europaea, respectively (Fig. 4a). In all treatments, SOD activities in S. persica were higher than those of S. europaea. The identities of the major SOD activity bands were tested by preincubating the gels with well-characterized SOD inhibitors: KCN is an inhibitor of Cu/Zn-SOD, whereas H2O2 inhibits both Cu/Zn-SOD and Fe-SOD. The upper band (Fig. 4b), in both species, represented a mitochondrial Mn-SOD. Two bands with higher mobility represented the chloroplastic Fe-SOD isoforms in both species. Three and two bands constituted the cytosolic Cu/Zn-SOD isoforms in S. persica and S. europaea, respectively. The intensity of the Mn-SOD isoform in S. persica was weaker than that of S. europaea. Fe-SOD and Cu/Zn-SOD isoform activities in both species were obviously stimulated by salinity.
CAT activities increased with increasing NaCl concentrations in both species of Salicornia (Fig. 5a). The maximum CAT activity in both species was obtained at 340 mM NaCl, which was ca. 120 and 140% of the control value in S. persica and S. europaea, respectively. The analyses on PAGE gels revealed two CAT isoforms for both species (Fig. 5b). In both species, A1 band was completely absent in control and at low salinities and was strongly activated at higher salinities (170 and 340 mM NaCl). In S. persica, A2 band was strong at control and 340 mM NaCl, whereas in S. europaea it was activated only at 170 mM NaCl.
Salinity considerably increased the total GPX activity in both Salicornia species as compared to that of control (Fig. 6a). The maximum GPX activity in both species was obtained at 340 mM NaCl, which was ca. 146 and 78% higher than that of the control in S. persica and S. europaea, respectively. The GPX activities in S. persica were not significantly higher than those of S. europaea (Fig. 6a). The GPX activities on the gels revealed three isoforms of peroxidase in both species (Fig. 6b). In S. persica, A1 isoform was activated by salinity, whereas A2 and A3 isoforms remained unchanged under NaCl treatment. In S. europaea, A1 isoform was activated at higher salinities (170 and 340 mM), A2 isoform was detected only at low salinities, and A3 band remained unchanged under salt treatment.
Discussion
The present investigation indicates the halophytic characteristics of Salicornia plants as evidenced by the positive effect of moderate salinities (85 and 170 mM NaCl) on plant growth (Fig. 2). It would appear that the growth response at moderate salinities may be largely the consequence of an increased uptake of solutes that are required to induce cell expansion, since this maintains the pressure potential in plant tissue (Khan et al. 2000). At high salinities, growth reduction might either be caused by a reduced ability to adjust osmotic potential as the result of saturation of the solute uptake system, or because of excessive demand for energy requirements of such systems (Gale and Zeroni 1984). RWC of S. persica and S. europaea decreased to 74 and 70% of the control value at 510 mM NaCl, respectively. Osmotic potential became increasingly more negative with the corresponding increase in media salinity, indicating that both species osmotically adjust in response to salinity. Growth and survival of halophytes is dependent on the high levels of ion accumulation in their tissues for the maintenance of turgor and osmotic adjustment (Flowers et al. 1977). Furthermore, Glenn et al. (1996) showed a strong, positive correlation between capacity for Na uptake and degree of salt tolerance among the different genotypes, and a negative relationship between K uptake capacity and salt tolerance. In the present study, both species of Salicornia accumulated Na+ by salt treatment, the accumulation of Na+ being higher in shoots than in roots (Table 2). The high capacity to translocate Na+ to the shoots indicates that Salicornia plants are not Na+ excluders and that they have a mechanism by which select sodium sequestration in the shoot vacuole (Parks et al. 2002). This study indicated that under salt stress, higher Na+ accumulation occured in S. europaea roots than that of S. persica (Table 2). This is probably due to the more control of Na+ transport to shoot in S. europaea. According to Marschner (1986), resorption of Na+ from xylem sap is an effective mechanism of restricting translocation to the leaf. When both species were grown with NaCl stress, while the accumulation of K+ and Pi in shoots and roots was decreased by salt treatment, K+ accumulation in shoots was more than in roots. On the other hand, salinity affects the plant growth by inducing nutrient (K+, Ca2+, and Mg2+) deficiency (Khan et al. 2000). Although phosphorus is not typically considered the most limiting nutrient of plant growth, it has been shown to limit the growth of Salicornia species when found in low concentrations. Brown et al. (2006) demonstrated that shoots and roots uptake of phosphorus decreased under salinity and drought conditions.
Increase in succulency of Salicornia species at moderate salinities might be related to the accumulation of proline (Fig. 2). However, enhanced levels of proline accumulation may not be enough to maintain water balance of both species at higher salinities. Many plants accumulated proline as nontoxic and protective osmolyte under saline conditions (Muthukumarasamy et al. 2000; Jaleel et al. 2008; Siddiqui et al. 2009; Khan et al. 2010). Proline is also considered to be involved in the protection of enzymes (Solomon et al. 1994), cellular structures (Van Resenburg et al. 1993), and to act as a free radical scavenger.
Peroxidation of lipid membranes of higher plants reflects free radical-induced oxidative damage at the cellular level under salt stress conditions (Halliwell 1987; Demiral and Türkan 2004). A decline in MDA levels was observed in both species at moderate salinities and a significant increase at higher salinities (340 and 510 mM) (Fig. 3). Lower MDA increases observed in salt-treated S. persica suggests a better protection from oxidative damage. Our results are in agreement with the findings of Shalata and Tal (1998) and Bor et al. (2003) who found a correlation between increased antioxidant enzyme activities and decreased lipid peroxidation levels in salt-tolerant tomato and wild beet under salt stress, respectively.
Salt tolerance is often correlated with a more efficient antioxidative system (Cakmak and Marschner 1992). Constitutive and/or induced activities of SOD, GPX, and CAT suggest better tolerance to salt stress in S. persica in comparison with S. europaea. Furthermore, lower level of MDA content, in S. persica in comparison with S. europaea could be related to the increased activities of antioxidative enzymes. Our result is consistent with the findings in cotton (Meloni et al. 2003), wild beet (Bor et al. 2003) and drought-tolerant P. acutifolius and drought-sensitive P. vulgaris (Türkan et al. 2005).
SOD activities increased due to the increase in salt concentrations in both species (Fig. 4). The contribution of individual SOD isoforms to total SOD activity was determined by performing SOD assays directly on protein extracts separated in native gels. Activities of Fe and Cu/Zn-SOD constituted the major part of total SOD activities in both species. Exposure to severe salt stress increased Fe-SOD activity in pea plants (Gomez et al. 1999), and the induction of chloroplastic Cu/Zn-SOD activities in pea plants might respond a protective role against the production of O2 − under salt stress (Hernández et al. 2001). As with our findings, the increased activities of Cu/Zn-SOD isoforms in Suaeda salsa may contribute to increase of total SOD under salt stress, while Mn-SOD isoform decreased and some isoforms around Mn-SOD disappeared under salt stress (Qiu-Fang et al. 2005).
The activities of CAT, which also decomposes H2O2 increased with increasing salinity (Fig. 5). CAT and GPX activities coordinated with SOD activities play a central protective role in the O2 − and H2O2 scavenging process (e.g. Liang et al. 2003; Azevedo Neto et al. 2006). Although the ratio of salt-induced increase in SOD and CAT activities was lower in S. persica, constitutive activities of both enzymes were higher than those in S. europaea, which might lead tolerant species to defend against the potential oxidative damage with no need to further increase of SOD and CAT activities. These results are in strong confirmation with the results of Demiral and Türkan (2004) who reported constitutively higher SOD and APX activities in salt-tolerant Pokkali than salt-sensitive IR-28. Moreover, GPX activation under salt stress (Fig. 6) was reported by several researchers (Lee et al. 2001; Quiroga et al. 2000; Mhadhbi et al. 2004), and it was suggested to play an important role in plant salt tolerance (Quiroga et al. 2000). NaCl-induced enhancement of GPX activities in salinized cells of Suaeda nudiflora Moq. indicated that these cells had a higher capacity for decomposition of H2O2 (Cherian and Reddy 2003).
In conclusion, based on the data obtained from biomass parameters, ion accumulation, and proline content, it is clear that S. persica was more tolerant to NaCl stress than S. europaea. It is possible that better salt stress tolerance of S. persica was associated with its ability to maintain higher activities of SOD, CAT, and GPX resulting in lower H2O2 production and lipid peroxidation.
Abbreviations
- ROS:
-
Reactive oxygen species
- PVP:
-
Polyvinylpyrrolidone
- NBT:
-
Nitroblue tetrazolium
- EDTA:
-
Ethylenediamine-N,N,N′,N′ tetraacetic acid
- DTT:
-
1,4 Dithiothreitol
- TCA:
-
Trichloroacetic acid
- MDA:
-
Malondialdehyde
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Aghaleh M, Niknam V, Ebrahimzadeh H, Razavi K (2009) Salt stress effects on growth, pigments, proteins and lipid peroxidation in Salicornia persica and S. europaea. Biol Plant 53:243–248
Akhani H (2003) Salicornia persica Akhani (Chenopodiaceae), a remarkable new species from central Iran. Linz Biol Bietr 35:607–612
Allen R (1995) Dissection of oxidative stress tolerance using transgenic plants. Plant Physiol 107:1049–1054
Anderson MD, Prasad TK, Stewart CR (1995) Changes in isozymes profiles of catalase, peroxidase, and gluthatione reductase during accumulation to chilling in mesocotyls of maize seedlings. Plant Physiol 109:1247–1257
Azevedo Neto AD, Prisco JT, Enéas-Filho J, Abreu CEB, Gomes-Filho E (2006) Effect of salt stress on oxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ Exp Bot 56:87–94
Aziz A, Larher F (1998) Osmotic stress induced changes in lipid composition and peroxidation in leaf discs of Brasica napus L. J Plant Physiol 153:754–762
Ball RA, Oosterhuis DM (2005) Measurement of root and leaf osmotic potential using the vapor-pressure osmometer. Environ Exp Bot 53:77–84
Bates LS, Walderd RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–208
Bor M, Özdemir F, TüKan I (2003) The effect of salt stress on lipid peroxidation and antioxidants in leave of sugar beet Beta vulgaris L. and wild beet Beta maritime L. Plant Sci 163:77–84
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein-dye binding. Anal Biochem 72:248–254
Brown CE, Pezeshki SR, DeLaune RD (2006) The effects of salinity and soil drying on nutrient uptake and growth of Spartina alterniflora in a simulated tidal system. Environ Exp Bot 58:140–148
Cakmak I, Marschner H (1992) Magnesium-deficiency and high-light intensity enhance activities of superoxide-dismutase, ascorbate peroxidase and glutathione-reductase in been leaves. Plant Physiol 98:1222–1227
Cheesman JM (1988) Mechanisms of salinity tolerance in plants. Plant Physiol 87:547–550
Cherian S, Reddy MP (2003) Evaluation of NaCl tolerance in the callus cultures of Suaeda nudiflora Moq. Biol Plant 46:193–198
Demiral T, Türkan I (2004) Does exogenous glycinbetaine affect antioxidative system of rice seedlings under NaCl treatment? J Plant Physiol 161:1089–1100
Fielding JL, Hall JL (1978) A biochemical and cytological study of peroxidase activities in roots of Pisum sativum. J Exp Bot 29:969–981
Flowers TJ, Yeo AR (1986) Ion relations of plants under drought and salinity. Aust J Plant Physiol 13:75–91
Flowers TJ, Troke PF, Yeo AR (1977) The mechanism of salt tolerance in halophytes. Annu Rev Plant Physiol 28:89–121
Gale J, Zeroni M (1984) Cultivation of plants in brackish water incontrolled environmental agriculture. In: Staples RC, Toenniessen GR (eds) Salinity tolerance on plants, pp 363–380. Wiley, New York, p 443
Glenn E, Pfister R, Browen J, Thompson T, O’ Leary L (1996) Na and K accumulation and salt tolerance of Atriplex canescens (Chenopodiaceae) genotype. Am J Bot 83:997–1005
Gomez JM, Hernández JA, Jimenez Del Rio LA, Sevilla F (1999) Differential response of antioxidative enzymes of chloroplasts and mitochondria to long-term NaCl stress of pea plants. Free Red Res 31:11–18
Halliwell B (1987) Oxidative damage, lipid peroxidation, and antioxidant protection in chloroplasts. Chem Phys Lipids 44:327–340
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. Arch Biochem Biophys 125:189–198
Hernández JA, Ferrer MA, Jimenez A, Barcelo AR, Sevilla F (2001) Antioxidant systems and O2 −/H2O2. Production in the apoplast of pea leaves. Is relation with salt-induced necrotic lesions in minor veins. Plant Physiol 127:817–831
Jackson ML (1962) Soil chemical analysis. Contable Co. Ltd, London
Jaleel CA, Kishorekumar A, Manivannan P, Sankar B, Gomathinayagam M, Panneerselvam R (2008) Salt stress mitigation by calcium chloride in Phyllanthus amarus. Acta Bot Croat 67:53–62
Jiang M, Zhang J (2001) Effect of abscisic acid on active oxygen species, antioxidant defense system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42:1262–1273
Khan MA, Ungar IA, Showlter AM (2000) The effect of salinity on growth, water status, and ion content of a leaf succulent perennial halophyte, Suaeda fruticosa (L.) Forssk. J Arid Environ 45:72–85
Khan MN, Siddiqui MH, Mohammad F, Naeem M, Khan MMA (2010) Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiol Plant 32:121–132
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature 227:680–685
Lee DL, Kim YS, Lee CB (2001) The inductive responses of the antioxidant enzymes by salt stress in the rice (Oryza sativa L.). J Plant Physiol 158:737–745
Liang YC, Chen Q, Liu Q, Zhang W, Ding R (2003) Exogenous silicon (Si) increases antioxidant enzyme activities and reduced lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J Plant Physiol 160:1157–1164
Lin CC, Kao CH (1999) NaCl induced changes in ionically bounds peroxidase activities in roots of rice seedlings. Plant Soil 216:147–153
Marschner H (1986) Mineral nutrition of higher plants. Academic Press, Boston
Meloni DA, Oliva MA, Martinez CA, Cambraia J (2003) Photosynthesis and activities of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 49:69–76
Mhadhbi H, Jebara M, Limam F, Aouani ME (2004) Rhizobial strain involvement in plant growth, nodule protein composition and antioxidant enzyme activities of chickpes-rhizobia symbioses: modulation by salt stress. Plant Physiol Biochem 42:717–722
Molassiotis AN, Sotiropoulos T, Tanou G, Kofidis G, Diamantidis G (2006) Antioxidant and anatomical responses in shoot culture of the apple rootstock MM 106 treated with NaCl, KCl, mannitol or sorbitol. Biol Plant 50:61–68
Muthukumarasamy M, Gupta SD, Pannerselvam R (2000) Enhancement of peroxidase, polyphenol oxidase and superoxide dismutase activities by triadimefon in NaCl stressed Raphanus sativus L. Biol Plant 43:317–320
Nobel PS (1991) Physiochemical and environmental plant physiology. Academic Press, San Diego
Oba G, Nordal I, Stenseth NC, Stave J, Bjora CS, Muthondeki JK, Bii WKA (2001) Growth performance of exotic and indigenous tree species in saline soil in Turkana, Kenya. J Arid Environ 47:499–511
Parks GE, Dietrich MA, Schumaker KS (2002) Increased vacuolar Na+/H+ exchange activities in Salicornia bigelovii Torr. in response to NaCl. J Exp Bot 53:1055–1065
Qiu-Fang Z, Yuan-Yuan L, Cai-Hong P, Cong-Ming L, Bao-Shan W (2005) NaCl enhances thylakoid-bound SOD activities in the leaves of C3 halophyte Suaeda salsa L. Plant Sci 168:423–430
Quiroga M, Guerrero C, Botella MA, Barcelo AR, Medina MI, Alonso FJ (2000) A tomato peroxidase involved in the synthesis of the lignin and suberin. Plant Physiol 122:1119–1127
Shalata A, Tal M (1998) The effect of salt stress on lipid peroxidatiopn and antioxidants in the of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii. Physiol Plant 104:169–174
Siddiqui MH, Mohammad F, Khan MN (2009) Morphological and physio–biochemical characterization of Brassica juncea L. Czern. & Coss. genotypes under salt stress. J Plant Interact 4:67–80
Solomon A, Beer S, Waisel Y, Jones GP, Poleg LG (1994) Effect of NaCl on the carboxylating activities of Rubisco from Tamarix jordanis in the presence and absence of proline related compatible solutes. Physiol Plant 90:189–204
Türkan Ì, Bor M, Özdemir Koca H (2005) Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci 168:223–231
Van Resenburg L, Kruger GHJ, Kruger H (1993) Proline accumulation as drought tolerance selection criterion: Its relationship to membrane integrity and chloroplast ultrastructure in Nicotiana tabacum L. J Plant Physiol 141:188–194
Weckx JEJ, Clijsters HMM (1997) Zn phytotoxicity induced oxidative stress in primary leaves of Phaseolus vulgaris. Plant Physiol Biochem 35:405–410
Wheatherley PE (1973) Studies in the water relations of cotton plants. I. The field measurement of water deficit in leaves. New Phytol 49:81–87
Winicov I, Bastol DR (1997) Salt tolerance in crop plants: new approaches through tissue culture and gene regulation. Acta Physiol Plant 19:432–449
Yu Q, Rengel Z (1999) Micronutrient deficiency influences plant growth and activities of superoxide dismutases in narrow-leafed lupins. Ann Bot 83:175–182
Acknowledgments
The financial support of this research was provided by College of Science, University of Tehran. The author thanks Dr. H. Akhani for providing the seeds of S. persica and Dr. Sh. Rashidi and Dr. F Shokohifar for their help in manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Aroca.
Rights and permissions
About this article
Cite this article
Aghaleh, M., Niknam, V., Ebrahimzadeh, H. et al. Effect of salt stress on physiological and antioxidative responses in two species of Salicornia (S. persica and S. europaea). Acta Physiol Plant 33, 1261–1270 (2011). https://doi.org/10.1007/s11738-010-0656-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0656-x