Abstract
Regulation of proline accumulation in plants under chilling stress remains unclear. In this paper, we treated Jatropha curcas seedlings under chilling stress with exogenous calcium chloride (CaCl2), the plasma membrane Ca2+-channel blocker lanthanum chloride (LaCl3), calmodulin antagonists, chlorpromazine (CPZ), and trifluoperazine (TFP) and investigated the effects of calcium and calmodulin (CaM) on proline accumulation and chilling tolerance. The results showed that CaCl2 treatment significantly enhanced chilling stress-induced proline accumulation. CaCl2 also induced an almost immediate and rapid increase of Δ1-pyrroline-5-carboxylate synthetase (P5CS) and glutamate dehydrogenase activities, the key enzymes in the glutamate pathway of proline biosynthesis, and up-regulated P5CS expression, but it decreased the activity of proline dehydrogenase (ProDH), a key enzyme of proline degradation, and inhibited ProDH expression. Treatment with LaCl3, CPZ, and TFP exhibited the opposite effects to those by CaCl2 treatment. Moreover, CaCl2, LaCl3, CPZ, and TFP had little effect on the activities of ornithine aminotransferase and arginase, the key enzymes in the ornithine pathway of proline biosynthesis. These results indicated that Ca2+-CaM might be involved in signal transduction events, leading to proline accumulation in J. curcas seedlings under chilling stress, and that Ca2+-induced proline accumulation is a combined result of the activation of the glutamate pathways of proline biosynthesis and the simultaneous inhibition of the proline degradation pathway. In addition, CaCl2 treatment increased tissue vitality, decreased the content of the lipid peroxidation product malondialdehyde (MDA), and alleviated electrolyte leakage in J. curcas seedlings under chilling stress, indicating that exogenous Ca2+ can enhance chilling tolerance, and proline might be a key factor in this increased chilling tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chilling stress (0–15 °C) is a major environmental factor that affects metabolism, growth, development, distribution, and cultivation of chilling-sensitive plants from tropical and subtropical regions (Ruelland and others 2009; Jeon and Kim 2013; Kalisz and others 2015). Chilling-sensitive plants, such as maize, tobacco, tomato, and Jatropha curcas L., can be irreparably damaged when the temperature drops below 10 °C, mainly due to chilling stress-induced dysfunctions at the cellular level that include damage to membranes, osmotic stress, generation of reactive oxygen species (ROS), protein denaturation, and accumulation of the lipid peroxidation product malondialdehyde (MDA); osmotic stress is considered as one of the major injuries of chilling stress (Jan and others 2009; Heidarvand and Amiri 2010; Janska and others 2010). To cope with osmotic stress caused by chilling stress, higher plants have developed mechanisms of osmotic adjustment through the accumulation of compatible solutes such as proline, betaine, and soluble sugars (Trovato and others 2008; Szabados and Savoure 2010). Among these plant-compatible osmolytes (or plant protectants), proline is considered of major importance, as it has been reported to accumulate in a large number of species in response to stresses such as excess salinity, drought, cold, nutrient deficiency, heavy metals, pathogen infection, and high acidity (Delauney and Verma 1993; Ashraf and Foolad 2007; Trovato and others 2008).
Proline fulfills diverse functions in plants. As an amino acid, it is a structural component of proteins, but it also functions as an osmoticum, a sink of energy and reducing power, a nitrogen-storage compound, a hydroxy-radical scavenger, and a compatible solute that protects enzymes (Kishor and others 2005; Lehmann and others 2010; Szabados and Savoure 2010). In higher plants, proline can be synthesized from either glutamate or ornithine. The key enzymes involved in proline biosynthesis are Δ1-pyrroline-5-carboxylate synthetase (P5CS) and glutamate dehydrogenase (GDH) in the glutamate pathway, and arginase and ornithine aminotransferase (OAT) in the ornithine pathway (Zhao and others 2001; Zhang and others 2008; Yang and others 2009). The onset of stress-induced proline accumulation is correlated with transcriptional activation of the P5CS gene (Yang and others 2009; Szabados and Savoure 2010). Metabolism and accumulation of proline also depend on its degradation, which are catalyzed by the mitochondrial enzyme proline dehydrogenase (ProDH), and the ProDH gene plays a key role in controlling proline levels in plants (Lehmann and others 2010; Szabados and Savoure 2010; Chen and others 2014).
Various studies have indicated proline accumulates in response to cold shock in several plants, and proline contributes to the maintenance of enzymes from denaturation, interacts with membrane systems, regulates cytosolic pH, balances the ratio of NADH/NAD+ functions as a source of energy, and helps plants to detoxify ROS (Konstantinova and others 2002; Ruiz and others 2002; Javadian and others 2010; Ao and others 2013). Although much is known about proline metabolism, information on signaling mechanisms that regulate proline synthesis and degradation in plants under chilling stress is still unclear. Several signaling molecules and ions including abscisic acid (ABA), H2O2, Ca2+, and phospholipase D have been suggested (Knight and others 1997; Trovato and others 2008; Yang and others 2009; Szabados and Savoure 2010).
Ca2+ is the most versatile intracellular messenger discovered so far, because it is involved in the regulation of almost all known cellular functions and reactions (Petersen and others 2005). In plant cells, the calcium ion is a ubiquitous intracellular second messenger involved in numerous signaling pathways. Variations in the cytosolic concentration of Ca2+ ([Ca2+]cyt) combine a large array of signals and responses (Lecourieux and others 2006; Kim 2013). Calmodulin (CaM), the ubiquitous calcium sensor protein, is involved in almost all intracellular events (Yang and Poovaiah 2003; Arshi and others 2010). It has been shown that CaM binds and regulates more than 300 target proteins and that its structural plasticity is crucial for enabling its interaction with diverse partners (Yang and Poovaiah 2003).
In plants, the Ca2+-CaM-mediated signal network affects many aspects of plant growth, development, and responses to environmental changes (Yang and Poovaiah 2003; Kim 2013). Exogenous application of calcium conferred enhanced tolerance to salt (Guimarães and others 2011; Upadhyaya and others 2011), drought (Issam and others 2012; Xu and others 2013), and cold (Wang and others 2009; Zhou and Guo 2009; Liu and others 2015) stresses. However, the studies on calcium-induced cold resistance in plants are still limited.
In recent years, a few studies suggested that the calcium messenger system was involved in proline accumulation of plants. Exogenous CaCl2 treatment led to a significant accumulation of proline in Cichorium intybus L. (Arshi and others 2010). Proline accumulation in the leaves of Cassia angustifolia Vahl. was 1.6 times higher after treatment with CaCl2 and NaCl than in the controls (treatment with NaCl alone) (Arshi and others 2005). A further increase in proline concentration was observed with the addition of calcium chloride, and calcium appears to confer greater osmoprotection to plants under water deficit (Jaleel and others 2007).
Jatropha curcas is a tropical plant (a chilling-sensitive plant) that grows well on marginal land. It is considered an important drought-tolerant plant that belongs to the tribe Jatropheae in the Euphorbiaceae family (Carels 2009; Mukherjee and others 2011). In preliminary experiments, we found that exogenous CaCl2 treatment promoted chilling stress-induced proline accumulation in J. curcas seedlings. It also increased chilling tolerance of J. curcas seedlings. Treatment with the calcium-channel blocker LaCl3 and CaM antagonists, chlorpromazine (CPZ), and trifluoperazine (TFP), exhibited contrary results to those of the CaCl2 treatment (data not shown). We hypothesize that the Ca2+-CaM messenger system plays an important role in the rapid accumulation of cellular proline induced by chilling stress. However, there is little information available on the relationship among Ca2+-CaM, chilling stress, and proline metabolism in plants. In the present study, the objective was to investigate the effects of calcium and CaM on proline accumulation and chilling tolerance. In addition, the possible metabolic pathways of Ca2+-CaM that promote chilling stress-induced proline accumulation were investigated.
Materials and Methods
Plant Material and Treatments
Seeds of J. curcas, a mix of cultivars, were collected from Yunnan Province, China. Seeds were surface sterilized in 1 % CuSO4 for 30 min, and then pre-soaked for imbibition in distilled water for 24 h. The pre-soaked seeds were sowed on six layers of wetted filter papers (a 0.2–0.3-cm layer of distilled water was present above the surface of filter paper) in covered trays (200 seeds per tray) and germinated in the dark at 25 °C for 7 days. The surface water layer was maintained by adding 50 mL of water per day. Seedlings of uniform size were then selected and transferred into pots (20 × 18 × 15 cm3, 20 seedlings per pot) containing silica sand wetted with 1/2 Hoagland’s solution. The pots were placed in a climate chamber (day/night temperature: 25/20 °C, relative humidity: 75 %, photoperiod: 16 h, photon flux density: 300 μmol m−2 s−1) and sequentially grown for 14 days. The seedlings were cultured on 1/2 Hoagland’s solution (200 mL per pot), and the surface water layer was maintained by adding 100 mL of 1/2 Hoagland’s solution per day.
In preliminary experiments, J. curcas seedlings under chilling stress were treated with 0–30 mM CaCl2, 0–1500 μM LaCl3, 0–1000 μM CPZ, and 0–1000 μM TFP for 96 h, and the proline content was determined. For the following experiments, 10 mM CaCl2, 500 μM LaCl3, 200 μM CPZ, and 200 μM TFP were chosen because these concentrations significantly induced or inhibited proline accumulation compared to the seedlings subjected to chilling treatment alone (data not presented).
Seedlings of uniform size (21 days after sowing, approximately 10.0 cm in height, and 60 seedlings per tray) were gently transferred to 1/2 Hoagland’s solution and subjected to five treatments for 96 h. These included (1) 2 °C, (2) 10 mM CaCl2 + 2 °C, (3) 500 μM LaCl3 + 2 °C, (4) 200 μM CPZ + 2 °C, and (5) 200 μM TFP + 2 °C. The control group (60 seedlings per tray) continued to be cultured in 1/2 Hoagland’s solution at 25 °C for 96 h.
Measurement of Tissue Vitality, Electrolyte Leakage, and Malondialdehyde Content
The vitality of J. curcas seedlings leaves was measured using triphenyl tetrazolium chloride (TTC): 0.2 g of leaf samples were cultured in 0.6 % TTC solution at 27 °C for 15 h, and then the TTC solution was drained off and leaf samples were homogenized in 95 % (v/v) ethanol. The crude homogenate was heated in a 80 °C water bath for 10 min to extract formazan. The homogenate was then diluted with 95 % ethanol to a final volume of 25 mL, and the mixture was centrifuged at 10,000×g for 10 min. The absorbance of the supernatant at 485 nm was determined spectrophotometrically. Electrolyte leakage was measured according to the method described by Gong and others (2001). The content of MDA was determined by the thiobarbituric acid reaction as described by Bailly and others (1996).
Measurement of Proline Content
Proline in leaves of J. curcas seedlings was extracted and measured according to the method described by Bates and others (1973).
Enzyme Assays
GDH, P5CS, OAT, arginase, and ProDH in leaves of J. curcas seedlings were extracted and assayed according to our previously described method (Yang and others 2009), and the activities of GDH, P5CS, OAT, arginase, and ProDH were expressed as nmol NADH oxidized mg−1 protein min−1, nmol NADPH oxidized mg−1 protein min−1, μmol NADH oxidized mg−1 protein min−1, nmol ornithine mg−1 protein min−1, and nmol NADP+ mg−1 protein min−1, respectively.
Assay of CaM Activity
CaM in leaves of J. curcas seedlings was assayed according to our previously described method (Gong and others 1997). One unit was defined as the amount of CaM that stimulates 0.01 units of 3′,5′-cyclic nucleotide phosphodiesterase to 50 % of the maximum activity of the enzyme when saturated with CaM in the presence of 100 μM Ca2+ in a 3-mL reaction volume at 30 °C, pH 7.5.
RNA Isolation and Quantitative Real-Time RT-PCR (RT-qPCR)
Seedlings of J. curcas were treated with five treatments as described above. Total RNA was isolated from leaves of J. curcas seedlings after 24, 48, 72, and 96 h of treatments with an RNAiso for Polysaccharide-rich Plant Tissue kit (TaKaRa Biotechnology, Dalian, China) according to manufacturer’s recommendation and used to generate a cDNA pool. RT-qPCR was performed using an ABI 7500 Fast Real-Time PCR instrument (Applied Biosystems, Foster City, CA, USA). The following gene-specific primers for RT-qPCR were designed using Primer Premier 5.0 software (Premier Biosoft International, Palo Alto, CA, USA): for P5CS (GenBank accession No. GU358610) (5′-GGCAGATGGACTCCTGTTAGA-3′ and 5′-TTTCATTTGACCGCTTGGC-3′, amplicon size: 164 bp), for ProDH (GenBank accession No. KF879446) (5′-CGAGGCTGTAAAGTGTGTAAGG-3′ and 5′-CAAGTAGGTTCAGAGGGCAAAT-3′, amplicon size: 209 bp), and for actin gene (GenBank accession No. HM044307), which was used as internal control (5′-GTGTTATGGTTGGGATGGGT-3′ and 5′-AAGCACTGGGTGTTCCTCTG-3′, amplicon size: 188 bp).
RT-qPCR was performed using One Step SYBR® PrimeScript™ RT-PCR Kit II (TaKaRa Biotechnology, Dalian, China) according to manufacturer’s recommendations. Relative expression levels were analyzed by the comparative CT method using Microsoft Excel 2010 as described by Livak and Schmittgen (2001).
Statistical Analysis
All experiments were repeated at least three times with two independent biological replicates. The results were processed statistically with SPSS 15.0 software (IBM Corp., Armonk, NY, USA). The data were subjected to analysis of variance (ANOVA) and Duncan’s multiple range test was employed to determine significance differences between treatments at P < 0.05 level. Figures were drawn by SigmaPlot 10.0 (Systat Software, San Jose, CA, USA), error bars represent standard error, and each point in figure represents the mean ± SE of at least three experiments.
Results
Effect of Ca2+ on Proline Accumulation and Metabolic Pathways in Leaves of J. curcas Seedlings Under Chilling Stress Conditions
During the treatment with 2 °C for 96 h, the proline content in leaves of J. curcas seedlings was about 2.83 times higher than that in plants not subjected to chilling stress (P < 0.01, Fig. 1). Interestingly, treatments with 10 mM CaCl2 for 96 h remarkably enhanced the accumulation of proline in leaves of J. curcas seedlings under chilling stress (P < 0.01, Fig. 1), whereas treatment with the plasma membrane Ca2+-channel blocker LaCl3 exhibited the opposite effect to that observed in CaCl2 treatment (P < 0.05, Fig. 1).
Effects of CaCl2 and LaCl3 on proline content in leaves of J. curcas seedlings under chilling stress. Seedlings were treated with 2 °C, 10 mM CaCl2 + 2 °C, or 500 μM LaCl3 + 2 °C for 96 h and proline content was measured. The control seedlings continued to be cultured in 1/2 Hoagland’s solution at 25 °C for 96 h. Each point represents the mean ± SE of at least six replicates of three independent experiments. Treatments marked with different letters at a given sampling date are significantly different at P < 0.05
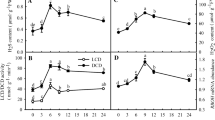
During chilling stress at 2 °C for 96 h, the activities of P5CS and GDH in leaves of J. curcas seedlings increased rapidly in the first 72 h and 48 h of stress conditions, respectively, and then this increase became relatively gentle in the subsequent 24 and 48 h of stress conditions, respectively (Fig. 2a, b). Treatment with 10 mM CaCl2 significantly increased P5CS (P < 0.01) and GDH (P < 0.01) activities in J. curcas seedlings under chilling stress conditions compared to the seedlings subjected to chilling treatment alone (Fig. 2a, b). However, treatment with 500 μM LaCl3 for 96 h significantly decreased the activities of P5CS and GDH (P < 0.05, Fig. 2a, b).
Effects of CaCl2 and LaCl3 on activities of the key enzymes of proline biosynthesis in leaves of J. curcas seedlings under chilling stress. Seedlings were treated with 2 °C, 10 mM CaCl2 + 2 °C, or 500 μM LaCl3 + 2 °C for 96 h and the activities of four key enzymes of proline metabolism were assayed: a GDH, b P5CS, c arginase, d OAT. Each point represents the mean ± SE of at least six replicates of three independent experiments. Treatments marked with different letters at a given sampling date are significantly different at P < 0.05
During the treatment with chilling stress at 2 °C for 96 h, arginase and OAT activities in leaves of J. curcas seedlings showed similar changes. In the early phase of chilling stress (0–24 h), there was a small change in the level of activities of arginase and OAT compared to the control. With prolonged chilling treatment (24–96 h), the activities of arginase and OAT increased dramatically (Fig. 2c, d), reaching levels that were 2.32 and 2.06 times higher, respectively, by the end of the treatment than those in plants not exposed to chilling stress (P < 0.01, Fig. 2c, d). In contrast, both CaCl2 and LaCl3 treatments had no significant effect on the activities of arginase and OAT (P > 0.05, Fig. 2c, d).
Chilling stress led to a gradual decline of ProDH activity in leaves of J. curcas seedlings (Fig. 3). Interestingly, compared to the seedlings exposed to chilling treatment alone, CaCl2 treatment significantly decreased the activity of ProDH under chilling stress conditions (P < 0.01), but treatment with 500 μM LaCl3 increased the ProDH activity (P < 0.01, Fig. 3).
Effects of CaCl2 and LaCl3 on ProDH activity in leaves of J. curcas seedlings under chilling stress. Seedlings were treated with 2 °C, 10 mM CaCl2 + 2 °C, or 500 μM LaCl3 + 2 °C for 96 h and the activity of ProDH was assayed. Each point represents the mean ± SE of at least six replicates of three independent experiments. Treatments marked with different letters at a given sampling date are significantly different at P < 0.05
To further determine the effect of Ca2+ on biosynthesis and degradation of proline under chilling stress, the mRNA levels of P5CS and ProDH in leaves of J. curcas seedlings were detected. As shown in Fig. 4, during the chilling stress treatment at 2 °C for 96 h, chilling stress significantly up-regulated P5CS expression. Compared to the seedlings exposed to chilling treatment alone, treatment with 10 mM CaCl2 remarkably up-regulated P5CS expression, but LaCl3 treatment down-regulated P5CS expression under chilling stress (P < 0.01, Fig. 4a). In contrast, ProDH expression was inhibited under chilling stress conditions, and CaCl2 treatment down-regulated ProDH expression, but LaCl3 treatment up-regulated ProDH expression in seedlings compared to those that had undergone chilling treatment alone (P < 0.01, Fig. 4b).
Effects of CaCl2 and LaCl3 on the expression of P5CS and ProDH genes in leaves of J. curcas seedlings under chilling stress. Seedlings were treated with 2 °C, 10 mM CaCl2 + 2 °C, or 500 μM LaCl3 + 2 °C for 24, 48, 72 and 96 h, and P5CS and ProDH genes expression were analyzed by RT-qPCR. Each point represents the mean ± SE of at least six replicates of three independent experiments. Treatments marked with different letters at a given sampling date are significantly different at P < 0.05
Effect of CaM Antagonists on Proline Accumulation and Metabolic Pathways in Leaves of J. curcas Seedlings Under Chilling Stress Conditions
CPZ and TFP have been commonly used as CaM antagonists of choice in many studies (Martínez-Luis and others 2007). In the present work, we studied the effects of 200 μM CPZ or TFP on CaM activity in leaves of J. curcas seedlings under chilling stress conditions. Compared with the control, chilling stress significantly increased CaM activity (P < 0.01), whereas CPZ and TFP decreased CaM activity compared to the seedlings subjected to chilling treatment alone (P < 0.05, Fig. 5a).
Effects of CPZ and TFP on CaM activity (a) and proline content (b) in leaves of J. curcas seedlings under chilling stress. Seedlings were treated with 2 °C, 200 μM CPZ + 2 °C, or 200 μM TFP + 2 °C for 96 h and proline content and CaM activity were measured. The control seedlings continued to be cultured in 1/2 Hoagland’s solution at 25 °C for 96 h. Each point represents the mean ± SE of at least six replicates of three independent experiments. Treatments marked with different letters at a given sampling date are significantly different at P < 0.05
Treatments with CPZ and TFP for 96 h significantly decreased the content of proline compared to that in the seedlings treated with chilling stress alone (P < 0.05) (Fig. 5b). They also induced a decrease in the activities of GDH and P5CS (P < 0.05, Fig. 6a, b), but both CPZ and TFP treatments had little effect on the activities of arginase and OAT (P > 0.05, Fig. 6c, d). In addition, CPZ and TFP treatments markedly increased ProDH activity in seedlings compared to that in seedlings under chilling treatment alone (P < 0.01, Fig. 7).
Effects of CPZ and TFP on activities of the key enzymes of proline biosynthesis in leaves of J. curcas seedlings under chilling stress. Seedlings were treated with 2 °C, 200 μM CPZ + 2 °C, or 200 μM TFP + 2 °C for 96 h and the activities of four key enzymes of proline metabolism were assayed: a GDH, b P5CS, c arginase, d OAT. Each point represents the mean ± SE of at least six replicates of three independent experiments. Treatments marked with different letters at a given sampling date are significantly different at P < 0.05
Effects of CPZ and TFP on ProDH activity in leaves of J. curcas seedlings under chilling stress. Seedlings were treated with 2 °C, 200 μM CPZ + 2 °C, or 200 μM TFP + 2 °C for 96 h and the activity of ProDH was assayed. Each point represents the mean ± SE of at least six replicates of three independent experiments. Treatments marked with different letters at a given sampling date are significantly different at P < 0.05
Treatments with CPZ and TFP also changed the expression level of P5CS and ProDH under chilling stress conditions. Thus, they down-regulated P5CS and up-regulated ProDH expression in seedlings compared to that in seedlings subjected to chilling treatment alone (P < 0.01, Fig. 8).
Effects of CPZ and TFP on the expression of P5CS and ProDH genes in leaves of J. curcas seedlings under chilling stress. Seedlings were treated with 2 °C, 200 μM CPZ + 2 °C, or 200 μM TFP + 2 °C for 24, 48, 72 and 96 h, and P5CS and ProDH genes expression were analyzed by RT-qPCR. Each point represents the mean ± SE of at least six replicates of three independent experiments. Treatments marked with different letters at a given sampling date are significantly different at P < 0.05
Effect of Ca2+ and CaM Antagonists on Some Physiological Parameters of J. curcas Seedlings Under Chilling Stress Conditions
To explore the effect of Ca2+ and CaM on chilling tolerance of J. curcas seedlings, seedlings were treated with 2 °C, 10 mM CaCl2 + 2 °C, 500 μM LaCl3 + 2 °C, 200 μM CPZ + 2 °C, and 200 μM TFP + 2 °C for 96 h, and tissue vitality, electrolyte leakage, and MDA content in leaves of J. curcas seedlings were assayed daily. The results showed that chilling stress at 2 °C for 96 h decreased tissue vitality (P < 0.01, Fig. 9a) and increased electrolyte leakage (P < 0.01, Fig. 9b) and MDA content (P < 0.01, Fig. 9c) in seedlings. Interestingly, treatment with 10 mM CaCl2 significantly increased tissue vitality (Fig. 9a) and decreased electrolyte leakage (Fig. 9b) and MDA content (Fig. 9c) compared to the seedlings subjected to chilling treatment alone. Treatments with LaCl3 (P < 0.05, Fig. 9), CPZ, and TFP exhibited opposite effects to those with CaCl2 treatment (P < 0.05, Fig. 10).
Effects of CaCl2 and LaCl3 on tissue vitality (a), electrolyte leakage (b) and MDA content (c) in leaves of J. curcas seedlings under chilling stress. Each point represents the mean ± SE of at least six replicates of three independent experiments. Treatments marked with different letters at a given sampling date are significantly different at P < 0.05
Effects of CPZ and TFP on tissue vitality (a), electrolyte leakage (b) and MDA content (c) in leaves of J. curcas seedlings under chilling stress. Each point represents the mean ± SE of at least six replicates of three independent experiments. Treatments marked with different letters at a given sampling date are significantly different at P < 0.05
Discussion
Proline accumulates in many plant species in response to environmental stress. The main biological function of proline accumulation is associated with the osmotic regulation of plant cells, the regulation of nitrogen and energy metabolism, and the protection of cell membrane system (Trovato and others 2008; Szabados and Savoure 2010). Studies have indicated variations in proline metabolism and accumulation in response to cold stress in several plants (Ruiz and others 2002; Javadian and others 2010; Ao and others 2013). Proline accumulation during cold acclimation showed a significant positive correlation with freezing tolerance in bermudagrass (Zhang and others 2011). Javadian and others (2010) reported that proline accumulation was greater than that of other amino acids under cold stress. They also observed higher proline accumulation in winter wheat cultivars than in spring ones, which was explained by proline’s major role in coping with cold stress and maintaining favorable osmotic potential between the cell and its surrounding. Ruiz and others (2002) found that cold shock led to a significant accumulation of proline in green bean plants where OAT and ProDH appeared as determinant in this accumulation. P5CS was essential for cold tolerance in rice (Hur and others 2004), and overexpression of P5CS in Eucalyptus saligna Sm. accelerated proline synthesis (Dibax and others 2010). Chen and others (2014) reported that increased P5CS and ProDH expression caused proline accumulation in chrysanthemum species under cold acclimation, without the need for OAT expression changes. Our results partly agree with these authors in that the present results clearly showed that chilling stress could lead to a significant accumulation of proline (Fig. 1), an early and rapid increase of activities of P5CS and GDH, the key regulatory enzymes of the glutamate pathway (Fig. 2a, b), and an up-regulation of P5CS expression in leaves of J. curcas seedlings (Fig. 4a), indicating that the glutamate pathway of proline biosynthesis was activated in the early phase of chilling stress. Interestingly, activities of arginase and OAT, the key enzymes of the ornithine pathway, significantly increased only after 24 h of chilling stress (Fig. 2c, d), indicating that the activation of the ornithine pathway of proline biosynthesis by chilling stress was delayed compared to the activation of the glutamate pathway. In addition, chilling stress treatment decreased the activity of ProDH, the key enzyme of proline degradation, and down-regulated ProDH expression (Figs. 3, 4b). These results suggest that the chilling stress-induced proline accumulation is the result of the combined sequential activation of the glutamate and ornithine pathways of proline biosynthesis and simultaneous inhibition of proline degradation.
Although the importance of proline accumulation in the adaptation of plants to abiotic stress including chilling stress have been demonstrated, little is known about the regulation of proline biosynthesis and degradation. Proline accumulation is generally believed not to be a primary response to stress and was found to be dependent on de novo protein synthesis, suggesting the existence of a signaling cascade controlling proline biosynthesis (Delauney and Verma 1993; Trovato and others 2008; Szabados and Savoure 2010). Several signaling molecules and ions, including ABA, NO, Ca2+, H2O2, phospholipase D, and salicylic acid (SA) are involved in the regulation of proline metabolism in plants under abiotic stress (Kishor and others 2005; Trovato and others 2008; Misra and Saxena 2009; Yang and others 2009; Lehmann and others 2010). The effect of ABA on the expression of P5CS and ProDH during abiotic stress has been described and reviewed in a number of studies (Verslues and Bray 2006; Lehmann and others 2010; Sharma and Verslues 2010). In banana fruit, NO treatment significantly enhanced the accumulation of proline under chilling stress, which resulted from the increased P5CS activities and decreased ProDH activity (Wang and others 2013). Treatment with 0.5 mM SA elevated proline content in the shoots of lentil seedlings possibly by enhancing pyrroline-5-carboxylate reductase activity and decreasing the activity of ProDH (Misra and Saxena 2009). The impact of SA on proline accumulation was further increased under high salinity conditions, leading to the assumption that the stress-protective effect of SA might partially be achieved via control of proline metabolism (Misra and Saxena 2009; Lehmann and others 2010). Proline content and the activity of P5CS were enhanced with CaCl2 treatment in Centella asiatica (L.) Urb. under salt stress (Murugan and Sathish 2005). Interestingly, the expression of P5CS1 in Arabidopsis seedlings was inhibited by calcium-chelator EGTA and calcium-channel blockers lanthanum and verapamil under osmotic stress (Knight and others 1997). The addition of CaM antagonists CPZ or TFP enhanced the increase in the levels of proline, indicating that the CaM-mediated signal transduction might be negatively involved in the regulation of proline accumulation via a modification in ProDH properties (Lee and Liu 1999).
Recently, several studies confirmed a correlation between proline metabolism and Ca2+-CaM messenger system under chilling stress. Nayyar and others (2005) reported that proline content in chickpea seedlings was significantly increased with CaCl2 treatment under chilling stress. Ruiz and others (2002) found that Ca2+-CaM-dependent NAD kinase induced the foliar accumulation of proline in response to cold shock, boosting OAT activity, and lowering ProDH activity in green bean plants. However, little is still known about the regulation of proline metabolism under chilling stress.
In the present study, CaCl2 treatment increased significantly accumulation of proline in J. curcas seedlings under chilling stress (Fig. 1). CaCl2 also induced a rapid increase of GDH and P5CS activities, the crucial enzymes of glutamate pathway (Fig. 2a, b), and up-regulated P5CS expression (Fig. 4a), whereas treatment with the plasma membrane Ca2+-channel blocker LaCl3 and CaM antagonists CPZ, and TFP exhibited the opposite effects to those of CaCl2 treatment (Figs. 1, 2a,b, 4a, 5b, 6a, b, 8a). These data indicate that the glutamate pathway of proline biosynthesis is activated by Ca2+ treatment, and Ca2+-CaM plays an important role in proline accumulation in J. curcas seedlings under chilling stress. However, CaCl2, LaCl3, CPZ, and TFP treatments had little effect on the activities of OAT and arginase, the key enzymes of the ornithine pathway (Figs. 2c, d, 6c, d), indicating that Ca2+-CaM has no significant effect on the ornithine pathway of proline biosynthesis under chilling stress. In addition, CaCl2 treatment decreased the activity of ProDH and inhibited the expression of ProDH (Figs. 3, 4b). However, LaCl3, CPZ, and TFP treatments increased ProDH activity and activated the expression of ProDH (Figs. 3, 4b, 7, 8b), suggesting that Ca2+-CaM is involved in the inhibition of proline degradation under chilling stress.
A number of studies have confirmed that Ca2+ plays a critical role in the regulatory response of plants to cold stress (Wang and others 2009; Zhou and Guo 2009). Schaberg and others (2011) found that Ca2+ modulated the stress-induced growth performance and photosynthetic efficiency of red spruce foliage during the cold season. Shi and others (2014) reported that CaCl2 treatment alleviated the ROS burst and cell damage triggered by chilling stress via activating antioxidant enzymes, the non-enzymatic glutathione antioxidant pool in bermudagrass. In this study, exogenous application of CaCl2 increased tissue vitality and decreased electrolyte leakage and MDA content (Fig. 9), while LaCl3 (Fig. 9), and CPZ and TFP (Fig. 10) reversed CaCl2 effects in J. curcas seedlings under chilling stress. These results showed that calcium could improve cold tolerance of J. curcas seedlings. However, the mechanism of calcium-induced cold stress tolerance remains unclear. The results presented herein and in other studies (Ruiz and others 2002; Nayyar and others 2005) indicate that calcium treatment increases significantly the accumulation of proline. In addition, exogenous proline increases chilling tolerance in plants under chilling stress (Kumar and Yadav 2009; Jonytienė and others 2012). Therefore, we speculate that proline is a key factor in calcium-induced chilling tolerance. However, in plants, the acquisition of chilling tolerance is a complex event, involved in many physiological, biochemical, and molecular aspects, while the molecular mechanism of calcium-induced chilling tolerance in J. curcas seedlings needs to be further investigated in future studies.
Our results indicate a possible involvement of Ca2+-CaM in signal transduction events leading to proline accumulation and regulation of proline biosynthesis and degradation in J. curcas seedlings under chilling stress. The Ca2+-CaM-induced proline accumulation in J. curcas seedlings under chilling stress might be the result of the combined activation of the glutamate pathways of proline biosynthesis and inhibition of the proline degradation pathway. In addition, exogenous Ca2+ can improve chilling tolerance of J. curcas seedlings and the acquisition of this chilling tolerance may be involved in proline.
References
Ao PX, Li ZG, Gong M (2013) Involvement of compatible solutes in chill hardening-induced chilling tolerance in Jatropha curcas seedlings. Acta Physiol Plant 35:3457–3464
Arshi A, Abdin MZ, Iqbal M (2005) Ameliorative effects of CaCl2 on growth, ionic relations, and proline content of senna under salinity stress. J Plant Nutr 28:101–125
Arshi A, Ahmad A, Aref IM, Iqbal M (2010) Effect of calcium against salinity-induced inhibition in growth, ion accumulation and proline contents in Cichorium intybus L. J Environ Biol 31:939–944
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Bailly C, Benamar A, Corbineau F, Dome D (1996) Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seed as related to deterioration during accelerated aging. Physiol Plant 97:104–110
Bates LS, Waldren LS, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Carels N (2009) Jatropha curcas: a review. Adv Bot Res 50:39–86
Chen Y, Jiang JF, Chang QS, Gu CS, Song AP, Chen SM, Dong B, Chen FD (2014) Cold acclimation induces freezing tolerance via antioxidative enzymes, proline metabolism and gene expression changes in two chrysanthemum species. Mol Biol Rep 41:815–822
Delauney AJ, Verma DPS (1993) Proline biosynthesis and osmoregulation in plants. Plant J 4:213–215
Dibax R, Deschamps C, Bespalhok JC, Vieira LGE, Molinari HBC, De Campos MKF, Quoirin M (2010) Organogenesis and Agrobacterium tumefaciens-mediated transformation of Eucalyptus saligna with P5CS gene. Biol Plant 54:6–12
Gong M, Chen SN, Song YQ, Li ZG (1997) Effect of calcium and calmodulin on intrinsic heat tolerance in relation to antioxidant systems in maize seedlings. Aust J Plant Physiol 24:371–379
Gong M, Chen B, Li ZG, Guo LH (2001) Heat-shock-induced cross adaptation to heat, chilling, drought and salt in maize seedlings and involvement of H2O2. J Plant Physiol 158:1125–1130
Guimarães FVA, de Lacerda CF, Marques EC, de Miranda MRA, de Abreu CEB, Prisco JT, Gomes-Filho E (2011) Calcium can moderate changes on membrane structure and lipid composition in cowpea plants under salt stress. Plant Growth Regul 65:55–63
Heidarvand L, Amiri RM (2010) What happens in plant molecular responses to cold stress? Acta Physiol Plant 32:419–431
Hur J, Jung KH, Lee CH, An GH (2004) Stress-inducible OsP5CS2 gene is essential for salt and cold tolerance in rice. Plant Sci 167:417–426
Issam N, Kawther M, Haythem M, Moez J (2012) Effects of CaCl2 pretreatment on antioxidant enzyme and leaf lipid content of faba bean (Vicia faba L.) seedlings under cadmium stress. Plant Growth Regul 68:37–47
Jaleel CA, Manivannan P, Sankar B, Kishorekumar A, Panneerselvam R (2007) Calcium chloride effects on salinity-induced oxidative stress, proline metabolism and indole alkaloid accumulation in Catharanthus roseus. CR Biol 330:674–683
Jan N, Hussain M, Andrabi KI (2009) Cold resistance in plants: a mystery unresolved. Electron J Biotechnol 12:1–15
Janska A, Marsik P, Zelenkova S, Ovesna J (2010) Cold stress and acclimation-what is important for metabolic adjustment? Plant Biol 12:395–405
Javadian N, Karimzadeh G, Mahfoozi S, Ghanati F (2010) Cold induced changes of enzymes, proline, carbohydrates, and chlorophyll in wheat. Russ J Plant Physiol 57:540–547
Jeon J, Kim J (2013) Cold stress signaling networks in Arabidopsis. J Plant Biol 56:69–76
Jonytienė V, Burbulis N, Kuprienė R, Blinstrubienė A (2012) Effect of exogenous proline and de-acclimation treatment on cold tolerance in Brassica napus shoots cultured in vitro. J Food Agric Environ 10:327–330
Kalisz A, Sękara A, Grabowska A, Cebula S, Kunicki E (2015) The effect of chilling stress at transplant stage on broccoli development and yield with elements of modeling. J Plant Growth Regul 34:532–544
Kim KN (2013) Stress responses mediated by the CBL calcium sensors in plants. Plant Biotechnol Rep 7:1–8
Kishor PBK, Sangam S, Amrutha RN, Laxmi PS, Naidu KR, Rao KRSS, Roa S, Reddy KJ, Theriappan P, Sreenivasulu N (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 88:424–438
Knight H, Trewavas AJ, Knight MR (1997) Calcium signalling in Arabidopsis responding to drought and salinity. Plant J 12:1067–1078
Konstantinova T, Parvanova D, Atanassov A, Djilianov D (2002) Freezing tolerance tobacco transformed to accumulate osmoprotectants. Plant Sci 163:157–164
Kumar V, Yadav SK (2009) Proline and betaine provide protection to antioxidant and methylglyoxal detoxification systems during cold stress in Camellia sinensis (L.) O. Kuntze. Acta Physiol Plant 31:261–269
Lecourieux D, Ranjeva R, Pugin A (2006) Calcium in plant defence-signalling pathways. New Phytol 171:249–269
Lee TM, Liu CH (1999) Regulation of NaCl-induced proline accumulation by calmodulin via modification of proline dehydrogenase activity in Ulva fasciata (chlorophyta). Aust J Plant Physiol 26:595–600
Lehmann S, Funck D, Szabados L, Rentsch D (2010) Proline metabolism and transport in plant development. Amino Acids 39:949–962
Liu YF, Zhang GX, Qi MF, Li TL (2015) Effects of calcium on photosynthesis, antioxidant system, and chloroplast ultrastructure in tomato leaves under low night temperature stress. J Plant Growth Regul 34:263–273
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Martínez-Luis S, Pérez-Vásquez A, Mata R (2007) Natural products with calmodulin inhibitor properties. Phytochemistry 68:1882–1903
Misra N, Saxena P (2009) Effect of salicylic acid on proline metabolism in lentil grown under salinity stress. Plant Sci 177:181–189
Mukherjee P, Varshney A, Johnson TS, Jha TB (2011) Jatropha curcas: a review on biotechnological status and challenges. Plant Biotechnol Rep 5:197–215
Murugan K, Sathish DK (2005) Ameliorative effect by calcium on NaCl salinity stress related to proline metabolism in the callus of Centella asiatica L. J Plant Biochem Biotechnol 14:205–207
Nayyar H, Bains TS, Kumar S (2005) Chilling stressed chickpea seedlings: effect of cold acclimation, calcium and abscisic acid on cryoprotective solutes and oxidative damage. Environ Exp Bot 54:275–285
Petersen OH, Michalak M, Verkhratsky A (2005) Calcium signalling: past, present and future. Cell Calcium 38:161–169
Ruelland E, Vaultier MN, Zachowski A, Hurry V (2009) Cold signalling and cold acclimation in plants. Adv Bot Res 49:35–150
Ruiz JM, Sánchez E, García PC, López-Lefebre LR, Rivero RM, Romero L (2002) Proline metabolism and NAD kinase activity in greenbean plants subjected to cold-shock. Phytochemistry 59:473–478
Schaberg PG, Minocha R, Long S, Halman JM, Hawley GJ, Eagar C (2011) Calcium addition at the Hubbard Brook experimental forest increases the capacity for stress tolerance and carbon capture in red spruce (Picea rubens) trees during the cold season. Trees 25:1053–1061
Sharma S, Verslues PE (2010) Mechanisms independent of abscisic acid (ABA) or proline feedback have a predominant role in transcriptional regulation of proline metabolism during low water potential and stress recovery. Plant Cell Environ 33:1838–1851
Shi ST, Ye TT, Zhong B, Liu X, Chan ZL (2014) Comparative proteomic and metabolomic analyses reveal mechanisms of improved cold stress tolerance in bermudagrass (Cynodon dactylon (L.) Pers.) by exogenous calcium. J Integr Plant Biol 56:1064–1079
Szabados L, Savoure A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Trovato M, Mattioli R, Costantino P (2008) Multiple roles of proline in plant stress tolerance and development. Rend Lincei Sci Fis 19:325–346
Upadhyaya H, Panda SK, Dutta BK (2011) CaCl2 improves post drought recovery potential in Camellia sinensis (L.) O. Kuntze. Plant Cell Rep 30:495–503
Verslues PE, Bray EA (2006) Role of abscisic acid (ABA) and Arabidopsis thaliana ABA-insensitive loci in low water potential induced ABA and proline accumulation. J Exp Bot 57:201–212
Wang Y, Yang ZM, Zhang QF, Li JL (2009) Enhanced chilling tolerance in Zoysia matrella by pre-treatment with salicylic acid, calcium chloride, hydrogen peroxide or 6-benzylaminopurine. Biol Plant 53:179–182
Wang YS, Luo ZS, Du RX, Liu Y, Ying TJ, Mao LC (2013) Effect of nitric oxide on antioxidative response and proline metabolism in banana during cold storage. J Agric Food Chem 61:8880–8887
Xu C, Li X, Zhang L (2013) The effect of calcium chloride on growth, photo- synthesis, and antioxidant responses of Zoysia japonica under drought conditions. PLoS One 8:e68214
Yang TB, Poovaiah BW (2003) Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci 8:505–512
Yang SL, Lan SS, Gong M (2009) Hydrogen peroxide-induced proline and metabolic pathway of its accumulation in maize seedlings. J Plant Physiol 166:1694–1699
Zhang LP, Mehta SK, Liu ZP, Yang ZM (2008) Copper-induced proline synthesis is associated with nitric oxide generation in chlamydomonas reinhardtii. Plant Cell Physiol 49:411–419
Zhang XZ, Wang KH, Ervin EH, Waltz C, Murphy T (2011) Metabolic changes during cold acclimation and deacclimation in five bermudagrass varieties. I. Proline, total amino acid, protein, and dehydrin expression. Crop Sci 51:838–846
Zhao FG, Cheng S, Liu YL (2001) Ornithine pathway in proline biosynthesis activated by salt stress in barley seedings. Acta Bot Sin 43:36–40
Zhou B, Guo Z (2009) Calcium is involved in the abscisic acid-induced ascorbate peroxidase, superoxide dismutase and chilling resistance in Stylosanthes guianensis. Biol Plant 53:63–68
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 31260169, 31260064).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, SL., Lan, SS., Deng, FF. et al. Effects of Calcium and Calmodulin Antagonists on Chilling Stress-Induced Proline Accumulation in Jatropha curcas L.. J Plant Growth Regul 35, 815–826 (2016). https://doi.org/10.1007/s00344-016-9584-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-016-9584-3