Abstract
Activation of complex metabolic pathways and antioxidant activities is necessary for enhancing quality and health promoting capacity of food crops. Plant growth regulators are the main factors affecting the expression of different genes and related biological activities. Effect of foliar spray with 24-epibrassinolide (EBL) during growth stages on total antioxidant activity, phenolics, anthocyanins, some anti-stress and antioxidant enzymes activity, fruit quality indices, and pathogen extension in strawberry fruit during cold storage was investigated. EBL significantly enhanced fruit total antioxidant activity, total phenolics, and total anthocyanins contents. Activity of superoxide dismutase, catalase, phenylalanine ammonia-lyase, and polyphenol oxidase enzymes was enhanced by 270%, 81%, 138%, and 94% in response to EBL treatment, respectively. Treated fruits showed lower decay extension, microbial count, weight loss, and higher postharvest life during storage. A positive correlation was observed between the color parameters, total anthocyanins, and antioxidant capacity of the fruits. EBL at 1 µM showed a good potential for enhancing strawberry fruit nutritional and overall quality, phytochemical compounds, and postharvest life.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Strawberries (Fragaria × ananassa Duch) are rich sources of important bio-compounds such as antioxidants, phenolics, and anthocyanins, making them desirable for improving human health. However, because of high metabolic activities and sensitivity against pathogens, the bioactive compounds and phytochemicals of the fruit rapidly decrease during storage and transport (Crecente-Campo et al. 2012; Basu et al. 2014).
Due to the presence of different cell walls, cuticles, and phytochemicals, including different antioxidants, the fruit may exhibit different structural and biochemical defense mechanism against pathogens. However, progress in senescence processes such as tissue softening, increased soluble sugars and sweetness, and decreased acidity and defense metabolites such as phenolics and antioxidants make the fruit more susceptible to pathogen attack and postharvest losses (Qin et al. 2009; Tian et al. 2013).
Enhancing the quality and phytochemical content of the fruit is not only crucial for the production of high-quality fruit but also is necessary for extending the crop shelf life, and has been considered as a practical strategy for production of foods with no chemical residues (Asghari and Soleimani Aghdam 2010; Asghari and Zahedipour 2016). It is possible to enhance the quality and defense mechanisms of the crop by management tools including different plant growth regulators and phytohormones (Oliveira et al. 2016; Zahedipour et al. 2019). Modulation of different resistance-related genes and enzymes in plants and harvested crops is controlled by phytohormones and growth regulators. A number of plant growth regulators such as salicylates (Babalar et al. 2007; Asghari and Soleimani Aghdam 2010), polyamines (PAs) (Pieterse et al. 2009), jasmonates (Asghari and Hasanlooe 2015), polyphenols (Ahammed et al. 2018), brassinosteroids (BRs) (Asghari and Zahedipour 2016), nitric oxide (NO) (Pieterse et al. 2009), melatonin (Ahammed et al. 2019), organic acids (Song et al. 2018), and ethylene (ET) (Iqbal et al. 2017) have been shown to be involved in plant immunity system activation and phytochemical production pathways.
Brassinosteroids are a group of phytochemicals playing key roles in different growth and developmental processes of plants (Choudhary et al. 2012; Gruszka 2018). These phytohormones have been shown to enhance both resistance mechanisms and growth parameters of the plants, making them as stress-mediating and growth-promoting agents (Asghari and Zahedipour 2016). They have important roles in activating crop defense responses, including different enzymatic and non-enzymatic antioxidant systems, against various biotic and abiotic stresses in plants and harvested crops (Bajguz and Hayat 2009; Asghari and Zahedipour 2016; Asghari and Rezaei-Rad 2018). Treatment of strawberry plants with 24-epibrassinolide (EBL), an active brassinosteroids, showed that EBL acts as a growth-promoting and relatively stress-mediating agent at low concentrations, but strongly modulates stress resistance at higher doses (Asghari and Zahedipour 2016). Similarly, the role of exogenous brassinosteroids in enhancing camellia plants defense against Colletotrichum gloeosporioides was reported (Zhang et al. 2018).
There are contradictory reports regarding the effects of BRs on phytochemical content, postharvest quality, and shelf life of perishable crops. Most studies indicate the senescence promoting effects of BRs due to enhancing ethylene production (Symons et al. 2006; Chai et al. 2013; Zhu et al. 2015; He et al. 2018). On the other hand, there are reports on positive effects of BRs in enhancing postharvest life and decreasing decay extension in some crops (Zhu et al. 2010).
There are few reports regarding the effects of BRs on strawberry fruit quality, different phytochemicals and antioxidant fractions, and postharvest physiology. In a previous study, the positive and dual effects of EBL on strawberry plant growth and resistance mechanisms were reported (Asghari and Zahedipour 2016), but the effects on different aspects of quality parameters, phytochemical contents, and resistance mechanisms against pathogens and postharvest stresses remain to be illustrated. In line with the previous study, the current experiment was conducted to illustrate the effects of foliar spray with different levels of EBL, as a safe and environmental friendly phytochemical compound, on strawberry fruit quality indices, pigmentations, phytonutrients, antioxidants, bioactive compounds, and postharvest physiology. Since BRs have shown some opposite and dual effects on quality of some studied crops, different levels of EBL were used to see if it may have dual effects on strawberry fruit quality parameters, different antioxidant fractions, color, and postharvest life or not. Also, the relations between changes in fruit quality parameters such as total soluble solids, total acidity and color, enzymatic and non-enzymatic antioxidants, phenolics, anthocyanin contents, and susceptibility to postharvest decays and weight loss were studied.
Materials and Methods
Treatment of Plants with EBL, Harvesting, and Storage of the Fruits
The experiment was carried out in a commercial strawberry production greenhouse, located in Urmia, Iran. Strawberry plants (Fragaria × ananassa Dutch, cv. Sabrosa) were cultivated in a coco-pit and perlite (1:1) growth medium, and fertilized with a complete nutrient solution containing a mixture of Calcinit™ (15.5% N, 19% Ca) and Superba™ Red (7-4-22% NPK + micronutrients) (Yara International, Oslo, Norway). Strawberry fruits were sprayed with EBL solution (0, 1, and/or 4 µM) 15 days after full bloom and also at ripening stage just 2 days before harvest. All fruits were harvested at commercial ripening, when more than 75% of fruit was red colored, in the morning and immediately transferred to postharvest laboratory of Urmia University. Fruits were selected for uniformity in size, shape, and color and absence of defects, placed in 250 mL polyethylene boxes (approximately 150 g weight of berries for each box) and stored at 1 ± 0.5 °C with 90–95% RH for 14 days. Each treatment was conducted on 4 plants as replicates and two observations were recorded for each replicate. After 14 days, fruits were removed from cold storage and, to simulate the commercial situations, kept at room temperature for 24 h, and then were evaluated.
Determination of SOD Enzyme Activity
Superoxide dismutase enzyme activity was determined according to the method described by Giannopolitis and Ries (1977). Fresh fruit tissue (4 g) was homogenized at 4 °C in extraction buffer (0.1 mM EDTA and 25 mM (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (pH 7.8)) with mortar and pestle. The homogenate was centrifuged at 10,000g for 10 min at 4 °C and the supernatant used as the crude enzyme extract. The reaction mixture contained 25 µM HEPES (pH 7.6), 50 mM Na2CO3 (pH 10.2), 12 mM methionine, 75 µM Nitro Blue Tetrazolium (NBT), 1 µM riboflavin, and 0.1 mM EDTA. Finally, the enzyme extract was added the tubes were shaken and then placed below a light bank consisting of fluorescent lamps (8000 Lux), at 22 °C for 20 min. SOD activity was determined by measuring the ability to inhibit the NBT photochemical reduction at 560 nm and the result reported as U kg−1 protein.
CAT Enzyme Activity
Catalase activity was evaluated by monitoring the disappearance of H2O2 by recording the decrease in absorbance every 30 s at 240 nm (Dhindsa and Matowe 1981). Frozen fruit tissue (2 g) was homogenized in 0.5 M phosphate buffer (pH 7.2), containing 1% (W/V) polyvinylpyrrolidone (PVP). The homogenate was centrifuged at 10,000g for 10 min at 4 °C. The reaction mixture contained 2.5 mL 0.5 M sodium phosphate buffer (pH 7.0), 0.3 mL 15 mM H2O2, and 0.1 mL of enzyme extract. CAT activity was expressed in unit kg−1. One unit of CAT activity was expressed as the amount of enzyme that decomposed 1 µM of H2O2 per min at 25 °C.
Polyphenol Oxidase (PPO) Activity Assay
The method described by Singh et al. (1999) was used for PPO activity assay. Fruit tissue (4 g) was pulverized in a cold mortar and pestle with extraction buffer (68.5 mL NaH2PO4 (0.2 M) and 31.5 mL NaHPO4 (0.2 M, pH 6.5) to assay PPO enzyme activity. The homogenate was centrifuged at 10,000g for 10 min at 4 °C. The supernatant was used for PPO assay. The reaction mixture containing 2 mL pyrogallol 0.01 M, 1.5 mL phosphate sodium 0.2 M (pH 6.5), and 0.5 mL extract crude enzyme was incubated at 38 °C for 3 min, the absorbance of each solution was read at 334 nm, changes in A334 recorded for 3 min, and the result expressed as ΔAbs kg−1 protein.
Phenylalanine Ammonia-Lyase (PAL) Activity Assay
The activity of PAL enzyme was determined using the method of Karthikeyan et al. (2006) using borate buffer (H3BO3/Na2B4O7·10H2O) 0.1 M (pH 7), Polyvinyl-pyrrolidone (0.1%), and 2-Mercaptoethanol (1.4 mM). The extracts were homogenized and centrifuged at 10,000g for 15 min at 4 °C, and then supernatants were used for enzyme activity assay. To determine PAL activity, enzyme extract (0.4 mL) was added to 0.5 mL (0.1 M) sodium borate buffer (pH 8.8) and 0.5 mL l-phenylalanine (12 mM). The mixture was incubated at 30 °C for 30 min and the absorbance of each solution was measured at 290 nm. PAL enzyme activity was determined as nmol trans-cinnamic acid kg−1 protein.
Determination of Total Phenolics (TP) Content
Total phenolics content of fruit extracts was determined using the Folin–Ciocalteu method as described by Plessi et al. (2007), and was expressed as mg galic acid kg−1 FW. For extraction, 1 g of fruit sample was homogenized with a solution composed of methanol/HCl (V/V 2:28). Then, the mixture was centrifuged at 10,000g and 4 °C for 10 min. The supernatant was used for the assay of TP content. For the assay, 0.1 mL of this extract was mixed with 0.5 mL Folin–Ciocalteu reagent and 7 mL distilled water. After the incubation for 2 min at room temperature in the dark, 1 mL sodium carbonate saturated solution was added and the sample was incubated again for 2 h at room temperature. The absorbance of the sample was read at 760 nm using a spectrophotometer (model; Analytik Jena Specord 200, Germany).
Total Anthocyanin Content of the Fruits
Fruit total anthocyanin content was determined based on a pH-differential method with some modifications (Plessi et al. 2007). For extraction of anthocyanin, 1 g FW of fruit sample was homogenized with solution composed of methanol/HCl (V/V 2:98). After mixing, the solution was centrifuged at 10,000g and 4 °C for 10 min. Then two dilutions of the extracts (V/V 1:10) were prepared, one with MES (2-[N-morpholino]-ethanesulfonate) buffer (pH 1) and the other with (pH 4.5), separately. KOH (1 M) and HCl (1 M) was used to regulate the pH of MES buffer. After the incubation for 5 h at 4 °C, the absorbance of each solution was measured at 510 nm.
Assay of Ascorbic Acid (AA) Content
Total ascorbic acid was measured by absorbance at 540 nm using a standard curve according to Terada et al. (1978). The TAA content was expressed as mg ascorbic acid kg−1 FW. Fresh fruit tissue (5 g) was homogenized and mixed with 100 mL metaphosphoric acid (6%) containing 2 M acetic acid to measure AA content. The mixture was centrifuged at 18,000g for 15 min at 4 °C. Then the supernatant was filtered and 1 mL aliquot of the supernatant was mixed with 0.05 mL 2, 6-dichlorophenolindophenol (0.2%), and the solution was incubated at room temperature for 1 h. Finally, 1 mL thiourea (2%) in metaphosphoric acid (5%) and 0.5 mL of dinitrophenylhydrazine (2%) in 4.5 mL L−1 sulfuric acid were added to the solution and incubated at 60 °C for 3 h. The reaction was stopped by placing the tubes in ice bath and slowly adding 2.5 mL of ice cold sulfuric acid (2%).
Total Antioxidant Activity (TAA) Assessment
2, 2-diphenyl-1-picrylhydrazyl radical (DPPH·) method was used to determine the TAA of sample extracts (Panico et al. 2009). In the free radical form, DPPH· disappears with acceptance of an electron from an antioxidant compound to become a stable diamagnetic molecule. In this study, aliquots of the fruit juice were mixed with 3 mM DPPH· solution (2.37 mg DPPH· in 2 mL ethanol). For the sample solution, 28 µL whole juice was mixed with 28 µL DPPH· solution and 944 µL ethanol. This solution was incubated in the dark at room temperature for 10 min. The absorbance of each solution was read at 515 nm. DPPH· blank solution containing 972 µL ethanol and 28 µL DPPH· was prepared and used freshly. The percentage decrease in absorbance was recorded for each sample, and percentage quenching of DPPH· radical was calculated on the basis of the observed decrease in absorbance according to the formula:
A0 is the absorbance value of the DPPH· blank solution, A1 is the absorbance value of sample solution.
Decay Extension Assay
In order to determine decay extension rate, each sample was homogenized in aseptic condition (Ribeiro et al. 2007). In order to inoculation, 1 mL of each sample was transferred to petri dishes containing 15 mL Plate Count Agar and cooled at 44–47 °C. The samples mixed immediately to obtain evenly dispersed colonies during incubation. After complete solidification, the plates incubated at 30 ± 1 °C for 72 h. The result was expressed as Log Colony Forming Units L−1.
Determination of Fruit Weight Loss, Total Soluble Solids (TSS), pH, and Total Acidity (TA)
Fruit weight was recorded, and the percentage weight loss from harvest was calculated. TSS and pH were determined at room temperature using a refractometer (Carl Zeiss, Jena, Germany) and pH meter (AZ-8601, China), respectively. For TA content measurement, 5 mL aliquot of strawberry juice was diluted with 95 mL distilled water and titration was carried out to pH 8.2 using 0.1 M NaOH.
Fruit Surface Color Evaluation
A digital chroma-meter (model:CR-400, Konica Minolta, Japan) was used to evaluate fruit surface color (Crecente-Campo et al. 2012). Fruits were sampled from each treatment and three readings were taken on different sides of each fruit for the surface color. The L* (lightness), a* (greenness [−] to redness [+]), and b* (blueness [−] to yellowness [+]) were measured. Chroma [C* = (a*2 + b*2)0.5] and hue angle [h° = (arctan b*/a*)] were calculated from CIE a* and b* values. Total color differences were calculated according to the following equation: [ΔE* = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2.
Correlation Analysis
The correlation analysis was performed to explain the internal reasons why the L* and C* values of treated strawberries with EBL were changed (Table 4). Strawberries with high concentration of anthocyanins are darker, less vivid, and tend to be redder (low L*, C* and h° value). Increase in anthocyanins content enhances light absorption (Crecente-Campo et al. 2012; Fernandez-Lara et al. 2015).
Statistical Analysis
The experiment was carried out in a completely randomized design with 3 EBL levels (0, 1 and 4 µM) and four replications. All statistical analyses were performed according to the general linear model procedure of statistical analysis system (SAS, version 9.2) and mean separations were performed by Duncan’s multiple range test. Differences at P < 0.01 and P < 0.05 were considered as significant.
Results
Antioxidant and Anti-stress Enzymes Activity
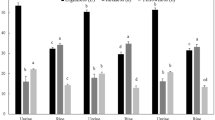
The activity of SOD, CAT, PPO, and PAL, as important anti-stress enzymes in plants and harvested crops, was significantly affected by pre-harvest EBL treatment (P < 0.01). The activity of these enzymes was about 270%, 81%, 94%, and 138% higher than the control in EBL-treated fruits, respectively (Fig. 1a–d). After 14 days of cold storage followed by 24 h storage at room temperature, fruits treated with 1 µM EBL showed the highest SOD, CAT, and PAL activity. PPO enzyme activity was enhanced in a concentration-dependent manner by EBL treatment.
Effect of foliar spray with EBL on SOD (a), CAT (b), PPO (c), and PAL (d) enzymes activity after storage. Columns followed by different letters in each group are significantly different at P < 0.01 level, according to Duncan’s multiple range test. Error bars represent standard error for the selected chart series in each group (n = 4)
With increase in the concentration of EBL from 1 to 4 µM, a decrease in the activity of SOD, CAT, and PAL enzymes was recorded.
TP, Total Anthocyanins, AA Content, and TAA
Pre-harvest treatment with EBL effectively enhanced fruit main antioxidant fractions including TP content (44.5%), anthocyanins (86%), and antioxidant activity (15.63%) (P < 0.01), and there was no significant difference between different levels of EBL in enhancing these bioactive compounds (Fig. 2a–c). There was a positive correlation between fruit phenolics, anthocyanins, antioxidant enzymes, and total antioxidant content (Table 1).
Effect of foliar spray with EBL on TP content (a), total anthocyanin content (b), TAA (c), AA concentration (d), and microbial count (e) of strawberries after storage. Columns followed by different letters in each group are significantly different at P < 0.01 level, according to Duncan’s multiple range test. Error bars represent standard error for the selected chart series in each group (n = 4)
Foliar treatment of the fruit with EBL decreased vitamin C content of the fruits, so that the AA content of the fruit treated with 4 µM EBL was significantly (P < 0.01) (18.3%) lower than the control.
Microbial Count (Decay Extension Rate)
24-epibrassinolide treatment significantly reduced the microbial count on strawberries during cold storage (P < 0.01; Fig. 2e). After 14 days of cold storage followed by a 24-h storage at room temperature, microbial count was approximately 11 and 5% lower than control in fruits treated with 1 and 4 µM, respectively. There was a negative correlation between fruit anti-stress and antioxidant components including anthocyanins, TAA, PAL, CAT, and SOD activity with microbial count (Table 1).
pH, TSS, TA, and Fruit Color
pH and TSS were not affected by EBL treatment, but fruit treated with EBL at 1 µM showed about 83% lower weight loss in comparison with the control (P < 0.01). The rate of weight loss in fruits pretreated with 4 µM EBL was more than 1 µM, indicating the adverse effects of higher EBL concentrations on fruit weight loss (Table 2). Foliar spray of the fruits with 1 µM EBL during growth stages significantly enhanced fruit acidity (19%) (Table 2; P < 0.01).
The treatment significantly enhanced the formation of red color in strawberry fruits. Compared with the control, the L* value was decreased in treated strawberries. In addition, decrease in C* and h° values in treated samples was recorded and with the increase in EBL level the effect on decreasing these values was increased. Statistical analysis of the whole data revealed that chromatic changes (ΔE*) were not significant. Strawberries treated with EBL tended to be more reddish and less vivid than control fruits (Table 3).
The anthocyanin content was found to be negatively correlated to C* and L* value with r = − 0.61, P < 0.05 and r = − 9.71, P < 0.01, respectively. Exogenous EBL had a significant effect on PPO activity (Fig. 1c). The activity of PPO enzyme is associated with formation of brown pigments (melanins) influencing fruit color quality. However, there was no significant correlation between PPO activity and color indicators like L*, C*, and also ΔE value in stored strawberries pretreated with EBL (Table 4).
Discussion
Decays caused by pathogen are the major reasons for wastage of food crops, especially perishable horticultural commodities with higher water content. Fruit, with a sensitive structure and delicate protection layer, is very perishable and susceptible to pathogen attacks (Jimenez-Zamora et al. 2016). In this study, strawberry fruits treated with 1 µM EBL during growth stages showed the lowest decay extension and weight loss during storage. There was a negative correlation between fruit anti-stress and antioxidant components including anthocyanins, total phenolic compounds, TAA, PAL, PPO, CAT, and SOD activity with decay extension rate and fruit weight loss, indicating the role of these bio-compounds in prevention of decay extension in fruit, as well as the role of BRs in enhancing disease resistance capacity of harvested fruits. The results show that EBL at lower concentration was able to decrease the senescence rate and increase postharvest life of the fruit. CAT and SOD dismutase are of main enzymatic antioxidants of fresh fruits playing pivotal roles in detoxifying different active oxygen species (Giannopolitis and Ries 1977; Ahammed et al. 2018, 2019). Enhancing the activity of these enzymes by the use of lower EBL levels promotes the plant and fruit resistance against pathogens and postharvest stresses and increases the nutritional quality and health promoting properties of the fruits (Qin et al. 2009; Choudhary et al. 2012; Zhang et al. 2018).
Phenolics, phytoalexins, tannins, organic acids, chitinases, lignins, different antioxidants, anthocyanins, and some other secondary metabolites with anti-stress, antifungal, and antioxidant activities are among the most important phytochemicals playing crucial roles in making nutritional quality and health promoting capacity of the fresh crops (Hancock and Viola 2005; Weng and Yen 2012; Ahammed et al. 2018; Zhang et al. 2018). These phytochemicals are at the same time the main components of crop defense systems against different biotic and abiotic stresses including postharvest weight loss and disease extension (Tomas-Barberan and Espin 2001; Balasundram et al. 2006; Oliveira et al. 2016). Production of structural phenolics such as cutins and formation of a complete cuticle layer as a consequence of increased PAL and PPO activity results in a decreased gas and water vapor transition between the fruit and environment, leading to decreased respiration and weight loss (Tian et al. 2013). On the other hand, exogenous EBL maintains cellular turgor pressure by improving antioxidant systems, which are responsible for protecting biological membranes against oxidative stress (Ding et al. 2016).
In the present study, correlation coefficient analysis showed a strong positive relation between different antioxidant enzymes, phenolics, anthocyanins, PPO, and PAL enzyme activity and postharvest life. Fruit with a higher antioxidants and phenolics exhibited more red color, higher organic acid content, lower postharvest weight loss, and decay extension. TSS is an indicator of senescence process and with increase in senescence rate the TSS content of the fruit increase as the result of cell wall degrading enzymes (Zhu et al. 2015; Iqbal et al. 2017). The lower TSS content in EBL-treated fruits indicates the lower cell wall degrading rate and lower senescence rate. In most of the previous studies, harvested fruits have been treated with a relatively higher BRs concentrations and the senescence promoting effects of these phytohormones were reported (Symons et al. 2006; Zhu et al. 2010, 2015; Chai et al. 2013; He et al. 2018). Most of the reports show that BRs are able to enhance the ripening and senescence process of fruits by enhancing ethylene production and action, but as a new approach, in the current study, the fruit and foliar parts of the plants were treated with lower levels of EBL and obtained opposite results. The findings of this study indicated that very low levels of BRs are able to enhance the quality of fruits by improving different metabolic pathways for biosynthesis of quality parameters including color, taste and flavor, sugars, organic acids, phenolics, and different antioxidants.
Different phenolic compounds including simple phenolics, polyphenols, flavonoids, and anthocyanins play important roles in cell antioxidant, anti-stress, and anti-pathogen activities (Ahammed et al. 2018; Wang et al. 2018; Zhang et al. 2018). PAL and PPO are the main players in biosynthetic routes of different phenolics and polyphenols, and BRs enhance gene expression and activity of these enzymes resulting in biosynthesis and accumulation of different phenolic compounds in plant and fruit cells (Choudhary et al. 2012; Asghari and Zahedipour 2016; Asghari and Rezaei-Rad 2018; Gruszka 2018). Increase in polyphenols and different phenolics is one of the reasons for decreased decay extension during postharvest storage (Zhang et al. 2018) and the substantial increase in total phenolics and anthocyanin content of fruit treated with 1 µM EBL is the consequence of 150% increase in PAL activity as the main enzyme in phenylpropanoid pathway.
Anthocyanins, as colorful phenolic compounds, are the major components of color in some fruits including the strawberries and also are powerful antioxidants acting as powerful anticancer and anti-inflammatory agents in human cells (Miguel 2011; Lin et al. 2017). Therefore, these phytochemicals play an important role in fruit overall and nutritional quality. Anthocyanins strongly enhance crop’s natural resistance against pathogens and decrease the adverse effects of postharvest conditions by directly scavenging free radicals, detoxifying active oxygen species, and activating resistance genes (Jaakola 2013). In this study, strawberries treated with the lower concentration of EBL tended to have the highest anthocyanins and be more reddish and less vivid than controls (Table 3), indicating the positive roles of BRs in anthocyanin biosynthesis. These fruits also showed the highest antioxidant capacity, organic acids, TSS, and activity of PPO and antioxidant enzymes.
With increase in EBL concentration, the positive effects on enhancing fruit quality were decreased in the present study. This may be due to the growth inhibitory effects of higher BRs. Previous reports indicate that BRs at higher concentrations may enhance the production and accumulation of growth inhibitors, mainly ethylene and ABA. Increase in growth inhibitors may result in decreased photosynthesis, assimilate production, and quality formation (Symons et al. 2006; Tian et al. 2013; Iqbal et al. 2017; He et al. 2018; Lv et al. 2018). This may be the reason for decrease in vitamin C content of the fruits in response to 4 µM EBL, indicating the adverse effects of higher BR levels on vitamin C production or stimulatory effects on vitamin C consumption rates (Fig. 2d).
These results indicated that the substantial increase in anthocyanin content, in response to EBL (1 µM) treatment, is a major reason for changes in color parameters in treated samples. In addition, the increase in activity of PPO and subsequent melanin production leading to a decrease in color parameters in response to higher (4 µM) EBL level is related to increased ethylene production rate. The appearance of melanins is important in light absorption and could change the color parameters values (Newman et al. 2011).
Conclusions
In contrast to the results of previous studies regarding the senescence promoting effects of BRs in some fruits, the results of this study showed that EBL, as a safe and environmental friendly compound, at 1 µM is able to enhance the overall and nutritional quality, health promoting capacity, and postharvest life of the strawberries. Negative correlation coefficients between fruit quality parameters, different enzymatic antioxidants, total acidity, phenolics, antioxidants, and decay extension indicate that by the use of BRs at very low concentrations during fruit growth and development stages, the quality, health promoting capacity, and safety of strawberries may be simultaneously improved. With increase in public awareness regarding the adverse effects of pesticides and other agricultural chemicals on human health and environment safety, the importance of using phytohormones and generally regarded as safe compounds as alternatives for these chemicals is more pronounced (Asghari 2019). Foliar spray of strawberry plants and fruits with very low EBL levels can be considered as an important and effective mean for producing strawberries with a very higher antioxidant and anticancer capacity and maintaining the fruit quality and safety for a longer period after harvest. The need for very lower levels of BRs for improving the quality and safety of the fruit is also very important from the economic point of view.
Future studies on the effects of lower BR levels on quality parameters, stress resistance mechanisms, gene expression, and enzyme activity of different metabolite pathways in different crops can further illustrate the mechanisms of actions of these phytohormones.
References
Ahammed GJ, Li Y, Li X, Han W-Y, Chen S (2018) Epigallocatechin-3-gallate alleviates salinity-retarded seed germination and oxidative stress in tomato. J Plant Growth Regul 37:1349–1356. https://doi.org/10.1007/s00344-018-9849-0
Ahammed GJ, Xu W, Liu A, Chen S (2019) Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ Exp Bot 161:303–311. https://doi.org/10.1016/j.envexpbot.2018.06.006
Asghari M (2019) Impact of jasmonates on safety, productivity and physiology of food crops. Trends Food Sci Technol 91:169–183. https://doi.org/10.1016/j.tifs.2019.07.005
Asghari M, Hasanlooe AR (2015) Interaction effects of salicylic acid and methyl jasmonate on total antioxidant content, catalase and peroxidase enzymes activity in Sabrosa strawberry fruit during storage. Sci Hort 197:490–495. https://doi.org/10.1016/j.scienta.2015.10.009
Asghari M, Rezaei-Rad R (2018) 24-Epibrassinolide enhanced the quality parameters and phytochemical contents of table grape. J Appl Bot Food Qual 91:226–231. https://doi.org/10.5073/JABFQ.2018.091.030
Asghari M, Soleimani Aghdam M (2010) Impact of salicylic acid on post-harvest physiology of horticultural crops. Trends Food Sci Technol 21:502–509. https://doi.org/10.1016/j.tifs.2010.07.009
Asghari M, Zahedipour P (2016) 24-Epibrassinolide acts as a growth-promoting and resistance-mediating factor in strawberry plants. J Plant Growth Regul 34:1–8. https://doi.org/10.1007/s00344-016-9577-2
Babalar M, Asghari M, Talaei A, Khosroshahi A (2007) Effect of pre- and postharvest salicylic acid treatment on ethylene production, fungal decay and overall quality of Selva strawberry fruit. Food Chem 105(2):449–453. https://doi.org/10.1016/j.foodchem.2007.03.0
Bajguz A, Hayat S (2009) Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem 47:1–8. https://doi.org/10.1016/j.plaphy.2008.10.002
Balasundram N, Sundram K, Samman S (2006) Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem 99:91–203. https://doi.org/10.1016/j.foodchem.2005.07.042
Basu A, Nguyen A, Betts NM, Lyons TJ (2014) Strawberry as a functional food: an evidence-based review. Crit Rev Food Sci Nutr 54:790–806. https://doi.org/10.1080/10408398.2011.608174
Chai Y, Zhang Q, Tian L, Li CL, Xing Y, Qin L, Shen YY (2013) Brassinosteroid is involved in strawberry fruit ripening. Plant Growth Regul 69(1):63–69. https://doi.org/10.1007/s10725-012-9747-6
Choudhary SP, Yu JQ, Yamaguchi-Shinozaki K, Shinozaki K, Tran LSP (2012) Benefits of brassinosteroid crosstalk. Trends Plant Sci 17:594–605. https://doi.org/10.1016/j.tplants.2012.05.012
Crecente-Campo J, Nunes-Damaceno M, Romero-Rodriguez MA, Vázquez-Odériz ML (2012) Color, anthocyanin pigment, ascorbic acid and total phenolic compound determination in organic versus conventional strawberries (Fragaria × ananassa Duch, cv Selva). J Food Compost Anal 28:23–30. https://doi.org/10.1016/j.jfca.2012.07.004
Dhindsa RS, Matowe W (1981) Drought tolerance in two mosses: correlated with enzymatic defense against lipid peroxidation. J Exp Bot 32:79–91. https://doi.org/10.1093/jxb/32.1.79
Ding Y, Zhu Z, Zhao J, Nie Y, Zhang Y, Sheng J, Meng D, Mao H, Tang X (2016) Effects of postharvest brassinolide treatment on the metabolism of white button mushroom (Agaricus bisporus) in relation to development of browning during storage. Food Bioprocess Technol 9:1–8. https://doi.org/10.1007/s11947-016-1722-1
Fernandez-Lara R, Gordillo B, Rodriguez-Pulido FJ, Gonzalez-Miret ML, del Villar-Martinez AA, Davila-Ortiz G, Heredia FJ (2015) Assessment of the differences in the phenolic composition and color characteristics of new strawberry (Fragaria × ananassa Duch.) cultivars by HPLC–MS and Imaging Tristimulus Colorimetry. Food Res Int 76:645–653. https://doi.org/10.1016/j.foodres.2015.07.038
Giannopolitis CN, Ries SK (1977) Superoxide dismutases I. Occurrence in higher plants. Plant Physiol 59:309–314. https://doi.org/10.1104/pp.59.2.309
Gruszka D (2018) Crosstalk of the brassinosteroid signalosome with phytohormonal and stress signaling components maintains a balance between the processes of growth and stress tolerance. Int J Mol Sci 19:1–47. https://doi.org/10.3390/ijms19092675
Hancock RD, Viola R (2005) Improving the nutritional value of crops through enhancement of l-ascorbic acid (vitamin C) content: rationale and biotechnological opportunities. J Agric Food Chem 53:5248–5257. https://doi.org/10.1021/jf0503863
He Y, Li J, Ban Q, Han S, Rao J (2018) Role of brassinosteroids in Persimmon (Diospyros kaki L.) fruit ripening. J Agric Food Chem 66(11):2637–2644. https://doi.org/10.1021/acs.jafc.7b06117
Iqbal N, Khan NA, Ferrante A, Trivellini A, Francini A, Khan M (2017) Ethylene role in plant growth, development and senescence: interaction with other phytohormones. Front Plant Sci 8:475. https://doi.org/10.3389/fpls.2017.00475
Jaakola L (2013) New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci 18:477–483. https://doi.org/10.1016/j.tplants.2013.06.003
Jimenez-Zamora A, Delgado-Andrade C, Rufian-Henares JA (2016) Antioxidant capacity, total phenols and color profile during the storage of selected plants used for infusion. Food Chem 199:339–346. https://doi.org/10.1016/j.foodchem.2015.12.019
Karthikeyan M, Radhika K, Mathiyazhagan S, Bhaskaran R, Samiyappan R, Velazhahan R (2006) Induction of phenolics and defense-related enzymes in coconut (Cocos nucifera L.) roots treated with biocontrol agents. Braz J Plant Physiol 18:367–377. https://doi.org/10.1590/S1677-04202006000300003
Lin BW, Gong CC, Song HF, Cui YY (2017) Effect of anthocyanins on the prevention and treatment of cancer. Br J Pharmacol 174:1226–1243. https://doi.org/10.1111/bph.13627
Lv B, Tian H, Zhang F, Liu J, Lu S, Bai M, Li C, Ding Z (2018) Brassinosteroids regulate root growth by controlling reactive oxygen species homeostasis and dual effect on ethylene synthesis in Arabidopsis. PLoS Genet 14(1):e1007144. https://doi.org/10.1371/journal.pgen.1007144
Miguel MG (2011) Anthocyanins: antioxidant and/or anti-inflammatory activities. J Appl Pharm Sci 01(06):07–15
Newman SM, Tantasawat P, Steffens JC (2011) Tomato polyphenol oxidase B is spatially and temporally regulated during development and in response to ethylene. Molecules 16(1):493–517. https://doi.org/10.3390/molecules16010493
Oliveira MDM, Varanda CMR, Felix MRF (2016) Induced resistance during the interaction pathogen × plant and the use of resistance inducers. Phytochem Lett 15:152–158. https://doi.org/10.1016/j.phytol.2015.12.011
Panico AM, Garufi F, Nitto S, Di Mauro R, Longhitano RC, Magri G, Catalfo A, Serrentino ME, De Guidi G (2009) Antioxidant activity and phenolic content of strawberry genotypes from Fragaria × ananassa. Pharm Biol 47:203–208. https://doi.org/10.1080/13880200802462337
Pieterse CM, Leon-Reyes A, Van der Ent S, Van Wees SC (2009) Networking by small-molecule hormones in plant immunity. Nat Chem Biol 5:308–316. https://doi.org/10.1038/nchembio.164
Plessi M, Bertelli D, Albasini A (2007) Distribution of metals and phenolic compounds as a criterion to evaluate variety of berries and related jams. Food Chem 100:419–427. https://doi.org/10.1016/j.foodchem.2005.09.018
Qin G, Meng X, Wang Q, Tian S (2009) Oxidative damage of mitochondrial proteins contributes to fruit senescence: a redox proteomics analysis. J Proteome Res 8:2449–2462. https://doi.org/10.1021/pr801046m
Ribeiro C, Vicente AA, Teixeira JA, Miranda C (2007) Optimization of edible coating composition to retard strawberry fruit senescence. Postharvest Biol Technol 44:63–70. https://doi.org/10.1016/j.postharvbio.2006.11.015
Singh N, Singh R, Kaur K, Singh H (1999) Studies of the physico-chemical properties and polyphenol oxidase activity in seeds from hybrid sunflower (Helianthus annuus) varieties grown in India. Food Chem 66:241–247. https://doi.org/10.1016/S0308-8146(99)00057-66
Song J, Zhang H, Duan C, Cui X (2018) Exogenous application of succinic acid enhances tolerance of Larix olgensis seedling to lead stress. J For Res 29(6):1497–1505. https://doi.org/10.1007/s11676-017-0579-0
Symons GM, Davies C, Shavrukov Y, Dry IB, Reid JB, Thomas MR (2006) Grapes on steroids. Brassinosteroids are involved in grape berry ripening. Plant Physiol 140(1):150–158. https://doi.org/10.1104/pp.105.070706
Terada M, Watanabe Y, Kunitomo M, Hayashi E (1978) Differential rapid analysis of ascorbic acid and ascorbic acid 2-sulfate by dinitrophenylhydrazine method. Anal Biochem 4:604–608. https://doi.org/10.1016/0003-2697(78)90083-0
Tian S, Qin G, Li B (2013) Reactive oxygen species involved in regulating fruit senescence and fungal pathogenicity. Plant Mol Biol 82:593–602. https://doi.org/10.1007/s11103-013-0035-2
Tomas-Barberan FA, Espin JC (2001) Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J Sci Food Agric 81:853–876. https://doi.org/10.1002/jsfa.885
Wang P, Mu X, Du J, Gao YG, Bai D, Jia L, Zhang J, Ren H, Xue X (2018) Flavonoid content and radical scavenging activity in fruits of Chinese dwarf cherry (Cerasus humilis) genotypes. J For Res 29(1):55–63. https://doi.org/10.1007/s11676-017-0418-3
Weng CJ, Yen GC (2012) Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat Rev 38:76–87. https://doi.org/10.1016/j.ctrv.2011.03.001
Zahedipour P, Asghari M, Abdollahi B, Alizadeh M, Rezaei Danesh Y (2019) A comparative study on quality attributes and physiological responses of organic and conventionally grown table grapes during cold storage. Sci Hort 247:86–95. https://doi.org/10.1016/j.scienta.2018.11.077
Zhang L, Ahammed GJ, Li X, Wei J-P, Li Y, Yan P, Zhang L-P, Han W-Y (2018) Exogenous brassinosteroid enhances plant defense against Colletotrichum gloeosporioides by activating phenylpropanoid pathway in Camellia sinensis L. J Plant Growth Regul 37:1235–1243. https://doi.org/10.1007/s00344-018-9857-0
Zhu Z, Zhang Z, Qin G, Tian S (2010) Effects of brassinosteroids on postharvest disease and senescence of jujube fruit in storage. Postharvest Biol Technol 56:50–55. https://doi.org/10.1016/j.postharvbio.2009.11.014
Zhu T, Tan WR, Deng XG, Zheng T, Zhang DW, Lin HH (2015) Effects of brassinosteroids on quality attributes and ethylene synthesis in postharvest tomato fruit. Postharvest Biol Technol 100:196–204. https://doi.org/10.1016/j.postharvbio.2014.09.016
Acknowledgements
This work was supported by Urmia University (11966) and the National Natural Science Foundation of China (31200146). The research was supported by a grant from the basic research fund of Heilongjiang Province (HDRCCX-201612).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, Y., Asghari, M. & Zahedipour-Sheshgelani, P. Foliar Spray with 24-Epibrassinolide Enhanced Strawberry Fruit Quality, Phytochemical Content, and Postharvest Life. J Plant Growth Regul 39, 920–929 (2020). https://doi.org/10.1007/s00344-019-10033-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-019-10033-y