Abstract
This study aims to investigate the effects of cold and salicylic acid (SA) priming on osmolytes accumulation in wheat leaves under freezing stress and its underlying physiological mechanism. The results showed that cold and SA priming treatment significantly enhanced sucrose and free proline contents as compared with non-priming treatment under freezing, resulting in increased leaf water potential, reduced cell death and boosted freezing tolerance. Cold and SA priming-induced free proline accumulation under freezing not only depended on promoting its synthesis, but also on inhibiting its degradation. Interestingly, the synthesis and hydrolysis of sucrose were both increased by cold and SA priming treatment under freezing. Besides, cold and SA priming up-regulated the catabolism of glucose and the assimilation of ammonia as compared with non-priming treatment under freezing stress. Findings of the present study suggested that cold and SA priming could simultaneously promote-free proline and sucrose accumulation in wheat leaves by coordinating carbon and nitrogen metabolism under freezing conditions, and then conferring tolerance to freezing stress. These findings could provide a new insight into the mechanisms by which cold and SA priming enhanced freezing tolerance in wheat.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Late spring frost is an important limiting factor in winter wheat production worldwide, including the USA (Gu et al. 2008), Australia (Zheng et al. 2015), Italy (Bascietto et al. 2019) and China (Zhong et al. 2008). However, while slightly counterintuitive, the probability and severity of spring frost are predicted to increase due to global warming (Gu et al. 2008; Ji et al. 2017). In addition to thermodynamical inhibition of metabolic reactions, sub-zero temperatures can also cause other adverse effects such as reactive oxygen species (ROS) over-accumulation, cell dehydration and contraction (Ruellan et al. 2009; Shin et al. 2018). Among them, dehydration stress is the main cause of deterioration of the intracellular structures and death of tissues induced by sub-zero temperatures (Ruellan et al. 2009).
To cope with dehydration damage, wheat plants can induce the accumulation of compatible solutes (such as free proline, sucrose, glycine betaine and polyols) to support osmotic regulation and sustain cell turgor, which play an important role in maintaining water potential in the plant (Wani et al. 2018; Wang et al. 2019). In addition to their role in osmoregulation, free proline and sucrose can act as signaling molecules to regulate expression of stress-related genes, as ROS scavenger, and as nutrients to help plants recover from stresses (Couee et al. 2006; Islam et al. 2021; Szabados and Savourcb 2010). It is well-documented that the accumulation of free proline and sucrose plays a critical role in winter wheat response to freezing stress (Dörffling et al. 1990; Kamata and Uemura 2004; Kovács et al. 2011).
It is interesting to note that the accumulation of sucrose and free proline often occur simultaneously in plant response to environmental stresses including cold, drought and salt (Cai et al. 2004; Ehsan et al. 2012; Perezalfocea and Larher 1995; Wang et al. 2019). Sucrose and proline metabolism are important components of carbon and nitrogen metabolism, respectively (Martins et al. 2020; Vágújfalvi et al. 1999). Several studies have demonstrated that there was a close relationship between carbon and nitrogen metabolism in plants, which was critical for plant growth and development (Bao et al. 2015; Gálvez et al. 1999). It is reasonable to speculate that the coordination between carbon and nitrogen metabolism plays an important role in the simultaneous accumulation of sucrose and free proline in plant response to environmental stimulus.
Priming, defined as a temporal experience of an environmental stimulus (including exposure to the stress cues themselves as well as the exogenous application of biologically active chemicals), can effectively improve the plant’s level of resistance to future stress events (Baier et al. 2019; Zuther et al. 2019). Salicylic acid (SA) is an important phenolic derivative widely distributed in the plant kingdom and is known to be a crucial signalling molecule in response to abiotic and biotic stresses (Saleem et al. 2020; Sadiq et al. 2020; Zaid et al. 2019; Ahmad et al. 2019; Dong et al. 2014). Previous studies have shown that SA and cold temperature priming could effectively enhance wheat freezing tolerance (Sun et al. 2018; Wang et al. 2020a). Zuther et al. (2019) indicted that the accumulation of sucrose and free proline was involved in the cold priming-induced tolerance to freezing in Arabidopsis. A series of studies showed that exogenous SA could promote accumulation of free proline in plant leaves under both normal and cold conditions (Min et al. 2018; Ignatenko et al. 2019). Exogenous SA decreased sucrose content in tomato leaves under normal condition (Poór et al. 2011), but increased sucrose content in spinach leaves under cold condition (Min et al. 2018). However, the regulatory mechanisms of cold and SA priming on sucrose and free proline accumulation under freezing stress in wheat are poorly understood.
Therefore, we hypothesized that cold and SA priming can promote freeze-induced sucrose and free proline accumulation by intensifying the coordination between carbon and nitrogen metabolism, which is contributing to improving wheat freezing tolerance. To test this hypothesis, we investigated the effects of cold and SA priming on cell dehydration, free proline and sucrose accumulation, and key enzymes and metabolites involved in carbon and nitrogen metabolism under freezing stress in wheat leaves.
Materials and Methods
Experiment Design
The locally and widely planted winter wheat (Triticum aestivum L. cv Yangmai 16) was planted in plastic pots (22 cm in height and 25 cm in diameter), with a planting density of 7 plants per pot. The fertilizer and water management was carried out as described by Wang et al. (2020b). Wheat plants were grown outdoor until the application of priming treatments.
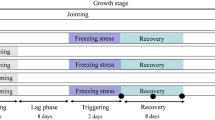
At the beginning of the jointing stage (when the 1st node was detectable, Zadoks 2010), one-third of the wheat plants were transferred into a growth chamber with a temperature of 16 °C/10 °C (day/night, set according to the ambient temperature) as control treatment (C); one-third of the wheat plants were primed with cold temperature (6 °C/2 °C, day/night) for 2 days as cold priming treatment (P); one-third of the wheat plants were primed with 100 μM SA (Sigma-Aldrich, St. Louis, MO) by foliage spraying for 2 days (twice a day with an interval of 12 h) under control temperature as SA priming treatment (S). Then, all the plants were grown under control temperature for recovery. After 8 days of recovery, half of each group of the plants were subjected to freezing stress for 2 days (the first day/night temperature was 2 °C/0 °C, and the second day/night temperature was − 2 °C/ − 4 °C), while the other half were kept at control temperature (16 °C/10 °C, day/night). Finally, six treatments composed: control (CC), SA priming without freezing stress (SC), cold priming without freezing stress (PC), freezing stress without priming (CF), SA priming plus freezing stress (SF) and cold priming plus freezing stress (PF). The light intensity of the chambers was set at 500 μmol m−2 s−1 with a photoperiod of 10 h. The latest fully expanded leaves were sampled at the end of freezing treatment for further biochemical and physiological analyses.
Freezing Tolerance Determination
The freezing tolerance of wheat leaves was determined by the LT50 (temperature at which 50% of ions in plant tissues leak out) according to Cai et al. (2004) with slightly modifications. Fresh leaves of C, S and P treatments at 8 days of recovery were cut into 1.5 cm lengths and wrapped in wet gauze, then placed in a glass tube and incubated at 4 °C for 2 h. After incubation, the leaves were treated at different freezing temperatures (0, − 3, − 6, − 9, − 12 and – 15 °C) for 2 h in a freezing bath (CDN-1007020F, Southeast Co. Ltd., Ningbo, China). Then, the leaves were thawed at 4 °C overnight and used to measure electrolyte leakage using a electrical conductivity bridge (DDS-307A, LEX Instruments Co. Ltd., Shanghai, China). The mean values of electrolyte leakage of each treatment were utilized to generate a sigmoid curve fitting the Logistic function, and the temperature corresponding to the inflection point of the curve is the LT50 (Sigmaplot 10.0, Systat Software Inc., CA, USA).
Leaf Water Potential and Histochemical Staining
The WP4C Dewpoint PotentiaMeter (Decagon Devices Inc., WA, USA) was used to detect water potential of the latest fully expanded leaves following the manufacturer’s instruction. Trypan blue and Evans blue staining were used to detect dead cells of the latest fully expanded leaves according to Koch and Slusarenko (1990) and Baker and Mock (1994), respectively.
Metabolites Contents
The contents of free proline and sucrose were determined by Lu et al. (2005) and Huber (1983) method, respectively. Ammonium measurements were carried out as described by Husted et al. (2000). Glutamate contents were analyzed using an L-8900 High Speed Amino Acid Analyzer (Hitachi Corp., Tokyo, Japan) as described by Zhong et al. (2018). 2-oxoglutarate (2-OG) contents were determined using an ACQUITY UPLC H-Class (Waters Corp., MA, USA) according to Lee and Foy (1986).
Enzyme Assays
The activities of sucrose-phosphate synthase (SPS) and sucrose synthase (SS, synthetic direction) were detected according to Wardlaw and Willenbrink (1994). The activities of acid-invertase (Ac-INV) and alkaline/neutral-invertase (A/N-INV) were measured according to Miron and Schaffer (1991) and Vargas et al. (2007), respectively. The activities of Δ1-pyrroline-5-carboxylate synthetase (P5CS) and proline dehydrogenase (PDH) were determined according to Mario et al. (1997) and Rena and Splittstoesser (1975), respectively. The activities of glutamine synthetase (GS), NADH-dependent glutamate dehydrogenase (NADH-GDH) and NADP-dependent isocitrate dehydrogenase (NADP-ICDH) were measured by the method of Lu et al. (2005).
Genes Expression
Total RNA extraction, cDNA synthesis and quantitative real time PCR (qRT-PCR) were performed as described by Wang et al. (2018). The specific primers of genes encoding ornithine- δ-aminotransferase (OAT), pyrroline-5-carboxylate dehydrogenase (P5CDH), hexokinase (HXK), citrate synthetase (CS), pyruvate kinase (PK) and malate dehydrogenase (MDH) are listed in Table S1. The relative expression levels of genes were calculated according to the 2 − ΔΔCt method (Wang et al. 2018), using Actin gene as a reference gene.
Statistical Analysis
Statistical analysis was performed using analysis of variance (ANOVA) followed by Duncan's multiple range test (SPSS18.0, SPSS Inc., IL, USA), and P < 0.05 was statistically significant.
Results
LT50, Leaf Water Potential and Cell Death
As shown in Fig. 1A, cold and SA priming treatment (S and P) decreased the LT50 from − 4.4 °C (non-priming treatment, C) to − 5.3 °C and − 5.8 °C, respectively, demonstrating the beneficial effect of cold and SA priming treatment on freezing tolerance enhancement. The LT50 was calculated by electrolyte leakage of leaves. Therefore, we hypothesized that priming with SA and cold temperature could alleviate freeze-induced tissue dehydration in wheat leaves.
Effects of SA and cold priming treatment on freeze-induced dehydration injury in wheat leaves. A LT50. The measurement was taken at the end of the lag phase (8 days of recovery from priming treatment). C no priming treatment, S SA priming treatment, P cold priming treatment. Each value of electrolyte leakage is the mean ± SE of three biological replicates; B Leaf water potential. Each value is the mean ± SE of three biological replicates. The different lowercase letters indicate statistically significant differences at P < 0.05 level; C Trypan blue staining; D Evans blue staining. B–D were determined at the end of the freezing treatment. C–D blue spots indicate dead cells. CC no SA or cold priming + no freezing stress, SC SA priming + no freezing stress, PC cold priming + no freezing stress, CF no SA or cold priming + freezing stress, SF SA priming + freezing stress, PF cold priming + freezing stress
As shown in Fig. 1B, the freezing treatment caused a significant decrease in leaf water potential. However, the SF and the PF treatments showed lower reduction of leaf water potential than the CF treatment (Fig. 1B). As compared to the CC treatment, the leaf water potential was decreased by 45.6%, 48.5% and 89.5% in the SF, the PF and the CF treatments, respectively. Evans blue and Trypan blue staining showed that more dead cells (as indicated by the blue spots) were present in the CF treatment as compared with the SF and the PF treatments (Fig. 1C, D). There was no significant difference in the leaf water potential and the number of dead cells between the primed and non-primed plants under non-freezing conditions (Fig. 1B–D). These results suggested that cold and SA priming could alleviate dehydration-induced cell death under freezing stress.
Activities and Expression Levels of Key Enzymes Involved in Proline and Sucrose Biosynthesis
The activity of SPS plays a crucial role in controlling sucrose biosynthesis in wheat (Vargas et al. 2007), and SS is an important enzyme considered to have reversible sucrose synthesis and cleavage functions (Kumutha et al. 2008). As shown in Fig. 2A and B, the SC and the PC treatments significantly increased the activities of SPS and SS (synthetic direction) as compared with the CC treatment. Freezing stress obviously enhanced the activities of SPS and SS (Fig. 2A, B). However, there were increases in SPS (67.3% and 72.3%, respectively) and SS (44.9% and 47.7%, respectively) activities in the SF and the PF treatments as compared with the CF treatment (Fig. 2A, B).
Effects of SA and cold priming on the activities and the expression levels of sucrose and free proline biosynthesis-related enzymes in wheat leaves. A Sucrose-phosphate synthase (SPS) activity; B Sucrose synthase (SS, synthetic direction) activity; C Δ1-pyrroline-5-carboxylate synthetase (P5CS) activity; D Relative expression level of OAT (encoding ornithine- δ-aminotransferase). A–D were determined at the end of the freezing treatment. CC no SA or cold priming + no freezing stress, SC SA priming + no freezing stress, PC cold priming + no freezing stress, CF no SA or cold priming + freezing stress, SF SA priming + freezing stress, PF cold priming + freezing stress. Each value is the mean ± SE of three biological replicates. For gene expression data, each biological replicate has three technical replicates. The different lowercase letters indicate statistically significant differences at P < 0.05 level
In higher plants, proline can be synthesized from glutamate and ornithine, which are, respectively, catalyzed by P5CS and OAT (Szabados and Savourcb 2010). As shown in Fig. 2C and D, the SC treatment clearly increased P5CS activity and the expression level of OAT as compared with the CC treatment. The PC treatment caused an obvious increase in the expression level of OAT, while it had no significant effect on P5CS activity as compared with the CC treatment (Fig. 2C, D). Freezing stress significantly up-regulated the expression level of OAT (2.31-fold), but it had no significant effect on P5CS activity as compared with the CC treatment (Fig. 2C, D). Here, the SF and the PF treatments showed relatively higher P5CS activity (73.1% and 87.3%, respectively) and expression level of OAT (1.52-fold and 1.45-fold, respectively) as compared with the CF treatment (Fig. 2C, D).
Free Proline and Sucrose Contents
Both free proline and sucrose contents were clearly enhanced by freezing stress (Fig. 3). Moreover, the SF and the PF treatments showed strikingly higher free proline and sucrose contents as compared with the CF treatment (Fig. 3). As compared to the CF treatment, the free proline and sucrose contents were increased by 64.9% and 13.1% under the SF treatment, while increased 60.5% and 20.9% under the PF treatment, respectively. The SC and the PC treatments significantly increased the contents of free proline (70.4% and 142%, respectively) and sucrose (28.0% and 32.8%, respectively) as compared with the CC treatment (Fig. 3). These results suggested that cold and SA priming could promote-free proline and sucrose accumulation under freezing conditions contributing to alleviating freeze-induced dehydration damage.
Effects of SA and cold priming on sucrose and free proline contents in wheat leaves. A Sucrose content; B Free proline content. A–B were determined at the end of the freezing treatment. CC no SA or cold priming + no freezing stress, SC SA priming + no freezing stress, PC cold priming + no freezing stress, CF no SA or cold priming + freezing stress, SF SA priming + freezing stress, PF cold priming + freezing stress. Each value is the mean ± SE of three biological replicates. The different lowercase letters indicate statistically significant differences at P < 0.05 level
Activities and Expression Levels of Key Enzymes Involved in Proline and Sucrose Catabolism
Sucrose can be hydrolyzed into glucose and fructose by INV in wheat (Vargas et al. 2007). As shown in Fig. 4A and B, the SC treatment had no significant effects on the activities of A/N-INV and Ac-INV as compared with the CC treatment. The PC treatment significantly increased the activities of A/N-INV and Ac-INV as compared with the CC treatment (Fig. 4A, B). Freezing stress obviously enhanced the activities of A/N-INV and Ac-INV (Fig. 4A, B). The SF and the PF treatments showed relatively higher A/N-INV (20.4% and 8.89%, respectively) and Ac-INV (12.4% and 10.3%, respectively) activities as compared with the CF treatment (Fig. 4A, B).
Effects of SA and cold priming on the activities and the expression levels of sucrose and free proline catabolism-related enzymes in wheat leaves. A Alkaline/neutral-invertase (A/N-INV) activity; B Acid-invertase (Ac-INV) activity; C Proline dehydrogenase (PDH) activity; D Relative expression level of P5CDH (encoding pyrroline-5-carboxylate dehydrogenase). A–D were determined at the end of the freezing treatment. CC no SA or cold priming + no freezing stress, SC SA priming + no freezing stress, PC cold priming + no freezing stress, CF no SA or cold priming + freezing stress, SF SA priming + freezing stress, PF cold priming + freezing stress. Each value is the mean ± SE of three biological replicates. For gene expression data, each biological replicate has three technical replicates. The different lowercase letters indicate statistically significant differences at P < 0.05 level
The degradation of proline is catalyzed by PDH and P5CDH in higher plants (Yang et al. 2016). As shown in Fig. 4C and D, the SC treatment significantly decreased PDH activity, and up-regulated the expression level of P5CDH as compared with the CC treatment. The PC treatment obviously up-regulated PDH activity and the expression level of P5CDH as compared with the CC treatment (Fig. 4C, D). The CF treatment significantly increased PDH activity (18.7%) and the expression level of P5CDH (4.47-fold) as compared with the CC treatment (Fig. 4C, D). Here, there were decreases in PDH activity (18.4% and 11.4%, respectively) and expression level of P5CDH (0.76-fold and 0.75-fold, respectively) in the SF and the PF treatments as compared with the CF treatment (Fig. 4C, D).
Ammonium Assimilation
The activity of GS and NADH-GDH play critical role in regulating the assimilation of ammonia for glutamate synthesis in plants (Miflin and Habash 2002). There was no substantial difference in the contents of ammonium and activities of GS and NADH-GDH between the primed and non-primed plants under non-freezing conditions, except for a significant increase of NADH-GDH activity in the SC treatment as compared with the CC treatment (Table 1). The CF treatment caused a significant increase in ammonium content (115%) and GS activity (12.2%), and a slight increase in NADH-GDH activity (8.0%) as compared with the CC treatment (Table 1). The SF and the PF treatments obviously increased the activities of GS (13.0% and 17.4%, respectively) and NADH-GDH (20.1% and 16.3%, respectively), and dramatically decreased ammonium content (46.8% and 39.2%, respectively) as compared with the CF treatment (Table 1). These results suggested that cold and SA priming promoted the assimilation of ammonium under freezing. Here, we investigated glutamate content in each treatment to further confirm this inference. Interestingly, the SF and the PF treatments induced a significant reduction by 28.7% and 26.5% in glutamate content in wheat leaves as compared with the CF treatment (Table 1).
NADP-ICDH Activity and 2-OG Content
In plants, 2-OG is an important carbon skeleton for glutamate synthesis (Miflin and Habash 2002), and cytosolic NADP-ICDH plays a key role in 2-OG synthesis (Wang et al. 2007). As shown in Fig. 5, the SC and the PC treatments insignificantly increased NADP-ICDH activity, and remarkably increased the content of 2-OG as compared with the CC treatment. The CF treatment induced a slight increase in NADP-ICDH activity (13.0%), but obviously enhanced the accumulation of 2-OG (61.3%) as compared with the CC treatment (Fig. 5). Interestingly, the SF and the PF treatments clearly increased NADP-ICDH activity by 23.0% and 35.1%, but significantly decreased 2-OG content by 14.9% and 11.9% as compared with the CF treatment, repectively (Fig. 5).
Effects of SA and cold priming on the activities of NADP-ICDH and the contents of 2-OG in wheat leaves. A NADP-dependent isocitrate dehydrogenase (NADP-ICDH) activity; B 2-oxoglutarate (2-OG) content. A–B were determined at the end of the freezing treatment. CC no SA or cold priming + no freezing stress, SC SA priming + no freezing stress, PC cold priming + no freezing stress, CF no SA or cold priming + freezing stress, SF SA priming + freezing stress, PF cold priming + freezing stress. Each value is the mean ± SE of three biological replicates. The different lowercase letters indicate statistically significant differences at P < 0.05 level
Expression Levels of Key Enzymes Involved in Carbon Metabolism
Expression levels of four key genes (HXK, CS, PK and MDH) involved in carbon metabolism were investigated here. There was no substantial difference in expression levels of these genes between the primed and non-primed plants under non-freezing conditions, except for a significant up-regulation of the expression level of HXK in the SC treatment as compared with the CC treatment (Fig. 6). The CF treatment significantly up-regulated the expression levels of HXK (1.81-fold) and CS (1.82-fold), while it had no significant effects on the expression level of PK and MDH as compared with the CC treatment (Fig. 6). The SF and the PF treatment evidently up-regulated the expression level of MDH (2.22-fold and 1.75-fold, respectively), and gently up-regulated the expression level of HXK, PK and CS as compared with the CF treatment (Fig. 6).
Effects of SA and cold priming on the expression levels of genes involved in glycolysis and the tricarboxylic acid (TCA) cycle in wheat leaves. A Relative expression level of HXK (encoding hexokinase); B Relative expression level of CS (encoding citrate synthetase); C Relative expression level of PK (encoding pyruvate kinase); D Relative expression level of MDH (encoding malate dehydrogenase). A–D were determined at the end of the freezing treatment. CC no SA or cold priming + no freezing stress, SC SA priming + no freezing stress, PC cold priming + no freezing stress, CF no SA or cold priming + freezing stress, SF SA priming + freezing stress, PF cold priming + freezing stress. Each value is the mean ± SE of three biological replicates, and each biological replicate has three technical replicates. The different lowercase letters indicate statistically significant differences at P < 0.05 level
Discussion
Despite the important role of sucrose and proline in winter wheat resisting freezing stress has been well recorded, the underlying mechanisms of freeze-induced sucrose and free proline accumulation are not completely understood. In the present study, the synthesis and degradation of sucrose were both clearly increased by freezing, as revealed by a significant higher SPS, SS (synthetic direction), A/N-INV and Ac-INV activities under freezing conditions than non-freezing conditions (Figs. 2A, B, and 4A, B). These results were similar to those of Savitch et al. (2000), who found that chilling stress prominently increased the activities of SPS, SS (synthetic direction), A/N-INV and Ac-INV in wheat leaves. Kaplan et al. (2007) indicated that starch degradation played an important role in chilling-induced sugar accumulation in Arabidopsis. The degradation of starch leaded to the accumulation of glucose-6-phosphate and fructose-phosphate (immediate precursors for sucrose biosynthesis), then to sucrose and then to fructose and glucose (Kaplan et al. 2007). In the light of the above studies, it appears that the accumulation of sucrose in wheat leaves under cold temperature stress might be due to a faster rate or an earlier start of its synthesis than degradation. Zhang et al. (2019) reported that freezing stress significantly increased sucrose content and SPS activity, but decreased INV activity in wheat ears at the booting stage. These studies suggested that the differential mechanisms of freeze-induced sucrose accumulation found in different organs of wheat.
Previous studies reported that the increased INV activity could contribute to producing more hexoses (fructose and glucose) that would be metabolized into energy and intermediate metabolites for plant resistance to stresses (Sehar et al. 2019; Savitch et al. 2000; Vargas et al. 2007). This was further confirmed by our results that the up-regulation of INV activity and the expression levels of HXK (encoding hexokinase, the crucial rate-limiting enzyme relating to the glycolytic pathway) and CS (encoding citrate synthetase, the crucial rate-limiting enzyme relating to the tricarboxylic acid cycle) induced by freezing treatment (Figs. 4A, B, and 6A, B). In addition, increased INV activity was associated with the transport of sucrose to the vacuole where it is used for fructan synthesis, which also played an important role in the regulation of freezing tolerance in wheat (Amani 2008; Vágújfalvi et al. 1999).
Previous studies indicated that both the glutamate pathway and the ornithine pathway were implicated in chilling-induced proline synthesis (Cao et al. 2012; Yang et al. 2016). However, in this study, freezing treatment caused a significant up-regulation of the expression level of gene encoding OAT, but it had no significant effect on P5CS activity (Fig. 2C, D). Yang et al. (2009) and (2016) found that the glutamate pathway was activated prior to the ornithine pathway when maize and Jatropha curcas seedlings responded to stress factors such as chilling temperature and hydrogen peroxide. Due to the activity of P5CS determined at the end of freezing treatment here, the role of the glutamate pathway in proline synthesis at the early time points of wheat plants response to freezing cannot be excluded. However, our results suggested that the ornithine pathway played an important role in proline synthesis at the later stage of freezing stress in wheat.
The accumulation of proline depends not only on its synthesis, but also on its degradation (Liu et al. 2020; Szabados and Savourcb 2010). Cao et al. (2012) and Zeng et al. (2015) indicated that the increase in proline level in loquat fruit and bamboo shoots under chilling stress were related with lower PDH activity. Intriguingly, freezing treatment significantly up-regulated the activity of PDH and the expression level of gene encoding P5CDH in this study (Fig. 4C, D). Szabados and Savourcb (2010) indicated that proline had a feedback effect on transcriptional regulation of proline metabolism. Thus, the up-regulation of activity of PDH and expression level of P5CDH at the end of freezing treatment might be induced by the accumulated free proline.
Due to glutamate is an important substrate for proline synthesis, the accumulation of proline is closely associated with ammonia metabolism (Martins et al. 2020). In the present study, freezing stress obviously enhanced ammonia content in wheat leaves (Table 1). This is in accordance with previous studies that the enhancement of photorespiration capacity and ROS-induced proteolysis under stress conditions could lead to an increase in intracellular ammonia (Skopelitis et al. 2006; Wang et al. 2007). The synthetic precursor of 2-OG is citrate, an intermediate metabolite of the tricarboxylic acid cycle (Gálvez et al. 1999). Therefore, freeze-enhanced the expression of HXK and CS and the activity of NADP-ICDH could contribute to the increase in 2-OG level (Figs. 5 and 6A, B). Previous studies indicated that accumulation of ammonia and 2-OG could regulate the activities of GS and NADH-GDH (Gálvez et al. 1999; Wang et al. 2016). Thus, the increased ammonia and 2-OG might induce the increasement of GS and NADH-GDH activities under freezing, resulting in a significant increase in glutamate content that could be used for proline synthesis (Table 1). These results were consistent with Lu et al. (2005), who found that the accumulation of free proline under chilling stress in rice was in good agreement with the induction of GS and NADP-ICDH activities. All these findings suggested that the coordination of carbon and nitrogen metabolism played an important role in the freeze-induced simultaneous accumulation of sucrose and free proline in wheat leaves.
It has been well-documented that winter wheat can acquire tolerance to sub-zero temperatures by a process known as cold acclimation or cold hardening (Kaplan et al. 2007; Savitch et al. 2000). Nevertheless, there are some differences between cold acclimation and cold priming, which have been well elucidated in several recent papers (Baier et al. 2019; Zuther et al. 2019). Previous studies demonstrated that accumulation of compatible solutes plays an important role in the cold acclimation-induced freezing tolerance in wheat (Vágújfalvi et al. 1999; Wardlaw and Willenbrink 1994). However, the effects of cold priming on compatible solutes accumulation under freezing remain largely unidentified. In this study, we found that cold priming treatment significantly increased free proline and sucrose contents in wheat leaves as compared with non-priming treatment under both non-freezing and freezing conditions (Fig. 3). This is in good agreement with a recent study by Zuther et al. (2019), who reported that the accumulation of osmoprotective compounds such as galactinol, sucrose and proline were involved in the cold priming-induced freezing tolerance in Arabidopsis. In addition, our previous studies reported that drought primed plants showed significantly higher levels of the compatible solutes such as free proline, sucrose and glycine betaine as compared with non-primed pants under the subsequent drought stress (Wang et al. 2019, 2018). Taken together, these results suggested that accumulation of compatible solutes is an important mechanism of the priming events inducing stress tolerance in wheat plants.
Numerous recent reports have shown that exogenous SA could promote accumulation of free proline under stressful conditions (Sedaghat et al. 2020; Min et al. 2018; Estaji and Niknam 2020). The regulatory effect of SA on accumulation of free proline in this study was consistent with the previous studies (Fig. 3B). However, previous reports about the regulatory effect of SA on sucrose level was inconsistent. Poór et al. (2011) and Dong et al. (2011) found that SA significantly decreased sucrose content in tomato and cucumber leaves, but increased its content in roots under salinity stress. La et al. (2019a) and Min et al. (2018) reported that SA markedly enhanced sucrose content in Brassica rapa and spinach leaves under drought and freezing stress, respectively. In this study, SA significantly increased sucrose level in wheat leaves under both non-freezing and freezing conditions (Fig. 3A). These results indicated that the regulatory effect of SA on sucrose level might be dependent on species and type of organs and stresses.
In the present work, it was shown that cold and SA priming-induced free proline accumulation by promoting its synthesis and inhibiting its degradation in wheat leaves as compared with non-priming treatment under freezing conditions (Figs. 2C, D, and 4C, D). The results were consistent with La et al. (2019b) who found that exogenous SA increased free proline content by up-regulating the expression level of P5CS and down-regulating the expression level of PDH in Brassica rapa leaves under drought stress. Previous studies indicated that exogenous SA could enhance sucrose level through increasing the activity of SPS and the degradation of starch in Brassica rapa leaves under drought stress (La et al. 2019a). Our results showed that cold and SA priming promoted sucrose accumulation by increasing the activities of SPS and SS (synthetic direction) in wheat leaves under freezing stress (Fig. 2A, B). However, the role of starch degradation in SA and cold priming-induced the accumulation of sucrose in wheat leaves under freezing need to be investigated further.
In this study, priming treatment significantly increased activities of GS and NADH-GDH, and decreased level of ammonia as compared with non-priming treatment under freezing stress (Table 1). This indicated that cold and SA priming improved ammonia assimilation under freezing stress, which could provide more synthetic precursor (glutamate) for the synthesis of proline. Meanwhile, cold and SA primed plants showed relatively higher activities of INV and NADP-ICDH, and higher expression level of genes involved in the glycolytic pathway (such as HXK and PK) and the tricarboxylic acid cycle (such as CS and MDH) as compared with non-primed plants under freezing stress (Figs. 4–6), indicating that cold and SA priming promoted the catabolism of sucrose and glucose under freezing stress. This might contribute to producing more energy and carbon skeleton (2-OG) for ammonia assimilation. Collectively, these results suggested that cold and SA priming could intensify the coordination between carbon and nitrogen metabolism to enhance the accumulation of compatible solutes in wheat leaves under the later freezing stress, and therefore effectively alleviated freeze-induced dehydration damage.
But interestingly, SA and cold primed plants showed significantly lower level of 2-OG as compared with non-primed plants under freezing conditions (Fig. 5B). This might be due to the accelerated ammonia assimilation by the priming treatment under freezing conditions (Table 1). In addition, the higher level of free proline in primed plants might explain why the primed plants accelerated ammonia assimilation but had lower glutamate contents as compared with the non-primed plants under freezing conditions (Table 1).
Our previous study found that cold priming significantly increased SA level in wheat leaves, and the results of pharmacological experiment indicated that SA signal was involved in the cold priming-induced tolerance to freezing in wheat (Wang et al. 2021). In the present study, the results indicated that SA and cold priming have similar mechanisms in inducing sucrose and free proline accumulation in wheat leaves to alleviate freeze-caused dehydration damage (Fig. 7). This could provide indirectly evidence for the involvement of SA in the cold priming-induced freezing tolerance, and should help the introduction of SA into practices of anti-freezing stress cultivation in wheat.
Schematics of SA and cold priming simultaneously enhanced freeze-induced sucrose and proline accumulation by intensifying the coordination of carbon and ammonia metabolism in wheat leaves. SPS sucrose-phosphate synthase, SS sucrose synthase, INV invertase, NADP-ICDH NADP-dependent isocitrate dehydrogenase; TCA cycle tricarboxylic acid cycle, 2-OG 2-oxoglutarate, NADH-GDH NADH-dependent glutamate dehydrogenase, GS glutamine synthetase, GOGAT glutamate synthase, P5CS Δ1-pyrroline-5-carboxylate synthetase, OAT ornithine- δ-aminotransferase, PDH proline dehydrogenase, P5CDH pyrroline-5-carboxylate dehydrogenase, ROS reactive oxygen species. Black arrows indicate the positive regulation of freezing temperature in metabolites level, enzymes activity and genes expression. Red arrows indicate the positive or negative regulation of SA and cold priming in metabolites level, enzymes activity and genes expression
Conclusions
In conclusion, the coordination between carbon and nitrogen metabolism played important role in the simultaneous accumulation of sucrose and free proline in wheat leaves response to freezing stress, and cold and SA priming could intensify this coordination to enhance sucrose and free proline levels, which was contributing to the alleviation of freeze-induced cell dehydration (Fig. 7). It has become clear that carbon and nitrogen metabolism are regulated to enable the coordination essential for plant growth and development. To the best of our knowledge, this is the first report owing that the important role of the coordination between carbon and nitrogen metabolism in wheat responded to freezing stress. However, the carbon and nitrogen metabolisms of plants involve many processes, the more detailed mechanisms of plant coordinated carbon and nitrogen metabolism in response to environmental stresses need further study.
References
Ahmad B, Zaid A, Sadiq Y, Bashir S, Wani SH (2019) Role of selective exogenous elicitors in plant responses to abiotic stress tolerance. In: Hasanuzzaman M, Hakeem K, Nahar K, Alharby H (eds) Plant abiotic stress tolerance. Springer, Cham
Amani A-L (2008) Activity of sucrose synthase and acid invertase in wheat seedlings during a cold-shock using micro plate reader assays. Aust J Basic Appl Sci 1(2):53–56
Baier M, Bittner A, Prescher A, Van BJ (2019) Preparing plants for improved cold tolerance by priming. Plant Cell Environ 42(3):782–800. https://doi.org/10.1111/pce.13394
Baker CJ, Mock NM (1994) An improved method for monitoring cell death in cell suspension and leaf disc assays using Evans blue. Plant Cell Tiss Org 39:7–12. https://doi.org/10.1007/BF00037585
Bao A, Liang Z, Zhao Z, Cai H (2015) Overexpressing of OsAMT1-3, a high affinity ammonium transporter gene, modifies rice growth and carbon-nitrogen metabolic status. Int J Mol Sci 16(12):9037–9063. https://doi.org/10.3390/ijms16059037
Bascietto M, Bajocco S, Ferrara C, Alivernini A, Santangelo E (2019) Estimating late spring frost-induced growth anomalies in European beech forests in Italy. Int J Biometeorol 63:1039–1049. https://doi.org/10.1007/s00484-019-01718-w
Cai Q, Wang S, Cui Z, Sun J, Ishii Y (2004) Changes in freezing tolerance and its relationship with the contents of carbohydrates and proline in overwintering centipedegrass (Eremochloa ophiuroides (Munro) Hack.). Plant Prod Sci 7(4):421–426. https://doi.org/10.1626/pps.7.421
Cao S, Cai Y, Yang Z, Zheng Y (2012) MeJA induces chilling tolerance in loquat fruit by regulating proline and γ-aminobutyric acid contents. Food Chem 133(4):1466–1470. https://doi.org/10.1016/j.foodchem.2012.02.035
Couee I, Sulmon C, Gouesbet G, Amrani AE (2006) Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J Exp Bot 57(3):449–459. https://doi.org/10.1093/jxb/erj027
Dong CJ, Wang XL, Shang QM (2011) Salicylic acid regulates sugar metabolism that confers tolerance to salinity stress in cucumber seedlings. Sci Hortic 129(4):629–636. https://doi.org/10.1016/j.scienta.2011.05.005
Dong C-J, Li L, Shang Q-M, Liu X-Y, Zhang Z-G (2014) Endogenous salicylic acid accumulation is required for chilling tolerance in cucumber (Cucumis sativus L.) seedlings. Planta 240:687–700. https://doi.org/10.1007/s00425-014-2115-1
Dörffling K, Schulenburg S, Lesselich G, Dörffling H (1990) Abscisic acid and proline levels in cold hardened winter wheat leaves in relation to variety-specific differences in freezing resistance. J Agron Crop Sci 165(4):230–239. https://doi.org/10.1111/j.1439-037X.1990.tb00857.x
Ehsan T, Foad F, Pichu R, Mcdonald GK (2012) A comparison of hydroponic and soil-based screening methods to identify salt tolerance in the field in barley. J Exp Bot 63(10):3853–3867. https://doi.org/10.1093/jxb/ers085
Estaji A, Niknam F (2020) Foliar salicylic acid spraying effect’ on growth, seed oil content, and physiology of drought-stressed Silybum marianum L. plant. Agr Water Manag 234:106116
Gálvez S, Lancien M, Hodges M (1999) Are isocitrate dehydrogenases and 2-oxoglutarate involved in the regulation of glutamate synthesis? Trends Plant Sci 4(12):484–490. https://doi.org/10.1016/S1360-1385(99)01500-9
Gu L, Hanson PJ, Post WM, Kaiser DP, Yang B, Nemani R, Pallardy SG, Meyers T (2008) The 2007 eastern US spring freeze: increased cold damage in a warming world? Bioscience 58(3):253–262. https://doi.org/10.1641/B580311
Huber SC (1983) Role of sucrose-phosphate synthase in partitioning of carbon in leaves. Plant Physiol 71(4):818–821. https://doi.org/10.1104/pp.71.4.818
Husted S, Schjoerring JK, Nielsen KH, Nemitz E, Sutton MA (2000) Stomatal compensation points for ammonia in oilseed rape plants under field conditions. Agr Forest Meteorol 105(4):371–383. https://doi.org/10.1016/S0168-1923(00)00204-5
Ignatenko A, Talanova V, Repkina N, Titov A (2019) Exogenous salicylic acid treatment induces cold tolerance in wheat through promotion of antioxidant enzyme activity and proline accumulation. Acta Physiol Plant 41(6):80. https://doi.org/10.1007/s11738-019-2872-3
Islam S, Zaid A, Mohammad F (2021) Role of triacontanol in counteracting the Ill effects of salinity in plants: A Review. J Plant Growth Regul 40(1):1–10. https://doi.org/10.1007/s00344-020-10064-w
Ji H, Xiao L, Xia Y, Song H, Liu B, Tang L, Cao W, Zhu Y, Liu L (2017) Effects of jointing and booting low temperature stresses on grain yield and yield components in wheat. Agr Forest Meteorol 243:33–42. https://doi.org/10.1016/j.agrformet.2017.04.016
Kamata T, Uemura M (2004) Solute accumulation in wheat seedlings during cold acclimation: contribution to increased freezing tolerance. Cryo-Lett 25(5):311–322
Kaplan F, Kopka J, Sung DY, Zhao W, Popp M, Porat R, Guy CL (2007) Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J 50:967–981. https://doi.org/10.1111/j.1365-313X.2007.03100.x
Koch E, Slusarenko A (1990) Arabidopsis is susceptible to infection by a downy mildew fungus. Plant Cell 2(5):437–445. https://doi.org/10.1105/tpc.2.5.437
Kovács Z, Simon-Sarkadi L, Sovány C, Kirsch K, Galiba G, Kocsy G (2011) Differential effects of cold acclimation and abscisic acid on free amino acid composition in wheat. Plant Sci 180(1):61–68. https://doi.org/10.1016/j.plantsci.2010.08.010
Kumutha D, Sairam RK, Ezhilmathi K, Chinnusamy V, Meena RC (2008) Effect of waterlogging on carbohydrate metabolism in pigeon pea (Cajanus cajan L.): upregulation of sucrose synthase and alcohol dehydrogenase. Plant Sci 175:706–716. https://doi.org/10.1016/j.plantsci.2008.07.013
La VH, Lee B-R, Islama MT, Park S-H, Lee H, Bae D-W, Kim T-H (2019a) Antagonistic shifting from abscisic acid- to salicylic acid-mediated sucrose accumulation contributes to drought tolerance in Brassica napus. Environ Exp Bot 162:38–47. https://doi.org/10.1016/j.envexpbot.2019.02.001
La VH, Lee B-R, Zhang Q, Park S-H, Islam MT, Kim T-H (2019b) Salicylic acid improves drought-stress tolerance by regulating the redox status and proline metabolism in Brassica rapa. Hortic Environ Biote 60:31–40. https://doi.org/10.1007/s13580-018-0099-7
Liu L, Huang L, Lin X, Sun C (2020) Hydrogen peroxide alleviates salinity-induced damage through enhancing proline accumulation in wheat seedlings. Plant Cell Rep 39:567–575. https://doi.org/10.1007/s00299-020-02513-3
Lee EH, Foy CD (1986) Aluminum tolerances of two snapbean cultivars related to organic acid content evaluated by high-performance liquid chromatography. J Plant Nutr 9(12):1481–1498. https://doi.org/10.1080/01904168609363544
Lu B, Yuan Y, Zhang C, Ou J, Zhou W, Lin Q (2005) Modulation of key enzymes involved in ammonium assimilation and carbon metabolism by low temperature in rice (Oryza sativa L.) roots. Plant Sci 169:295–302. https://doi.org/10.1016/j.plantsci.2004.09.031
Mario G-R, Tomomichi F, Christopher LP, D LR, M CJ, A BR, N CL, (1997) Cloning of a polycistronic cDNA from tomato encoding γ-glutamyl kinase and γ-glutamyl phosphate reductase. PNAS 94(15):8249–8254. https://doi.org/10.1073/pnas.94.15.8249
Martins M, Sousa B, Lopes J, Soares C, Machado J, Carvalho S, Fidalgo F, Teixeira J (2020) Diclofenac shifts the role of root glutamine synthetase and glutamate dehydrogenase for maintaining nitrogen assimilation and proline production at the expense of shoot carbon reserves in Solanum lycopersicum L. Environ Sci Pollut R 27:29130–29142. https://doi.org/10.1007/s11356-020-09136-x
Miflin BJ, Habash DZ (2002) The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J Exp Bot 53(370):979–987. https://doi.org/10.1093/jexbot/53.370.979
Min K, Showman L, Perera A, Arora R (2018) Salicylic acid-induced freezing tolerance in spinach (Spinacia oleracea L.) leaves explored through metabolite profiling. Environ Exp Bot 156:214–227. https://doi.org/10.1016/j.envexpbot.2018.09.011
Miron D, Schaffer AA (1991) Sucrose phosphate synthase, sucrose synthase, and invertase activities in developing fruit of Lycopersicon esculentum Mill. and the sucrose accumulating Lycopersicon hirsutum Humb. and Bonpl. Plant Physiol 95(2):623–627. https://doi.org/10.1104/pp.95.2.623
Perezalfocea F, Larher F (1995) Sucrose and proline accumulation and sugar efflux in tomato leaf discs affected by NaCl and polyethylene glycol 6000 iso-osmotic stresses. Plant Sci 107(1):9–15. https://doi.org/10.1016/0168-9452(95)04087-B
Poór P, Gémes K, Horváth F, Szepesi Á, Simon ML, Tari I (2011) Salicylic acid treatment via the rooting medium interferes with stomatal response, CO2 fixation rate and carbohydrate metabolism in tomato, and decreases harmful effects of subsequent salt stress. Plant Biol 13:105–114. https://doi.org/10.1111/j.1438-8677.2010.00344.x
Rena AB, Splittstoesser WE (1975) Proline dehydrogenase and pyrroline-5-carboxylate reductase from pumpkin cotyledons. Phytochemistry 14(3):657–661. https://doi.org/10.1016/0031-9422(75)83010-X
Ruellan E, Vaultier M, Zachowski A, Hurry V (2009) Cold signalling and cold acclimation in plants. Adv Bot Res 49(8):35–150. https://doi.org/10.1016/s0065-2296(08)00602-2
Sadiq Y, Zaid A, Khan MMA (2020) Adaptive physiological responses of plants under abiotic stresses: role of phytohormones. In: Hasanuzzaman M (ed) Plant ecophysiology and adaptation under climate change: mechanisms and perspectives I. Springer, Singapore
Saleem M, Fariduddin Q, Janda T (2020) Multifaceted role of salicylic acid in combating cold stress in plants: a review. J Plant Growth Regul. https://doi.org/10.1007/s00344-020-10152-x
Savitch LV, Harney T, Huner NPA (2000) Sucrose metabolism in spring and winter wheat in response to high irradiance, cold stress and cold acclimation. Physiol Plantarum 108(3):270–278. https://doi.org/10.1034/j.1399-3054.2000.108003270.x
Sedaghat M, Mokhtassi-Bidgoli A, Emam Y, Sarvestani T (2020) Foliar-applied GR24 and salicylic acid enhanced wheat drought tolerance. Russ J Plant Physiol 67:733–739. https://doi.org/10.1134/S1021443720040159
Sehar Z, Masood A, Khan NA (2019) Nitric oxide reverses glucose-mediated photosynthetic repression in wheat (Triticum aestivum L.) under salt stress. Environ Exp Bot 161:277–289. https://doi.org/10.1016/j.envexpbot.2019.01.010
Shin H, Min K, Arora R (2018) Exogenous salicylic acid improves freezing tolerance of spinach (Spinacia oleracea L.) leaves. Cryobiology 81:192–200. https://doi.org/10.1016/j.cryobiol.2017.10.006
Skopelitis DS, Paranychianakis NV, Paschalidis KA, Pliakonis ED, Delis ID, Yakoumakis DI, Kouvarakis A, Papadakis AK, Stephanou EG, Roubelakis-Angelakisa KA (2006) Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell 18(10):2767–2781. https://doi.org/10.2307/20076821
Sun L, Li X, Wang Z, Sun Z, Zhu X, Liu S, Song F, Liu F, Wang Y (2018) Cold priming induced tolerance to subsequent low temperature stress is enhanced by melatonin application during recovery in wheat. Molecules 23:1091. https://doi.org/10.3390/molecules23051091
Szabados LL, Savourcb A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15(2):89–97. https://doi.org/10.1016/j.tplants.2009.11.009
Vágújfalvi A, Kerepesi I, Galiba G, Tischner T, Sutka J (1999) Frost hardiness depending on carbohydrate changes during cold acclimation in wheat. Plant Sci 144(2):85–92. https://doi.org/10.1016/S0168-9452(99)00058-8
Vargas WA, Pontis HG, Salerno GL (2007) Differential expression of alkaline and neutral invertases in response to environmental stresses: characterization of an alkaline isoform as a stress-response enzyme in wheat leaves. Planta 226(6):1535–1545. https://doi.org/10.1007/s00425-007-0590-3
Wang Z-Q, Yuan Y-Z, Ou J-Q, Lin Q-H, Zhang C-F (2007) Glutamine synthetase and glutamate dehydrogenase contribute differentially to proline accumulation in leaves of wheat (Triticum aestivum) seedlings exposed to different salinity. J Plant Physiol 164:695–701. https://doi.org/10.1016/j.jplph.2006.05.001
Wang F, Gao J, Liu Y, Tian Z, Muhammad A, Zhang Y, Jiang D, Cao W, Dai T (2016) Higher ammonium transamination cpacity can alleviate glutamate inhibition on winter wheat (Triticum aestivum L.) root growth under high ammonium stress. PLoS ONE 11(8):e0160997. https://doi.org/10.1371/journal.pone.0160997
Wang X, Zhang X, Chen J, Cai J, Zhou Q, Dai T, Cao W, Jiang D (2018) Parental drought-priming enhances tolerance to post-anthesis drought in offspring of wheat. Front Plant Sci 9:261. https://doi.org/10.3389/fpls.2018.00261
Wang X, Mao Z, Zhang J, Hemat M, Huang M, Cai J, Zhou Q, Dai T, Jiang D (2019) Osmolyte accumulation plays important roles in the drought priming induced tolerance to post-anthesis drought stress in winter wheat (Triticum aestivum L.). Environ Exp Bot 166:103804–103813. https://doi.org/10.1016/j.envexpbot.2019.103804
Wang W, Wang X, Huang M, Cai J, Zhou Q, Dai T, Jiang D (2020a) Alleviation of field low-temperature stress in winter wheat by exogenous application of salicylic acid. J Plant Growth Regul. https://doi.org/10.1007/s00344-020-10144-x
Wang W, Wang X, Zhang J, Huang M, Cai J, Zhou Q, Dai T, Jiang D (2020b) Salicylic acid and cold priming induce late-spring freezing tolerance by maintaining cellular redox homeostasis and protecting photosynthetic apparatus in wheat. Plant Growth Regul 90:109–121. https://doi.org/10.1007/s10725-019-00553-8
Wang W, Wang X, Zhang X, Wang Y, Huo Z, Huang M, Cai J, Zhou Q, Jiang D (2021) Involvement of salicylic acid in cold priming-induced freezing tolerance in wheat plants. Plant Growth Regul 93:117–130. https://doi.org/10.1007/s10725-020-00671-8
Wani SH, Prateek T, Abbu Z, Challa GS, Anuj K, Vinay K, Jyoti U, Rohit J, Manoj B (2018) Transcriptional regulation of osmotic stress tolerance in wheat (Triticum aestivum L.). Plant Mol Biol 97:469–487. https://doi.org/10.1007/s11103-018-0761-6
Wardlaw IF, Willenbrink J (1994) Carbohydrate storage and mobilisation by the culm of wheat between heading and grain maturity: the relation to sucrose synthase and sucrose-phosphate synthase. Funct Plant Biol 21(3):255–271. https://doi.org/10.1071/pp9940255
Yang S-L, Lan S-S, Gong M (2009) Hydrogen peroxide-induced proline and metabolic pathway of its accumulation in maize seedlings. J Plant Physiol 166:1694–1699. https://doi.org/10.1016/j.jplph.2009.04.006
Yang S-L, Lan S-S, Deng F-F, Gong M (2016) Effects of calcium and calmodulin antagonists on chilling stress-induced proline accumulation in Jatropha curcas L. J Plant Growth Regul 35:815–826. https://doi.org/10.1007/s00344-016-9584-3
Zadoks JC (2010) A decimal code for the growth stages of cereals. Weed Res 14:415–421. https://doi.org/10.1111/j.1365-3180.1974.tb01084.x
Zaid A, Mohammad F, Wani SH, Siddique K (2019) Salicylic acid enhances nickel stress tolerance by up-regulating antioxidant defense and glyoxalase systems in mustard plants. Ecotox Environ Saf 180:575–587. https://doi.org/10.1016/j.ecoenv.2019.05.042
Zeng F, Jiang T, Wang Y, Luo Z (2015) Effect of UV-C treatment on modulating antioxidative system and proline metabolism of bamboo shoots subjected to chilling stress. Acta Physiol Plant 37(11):1–10. https://doi.org/10.1007/s11738-015-1995-4
Zhang W, Wang J, Huang Z, Mi L, Jiang D (2019) Effects of low temperature at booting stage on sucrose metabolism and endogenous hormone contents in winter wheat spikelet. Front Plant Sci 10:498. https://doi.org/10.3389/fpls.2019.00498
Zheng B, Chapman S, Christopher J, Frederiks T, Chenu K (2015) Frost trends and their estimated impact on yield in the Australian wheatbelt. J Exp Bot 66:3611–3623. https://doi.org/10.1016/j.proenv.2015.07.244
Zhong X, Mei X, Li Y, Yoshida H, Zhao P, Wang X, Han L, Hu X, Huang S, Huang J (2008) Changes in frost resistance of wheat young ears with development during jointing stage. J Agron Crop Sci 194(5):343–349. https://doi.org/10.1111/j.1439-037X.2008.00320.x
Zhong Y, Xu D, Yang KH, Yang D, Cai J, Wang X, Zhou Q, Cao W, Dai T, Jiang D (2018) Nitrogen topdressing timing modifies free amino acids profiles and storage protein gene expression in wheat grain. BMC Plant Biol 18:353. https://doi.org/10.1186/s12870-018-1563-3
Zuther E, Schaarschmidt S, Fischer A, Erban A, Pagter M, Mubeen U, Giavalisco P, Kopka J, Sprenger H, Hincha DK (2019) Molecular signatures associated with increased freezing tolerance due to low temperature memory in Arabidopsis. Plant Cell Environ 42:854–873. https://doi.org/10.1111/pce.13502
Acknowledgements
This study was supported by the National Key Research and Development Program of China (2017YFD0300205, 2018YFD0300800), the National Natural Science Foundation of China (31771693, U1803235), Jiangsu Provincial Key Research and Development Program (BE2018362, BE2020319), the Fundamental Research Funds for the Central Universities (KYZ201807), the China Agriculture Research System (CARS-03), China Postdoctoral Science Foundation (2021M692721), and Jiangsu Collaborative Innovation Center for Modern Crop Production (JCIC-MCP), and the 111 Project (B16026).
Author information
Authors and Affiliations
Contributions
WW, XW and DJ conceived and designed research. WW, ZL and MH conducted experiments. JC, QZ and ZH guided experiments. WW and AK wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling Editor: Saddam Hussain.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, W., Wang, X., Lv, Z. et al. Effects of Cold and Salicylic Acid Priming on Free Proline and Sucrose Accumulation in Winter Wheat Under Freezing Stress. J Plant Growth Regul 41, 2171–2184 (2022). https://doi.org/10.1007/s00344-021-10412-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10412-4