Abstract
The phytohormones brassinosteroids (BRs) regulate multiple aspects of plant growth, development, and responses to stress. However, the role of BRs in the defense response of tea (Camellia sinensis L.), one of the most important beverage crops, remains largely unknown. Previously, we reported that BRs improve tea quality both under normal and unfavorable temperature conditions. Here, we showed that 24-epibrassinolide (EBR, a bioactive BR) enhanced defense against Colletotrichum gloeosporioides in tea plants, which was associated with EBR-induced reduction in H2O2 accumulation in tea leaves. C. gloeosporioides-caused necrotic lesions and its actin gene expression increased over the postinoculation period, but exogenous EBR remarkably suppressed C. gloeosporioides spread. Time-course analysis of a key enzyme, phenylalanine ammonia-lyase (PAL), involved in phenylpropanoid biosynthesis, revealed that PAL activity gradually increased from 6 to 24 h postinoculation with C. gloeosporioides following an initial decline. Meanwhile, exogenous EBR sharply increased PAL activity of inoculated leaves compared with that of only C. gloeosporioides inoculation. Expression analysis of genes involved in phenylpropanoid pathway showed that both exogenous EBR and C. gloeosporioides inoculation increased transcript levels of CsPAL, CsC4H, and Cs4CL; however, combined treatment with EBR and C. gloeosporioides resulted in a greater increase. Furthermore, CsPR1 and CsLOX1 expression analyses revealed that EBR potentially activates systemic induced tolerance, but not the lipoxygenase pathway to enhance tea plant resistance to C. gloeosporioides. These findings indicate a positive role of BR in strengthening disease resistance and thus may have potential implications in the control of C. gloeosporioides-caused disease in tea plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Camellia sinensis L. is an evergreen woody perennial plant species, commercially cultivated for manufacturing tea, the most popular healthy drink in the world. It is cultivated across 58 countries in five continents with major production sites in Asia and Africa (Han et al. 2018). People around the globe consume tea because of its pleasant taste and numerous health benefits (Kim et al. 2009; Zhang and Tsao 2016). Tea is manufactured from young shoots and leaves which are more prone to abiotic and biotic stressors compared to mature leaves (Han et al. 2018; Li et al. 2018a). Because leaves are the final harvest in tea cultivation, pathogens that cause foliar diseases in tea, not only reduce tea yield but also tea quality. Tea cultivation is affected by hundreds of diseases (Li et al. 2016b). Among various disease causing agents, fungal pathogens from the genera Colletotrichum cause the most destructive leaf diseases in tea. The Colletotrichum species-caused diseases are commonly called ‘Anthracnose.’ One of the dominant species that causes anthracnose in Camellia sinensis is C. gloeosporioides. In China, C. gloeosporioides-caused disease causes a significant economic loss of tea every year (Guo et al. 2014). Although fungicides can effectively control C. gloeosporioides, the presence of pesticide residues in tea has emerged as a matter of public concern due to issues of food safety (Saha et al. 2012). Therefore, exploration of natural biomolecules that can strengthen tea defense against pathogens has received great attention from tea scientists (Li et al. 2016b).
The phenylpropanoid pathway plays a critical role in the defense response of plants to pathogens (Li et al. 2017a; Novo et al. 2017; Yogendra et al. 2015). In general, stimulation of phenylpropanoid metabolism leads to the synthesis and accumulation of a large number of secondary metabolites. Some of these compounds directly function as defense molecules that prevent pathogen expansion and minimize oxidative stress (Wei et al. 2017; Yogendra et al. 2015). Furthermore, accumulation of some other compounds, such as lignin, reinforce defensive structures, such as the cell wall by thickening lignin layers (Dixon et al. 2002; Yogendra et al. 2015). Phenylalanine ammonia-lyase (PAL), cinnamate-4-hydroxylase (C4H), and 4-coumarate-CoA ligase (4CL) are three critical enzymes that catalyze phenolic synthesis in the phenylpropanoid pathway (Wei et al. 2017). Derivatives of the phenylpropanoid pathways, such as flavonoids, have strong antimicrobial bioactivity, which restrict fungal pathogens by inhibiting spore germination, germ tube elongation, and mycelia growth (Li et al. 2017a; Novo et al. 2017). Tea plants are rich in secondary metabolites and the activation of phenylpropanoid pathways potentially helps tea plants to ward off pathogen infection (Han et al. 2018; Li et al. 2016a). For instance, anthracnose-resistant Camellia varieties show 5- to 10-fold larger levels of polyphenols, catechol, and salicylic acid content than that of susceptible varieties (Xinzhang 2012). In potato, an increased abundance of phenylpropanoid metabolites in stems results in enhanced resistance to Phytophthora infestans, the causal agent of late blight disease (Franke et al. 2012). Previous studies have shown that hormones and signal molecules can stimulate the phenylpropanoid pathway, leading to an enhanced tolerance to abiotic and biotic stresses (Ahammed et al. 2017; Li et al. 2017a). For instance, exogenous nitric oxide enhances disease resistance to Monilinia fructicola in peach fruit by activating the phenylpropanoid pathway (Li et al. 2017a). In our previous studies, we also found that application of 24-epibrassinolide increases PAL activity and flavonoid biosynthesis by triggering NO accumulation in tea leaves (Li et al. 2016a, 2017b).
Brassinosteroids are involved in multiple aspects of plant growth, development, and responses to stress (Ahammed et al. 2014; Li et al. 2016a, 2018b). Besides the critical roles of BR in abiotic stress tolerance, its function in disease resistance has been well documented in a range of plants species (Hideo et al. 2003; Li et al. 2018b; Song et al. 2018; Xia et al. 2009, 2011). BR strengthens defense against a broad range of biotic stressors, including fungi, virus, bacteria, and nematodes (Bjornson et al. 2016; Deng et al. 2016; Hideo et al. 2003; Song et al. 2018). In rice, BR application suppresses the fungal pathogens Magnaporthe grisea and Xanthomonas oryzae that cause blast and bacterial blight diseases, respectively (Hideo et al. 2003). Similarly, BR enhances resistance to the fungus Oidium sp., tobacco mosaic virus, and the bacteria Pseudomonas syringae pv. Tabaci in tobacco. In barley, suppression of BRASSINOSTEROID INSENSITIVE 1 (BRI1, BR receptor) enhances susceptibility to Fusarium culmorum as reflected by increased necrosis in leaves, indicating that a functional BRI1 is essential for disease resistance (Ali et al. 2014). However, overexpression of BRI1-associated kinase 1 (BAK1)-interacting receptor-like kinase 3 (BIR3) in tomato and Arabidopsis thaliana results in enhanced susceptibility to the necrotrophic fungus Botrytis cinerea (Huang et al. 2017). Song et al. (2018) showed that BR deficiency or lack of BR receptor increases susceptibility of tomato plants to root-knot nematode, Meloidogyne incognita. However, the role of BR in the defense response of tea plants to C. gloeosporioides remains largely unknown. In this study, we showed that exogenous BR could enhance defense against C. gloeosporioides in tea plants, which was associated with BR-induced activation of the phenylpropanoid pathway. Our results also suggest that BR potentially stimulates systemic-induced tolerance but not the lipoxygenase pathway to enhance tea plant resistance to C. gloeosporioides.
Materials and Methods
Plant Materials, Growth Conditions, and Treatments
Two-year-old tea seedlings of Longjing 43 (Camellia sisnensis (L.) O. Kuntze) grown in the greenhouse of the Tea Research Institute of the Chinese Academy of Agricultural Sciences (TRI, CAAS, N 30°10′, E 120°5′), Hangzhou, China were used in the current study. The growth conditions in the greenhouse were as follows: the mean maximum and minimum temperatures were 28.8 °C and 22.4 °C and relative humidity was about 70–80%. Tea seedlings were treated with 100 nM 24-epibrassinolide (EBR, Sigma-Aldrich, USA) at 24 h and 3 h prior to inoculation with C. gloeosporioides. EBR solution was prepared and sprayed following the method as described previously (Li et al. 2016a, 2017b). Control plants were prayed with distilled water. Each treatment comprised eighteen seedlings. The experiments were replicated three times independently. All plants were watered with Hoagland’s solution.
Culture of Fungi C. gloeosporioides and Methods of Inoculation
A pure culture of C. gloeosporioides isolates was obtained following the protocol that we described previously (Li et al. 2016b). To study the infection behavior of cultured C. gloeosporioides, a C. gloeosporioides spore suspension at a density of 1 × 105 spores per ml was sprayed onto the leaves. Tea seedlings were sealed hermetically by plastic wrap to make sure the humidity is 100%.
Determination of H2O2 Accumulation
Histochemical staining of H2O2 was performed as previously described (Xia et al. 2009). Tea leaves were vacuum infiltrated with 1 mg ml−1 3,3ʹ-diaminobenzidine (DAB) in 50 mM TRIS–acetate buffer (pH 3.8) and incubated at 25 °C in for 6 h. DAB-stained leaves were then decolorized in boiling ethanol (95%) for 15 min and photographed with a digital camera. H2O2 concentration in tea leaves was measured spectrophotometrically (Willekens et al. 1997).
Assay of PAL Enzyme Activity
A tea leaf sample (0.3 g) was homogenized in 3 ml 50 mM potassium phosphate buffer (pH 8.8, containing 2 mM ethylenediaminetetraac (EDTA), 2% polyvinyl polypyrrolidone (PVPP), and 0.1% mercaptoethanol). The resulting homogenates were centrifuged at 15,000 rpm for 20 min at 4 °C and crude enzyme extracts were obtained as the supernatants. The PAL activity was assayed with l-phenylalanine as substrate based on the yield of cinnamic acid and spectrophotometrically determined by monitoring the change in absorbance at 290 nm as described by Zheng et al. (2005).
Total RNA Extraction and Gene Expression Analysis
For gene expression analysis, leaf samples were collected at 12 h postinoculation and immediately frozen into liquid nitrogen and kept at − 80 °C until isolation of RNA. Total RNA from tea leaves was extracted using an RNA extraction kit (TIANGEN BIOTECH, Beijing, China), according to the manufacturer’s recommendations. Total RNA was reverse transcribed using a ReverTra Ace qPCR RT kit (Toyobo, Osaka, Japan), following the manufacturer’s instructions. Gene-specific quantitative real-time PCR (qRT-PCR) primers were designed based on their cDNA sequences. qRT-PCR was performed using the ABI 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). Each reaction (20 µl) consisted of 10 µl SYBR Green PCR Master Mix (Takara, Chiga, Japan), 2 µl diluted cDNA, 0.4 µl ROX reference Dye II (Takara, Chiga, Japan), and 0.2 µmol forward and reverse primers. The cycling conditions were as follows: 95 °C for 30 s, and 40 cycles of 95 °C for 5 s and 60 °C for 34 s. CsPTB was used as an internal control. Relative gene expression was calculated as previously described (Livak and Schmittgen 2001).
Statistical Analysis
The data were statistically analyzed using SAS 8.1 software package (SAS Institute Inc., Cary, NC, USA). Differences between treatments means were separated by the Tukey’ test at a significance level of P < 0.05.
Results
Exogenous Brassinosteroid Enhances Defense Against C. gloeosporioides
To explore the effects of BR on tea plant defense against C. gloeosporioides, we pretreated tea plants with exogenous 24-epibrassinolide (EBR, 100 nM) and then inoculated with C. gloeosporioides. Necrotic lesions caused by C. gloeosporioides expanded gradually over the postinoculation period (data not shown). It is evident from the leaf phenotypes (Fig. 1a) that the lesion area of controls was clearly larger than that of EBR-pretreated leaves, indicating that exogenous EBR remarkably suppressed C. gloeosporioides spread as reflected by very small lesions. We also assayed relative expression of C. gloeosporioides actin in tea leaves following exogenous EBR and/or C. gloeosporioides inoculation (Fig. 1b). The expression of C. gloeosporioides actin showed 3.6-, 6.4-, and 33.2-fold increases at 3, 6, and 9 days postinoculation (dpi), respectively, compared with that at 0 dpi. However, exogenous EBR significantly reduced C. gloeosporioides actin expression resulting in only at 1.6-, 2.0-, 6.3-fold changes at 3, 6, and 9 dpi, respectively, compared with that of only C. gloeosporioides-inoculated tea leaves. These results clearly indicate that exogenous EBR enhances tea plants defense against C. gloeosporioides.
Exogenous brassinosteroid suppressed C. gloeosporioides spread on tea leaves. a Phenotypes of leaves at 9 days postinoculation (dpi) with C. gloeosporioides. b Relative expression of C. gloeosporioides actin at different postinoculation time periods. Two-year-old tea seedlings were treated with 100 nM EBR (24-epibrassinolide) at 24 h and 3 h prior to inoculation with C. gloeosporioides. Gene expression data comprised six replicates. Means followed by the same letter are not significantly different according to Tukey’s test (P < 0.05)
Brassinosteroids Attenuate C. gloeosporioides-Induced H2O2 Accumulation in Tea Leaves
Reactive oxygen species (ROS) accumulation and subsequent oxidative stress are common phenomena that occur when a plant is challenged with biotic and abiotic stressors. Therefore, we analyzed H2O2 accumulation in tea leaves by using both histochemical and biochemical methods. In situ visualization of H2O2 using DAB staining showed that H2O2 accumulation increased over the postinoculation period and became highest at 9 dpi (Fig. 2a). Consistent with DAB staining results, H2O2 concentrations in tea leaves increased by 104.52% and 194.01% at 3 and 9 dpi, respectively (Fig. 2b). However, exogenous EBR remarkably decreased H2O2 accumulation in C. gloeosporioides-inoculated tea leaves as reflected by relatively light brown colored leaves and lower concentrations of H2O2. These findings suggest that exogenous EBR potentially alleviated C. gloeosporioides-induced oxidative stress by strengthening antioxidant potential.
Exogenous brassinosteroids minimized C. gloeosporioides-induced H2O2 accumulation in tea leaves. a Visualization of in situ H2O2 accumulation by 3,3′-diaminobenzidine (DAB) staining. The deep brown spots indicate the polymerization products produced by the reaction of DAB with H2O2. b H2O2 concentration. Two-year-old tea seedlings were treated with 100 nM EBR at 24 h and 3 h prior to inoculation with C. gloeosporioides. Biochemical data are expressed as mean ± SD (n = 6). Means denoted by the same letter did not significantly differ at P < 0.05 according to Tukey’s test
Brassinosteroids Stimulate Phenylpropanoid Pathway to Enhance Resistance to C. gloeosporioides
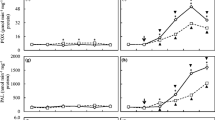
Activation of the phenylpropanoid pathway contributes to plant defense against pathogenic fungi. Therefore, we analyzed the activity of PAL, the first key enzyme in the phenyl propanoid pathway. Time-course analysis of PAL activity revealed that inoculation with C. gloeosporioides caused an initial decline in the early few hours; however, PAL activity, then, gradually increased with postinoculation time period (Fig. 3). At 24 h postinoculation, PAL activity in C. gloeosporioides-inoculated tea leaves increased by 48.74% compared with that of control. On the other hand, in the absence of C. gloeosporioides, exogenous EBR increased PAL activity during the initial 6 h, which then slowly declined over time. At 24 h postinoculation, PAL activity in only EBR-treated tea leaves was 17.30% higher than that of control. However, combined treatment of exogenous EBR and C. gloeosporioides increased PAL activity by 22.47% compared with only C. gloeosporioides inoculation.
Time-course analysis of phenylalanine ammonia-lyase (PAL) activity as influenced by exogenous brassinosteroid pretreatment and C. gloeosporioides inoculation. Two-year-old tea seedlings were treated with 100 nM EBR at 24 h and 3 h prior to inoculation with C. gloeosporioides. Data are expressed as mean ± SD (n = 6)
To further explore the mechanism of EBR-induced enhanced resistance to C. gloeosporioides, we assayed the transcript abundance of CsPAL, CsC4H, and Cs4CL, which encode the first three key enzymes of the phenylpropanoid pathway, respectively. As shown in Fig. 4, EBR, C. gloeosporioides, and EBR + C. gloeosporioides treatments caused 3.12-, 2.05-, and 4.03-fold increases in CsPAL, 1.84-, 1.57-, and 2.23-fold increases in CsC4H and 1.47-, 1.71-, and 2.65-fold increases in Cs4CL expression, respectively, as compared to the control. Interestingly, transcript levels of CsPAL, CsC4H, and Cs4CL in EBR and C. gloeosporioides-treated tea leaves were 1.96-, 1.42-, and 1.55-fold larger than that of only C. gloeosporioides-inoculated tea leaves, respectively. This implies that exogenous EBR activated transcription of key enzymes of the phenylpropanoid pathway, which potentially increased resistance to C. gloeosporioides in tea plants.
Exogenous brassinosteroids activated phenylpropanoid pathway in response to C. gloeosporioides infection in tea leaves. Relative expression of a PHENYLALANINE AMMONIA-LYASE (CsPAL), b CINNAMATE-4-HYDROXYLASE (CsC4H), and c 4-COUMARATE-COA LIGASE (Cs4CL). Two-year-old tea seedlings were treated with 100 nM EBR at 24 h and 3 h prior to inoculation with C. gloeosporioides. Transcript levels of genes were analyzed by qRT-PCR using gene-specific primer pairs (Supplementary Table S1). The samples were harvested for gene expression analysis at 12 h postinoculation with C. gloeosporioides. Data are the means ± SD of three biological replicates. Means denoted by the same letter did not significantly differ at P < 0.05 according to Tukey’s test
Brassinosteroids Induce Defense Gene Expression But Not LOX Pathway to Enhance Defense Against C. gloeosporioides
Three classical hormones, such as salicylic acid (SA), jasmonic acid (JA), and ethylene (ET), play critical roles in plant defense against pathogens (Yang et al. 2014). Most often, the SA and JA pathways function antagonistically to mediate a defense response. However, BR-induced resistance to Meloidogyne incognita is not dependent on SA, JA, and ET accumulation (Song et al. 2018). In our previous study, we found that exogenous caffeine enhances defense against C. gloeosporioides by increasing LOX activity and JA concentration in tea leaves (Li et al. 2016b). Furthermore, a close association between expression of defense genes, such as PATHOGENESIS-RELATED GENE 1 (PR1) and an effective defense response has been reported in various plant-pathogen systems (Ahammed et al. 2018; Xia et al. 2011). Therefore, we analyzed transcript abundance of CsPR1 and CsLOX1 following treatment with EBR and/or C. gloeosporioides. As shown in Fig. 5a, exogenous EBR and C. gloeosporioides inoculation increased the transcript levels of CsPR1 accounting for 10.93- and 21.93-fold changes, respectively, compared with the non-inoculated control. Meanwhile, EBR pretreatment on C. gloeosporioides-inoculated plants caused a 1.98-fold increase in CsPR1 expression as compared with that of only C. gloeosporioides-inoculated tea leaves. Moreover, we found 1.16- and 4.50-fold increases in CsLOX1 expression after exogenous EBR treatment and C. gloeosporioides inoculation, respectively, compared with the non-inoculated control (Fig. 5b). Unlike the transcript abundance of CsPR1, CsLOX1 expression was not altered by EBR-only treatment. Moreover, exogenous EBR rather attenuated C. gloeosporioides-induced upregulation in CsLOX1 expression, indicating that exogenous EBR potentially inhibits the LOX-mediated defense pathway to enhance resistance to C. gloeosporioides in tea plants.
Exogenous brassinosteroids augmented the expression of defense gene in C. gloeosporioides-inoculated tea leaves. Relative expression of a PATHOGENESIS-RELATED GENE 1 (CsPR1) and b LIPOXYGENASE 1 (CsLOX1). Two-year-old tea seedlings were treated with 100 nM EBR at 24 h and 3 h prior to inoculation with C. gloeosporioides. Transcript levels of genes were analyzed by qRT-PCR using gene-specific primer pairs (Supplementary Table S1). The samples were harvested for gene expression analysis at 12 h postinoculation with C. gloeosporioides. Data are the means ± SD of three biological replicates. Means denoted by the same letter did not significantly differ at P < 0.05 according to Tukey’s test
Discussion
Global tea production is being influenced by climate change (Han et al. 2018). The key drivers of climate change not only exert direct effects on tea physiology, but also affect tea production indirectly by altering insect and disease abundance. Previously, we showed that elevated CO2 increases susceptibility of tea plants to C. gloeosporioides by decreasing caffeine biosynthesis (Li et al. 2016b). In the current study, we found that exogenous BR improves tea plant resistance to C. gloeosporioides which is largely attributed to the BR-induced activation of the phenylpropanoid pathway (Figs. 1, 2, 3, 4). Moreover, exogenous BR upregulated expression of the defense gene, CsPR1, but downregulated expression of CsLOX1 to enhance the defense response of tea plants to C. gloeosporioides.
Plants have developed intricate strategies to restrict pathogen development to a limited area (Yogendra et al. 2015). These include activation of signaling molecules, transcriptional reprogramming, and biosynthesis of defense metabolites that limit pathogen infection. Most often a high concentration of secondary metabolites belonging to phenylpropanoid pathway largely contributes to the enhanced resistance in resistant genotypes (Li et al. 2017a; Novo et al. 2017; Wei et al. 2017; Yogendra et al. 2015). In the current study, inoculation with C. gloeosporioides caused necrotic lesions in tea leaves; however, exogenous EBR reduced the lesion expansion by limiting the pathogen spread beyond the site of infection (Fig. 1). Some evidence suggests that BRs alter the efficiency of innate immunity responses to microbe-associated molecular patterns (MAMPs) which are often perceived in association with the co-receptor, Brassinosteroid Insensitive1-Associated Kinase 1 (BAK1), leading to a rapid and transient production of apoplastic ROS as well as activation of mitogen-activated protein kinase (MAPK) cascades (Bjornson et al. 2016). The MAPK signaling pathway plays a critical role in defense against biotic stressors (Song et al. 2018; Yogendra et al. 2015). In potato, MAPKs stimulate the WRKY1 transcription factor which directly triggers 4-coumarate:CoA ligase (4CL) synthesis to enhance defense against Phytophthora infestans by secondary cell wall thickening (Yogendra et al. 2015). On the other hand, in tomato, suppression of MAPK1, MPAK2, and MAPK3 compromises BR-induced resistance to root-knot nematode (Song et al. 2018), suggesting that BR might activate MAPK and thus WRKY to stimulate the phenylpropanoid pathway to enhance defense against C. gloeosporioides in tea plants. However, at this point, such an assumption is just speculation and thus requires further molecular intervention to dissect the in-depth mechanism of BR-induced phenylpropanoid biosynthesis and subsequent disease resistance.
ROS play a dual role in plant stress responses (Xia et al. 2009). ROS overload causes oxidative burst and damage to proteins, lipids, and nucleic acids. On the other hand, a rapid and transient ROS accumulation in the apoplast mediates BR-induced disease resistance (Song et al. 2018; Xia et al. 2011). In the current study, C. gloeosporioides-caused necrotic lesions were associated with in situ accumulation of H2O2 (Fig. 2). However, exogenous EBR minimized H2O2 accumulation, possibly by activating the phenylpropanoid pathway that produces compounds with high antioxidative property. In cucumber, exogenous EBR enhances PAL activity and flavonoid content, leading to the attenuation of organic pollutant-induced ROS accumulation and oxidative stress (Ahammed et al. 2017). As the key enzymes of phenylpropanoid metabolism pathway, enhanced activity of PAL, C4H, and 4CL potentially increases the biosynthesis of polyphenols including flavonoids, in plants (Wei et al. 2017). Furthermore, nitric oxide plays a critical role in BR-induced flavonoid biosynthesis in tea plants (Li et al. 2017b). While exogenous NO enhances defense against fungal pathogen Monilinia fructicola by activating the phenylpropanoid pathway in peach fruits (Li et al. 2017a), scavenging of NO in the upper untreated leaves blocks BR-induced systemic virus resistance in tobacco (Deng et al. 2016). Therefore, it is plausible that BR-induced NO production and subsequent secondary metabolite synthesis might largely contribute to the BR-enhanced resistance to C. gloeosporioides in tea plants.
Plant hormones play a vital role in plant immune responses (Yang et al. 2014). These defense responses are not activated by a single hormone, rather through a complex intertwined network of pathways involving multiple hormones and signaling intermediates. The SA, JA, and ET pathways are primary mediators in the defense response, which function synergistically or antagonistically to fine-tune resistance of a plant species to a particular pathogen (Yang et al. 2014). Previous studies have revealed that the BR-induced systemic defense response is independent of SA, JA, and ET accumulation (Hideo et al. 2003; Xia et al. 2009, 2011), but possess some characteristics that are similar to SA-dependent systemic acquired resistance (SAR). For instance, exogenous EBR augments expression of stress- or defense-related genes such as PATHOGENESIS-RELATED 1 (PR1), leading to enhanced tolerance to wilt disease caused by Fusarium oxysporum in cucumber (Xia et al. 2011). EBR treatment results in a rapid and transient upregulation of PR-1 and PAL transcripts as early as at 3 h, which reaches the maximum level at 12 h without any significant effect on SA accumulation in cucumber (Xia et al. 2009). Likewise, we found that EBR treatment induced an increase in CsPR1 and CsPAL mRNA levels in the absence of C. gloeosporioides inoculation (Fig. 5a). However, combined treatment of EBR and C. gloeosporioides caused the maximum upregulation in CsPR1 expression, which was significantly higher than that of only EBR or C. gloeosporioides inoculation. In contrast to CsPR1, CsLOX1 expression was suppressed by exogenous EBR (Fig. 5b). Many oxylipins are derived through the 9-lipoxygenase (9-LOX) oxylipin pathway, which play important roles in plant defense. Previous studies have revealed close interactions between the 9-LOX and BR pathways in modulating plant defense in Arabidopsis (Marcos et al. 2015). Furthermore, a specific branch of the LOX pathway, the 13-LOX pathway, synthesizes JA in plants. Therefore, it cannot be excluded that exogenous EBR-induced suppressed LOX1 expression might result in decreased JA biosynthesis. Notably, BR suppresses JA biosynthesis in rice and thus attenuates plant defense against root-knot nematodes (Nahar et al. 2013). This observation contrasts with the report of Song et al. (2018), who demonstrated a positive role of BR in enhancing resistance to root-knot nematode in tomato. Therefore, BR-induced enhanced defense against C. gloeosporioides might be mediated by a complex interconnecting signal transduction pathway that demands further elucidation in tea plants.
In conclusion, we clarified a positive role of BR in strengthening plant resistance to C. gloeosporioides in tea plants. Exogenous EBR pretreatment substantially reduced C. gloeosporioides-induced lesion expansion in inoculated tea leaves and was accompanied by decreased transcript levels of C. gloeosporioides actin and a reduced accumulation of H2O2. Exogenous EBR activated the phenylpropanoid pathway as reflected by increased PAL activity and transcript levels of CsPAL, CsC4H, and Cs4CL. Moreover, expression of CsPR1 and CsLOX1 was upregulated and downregulated, respectively, by exogenous EBR in fungi-inoculated leaves, indicating that BR potentially activates the systemic defense response to enhance tea plant resistance to C. gloeosporioides. Taken together, our results suggest a positive role of BR in the immune response of tea plants which can be implicated in C. gloeosporioides-caused disease control in an eco-friendly manner.
References
Ahammed GJ, Xia X, Li X, Shi K, Yu J, Zhou Y (2014) Role of brassinosteroid in plant adaptation to abiotic stresses and its interplay with other hormones. Curr Protein Pept Sci 16:462–473
Ahammed GJ et al (2017) 24-Epibrassinolide alleviates organic pollutants-retarded root elongation by promoting redox homeostasis and secondary metabolism in Cucumis sativus L. Environ Pollut 229:922–931. https://doi.org/10.1016/j.envpol.2017.07.076
Ahammed GJ et al (2018) Tomato photorespiratory glycolate-oxidase-derived H2O2 production contributes to basal defence against Pseudomonas syringae. Plant Cell Environ 41:1126–1138. https://doi.org/10.1111/pce.12932
Ali SS, Gunupuru LR, Kumar GBS, Khan M, Scofield S, Nicholson P, Doohan FM (2014) Plant disease resistance is augmented in uzu barley lines modified in the brassinosteroid receptor BRI1. BMC Plant Biol 14:227. https://doi.org/10.1186/s12870-014-0227-1
Bjornson M, Dandekar AM, Chory J, Dehesh K (2016) Brassinosteroid’s multi-modular interaction with the general stress network customizes stimulus-specific responses in Arabidopsis. Plant Sci 250:165–177. https://doi.org/10.1016/j.plantsci.2016.06.007
Deng XG et al (2016) Orchestration of hydrogen peroxide and nitric oxide in brassinosteroid-mediated systemic virus resistance in Nicotiana benthamiana. Plant J 85:478–493. https://doi.org/10.1111/tpj.13120
Dixon RA, Lahoucine A, Parvathi K, Chang-Jun L, Srinivasa RMS, Liangjiang W (2002) The phenylpropanoid pathway and plant defence—a genomics perspective. Mol Plant Pathol 3:371–390. https://doi.org/10.1046/j.1364-3703.2002.00131.x
Franke RB, Dombrink I, Schreiber L (2012) Suberin goes genomics: use of a short living plant to investigate a long lasting polymer. Front Plant Sci 3:4. https://doi.org/10.3389/fpls.2012.00004
Guo M, Pan YM, Dai YL, Gao ZM (2014) First report of brown blight disease caused by Colletotrichum gloeosporioides on Camellia sinensis in Anhui Province. China Plant Dis 98:284–284. https://doi.org/10.1094/pdis-08-13-0896-pdn
Han W, Li X, Yan P, Zhang L, Ahammed GJ (2018) Tea cultivation under changing climatic conditions. In: Global tea science current status and future needs. Burleigh Dodds Science Publishing, Cambridge, pp 455–472 https://doi.org/10.19103/as.2017.0036.19
Hideo N et al (2003) Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J 33:887–898. https://doi.org/10.1046/j.1365-313X.2003.01675.x
Huang S, Nie S, Wang S, Liu J, Zhang Y, Wang X (2017) SlBIR3 negatively regulates PAMP responses and cell death in tomato. Int J Mol Sci. https://doi.org/10.3390/ijms18091966
Kim TI et al (2009) l-Theanine, an amino acid in green tea, attenuates beta-amyloid-induced cognitive dysfunction and neurotoxicity: reduction in oxidative damage and inactivation of ERK/p38 kinase and NF-kappaB pathways. Free Radical Biol Med 47:1601–1610. https://doi.org/10.1016/j.freeradbiomed.2009.09.008
Li X et al (2016a) Brassinosteroids improve quality of summer tea (Camellia sinensis L.) by balancing biosynthesis of polyphenols and amino acids. Front Plant Sci. https://doi.org/10.3389/fpls.2016.01304
Li X, Ahammed GJ, Li Z, Tang M, Yan P, Han W (2016b) Decreased biosynthesis of jasmonic acid via lipoxygenase pathway compromised caffeine-induced resistance to Colletotrichum gloeosporioides under elevated CO2 in tea seedlings. Phytopathology 106:1270–1277. https://doi.org/10.1094/PHYTO-12-15-0336-R
Li G, Zhu S, Wu W, Zhang C, Peng Y, Wang Q, Shi J (2017a) Exogenous nitric oxide induces disease resistance against Monilinia fructicola through activating the phenylpropanoid pathway in peach fruit. J Sci Food Agric 97:3030–3038. https://doi.org/10.1002/jsfa.8146
Li X et al (2017b) Nitric oxide mediates brassinosteroid-induced flavonoid biosynthesis in Camellia sinensis L. J Plant Physiol 214:145–151. https://doi.org/10.1016/j.jplph.2017.04.005
Li X et al (2018a) Freezing stress deteriorates tea quality of new flush by inducing photosynthetic inhibition and oxidative stress in mature leaves. Sci Hortic 230:155–160. https://doi.org/10.1016/j.scienta.2017.12.001
Li X et al (2018b) Brassinosteroids attenuate moderate high temperature-caused decline in tea quality by enhancing theanine biosynthesis in Camellia sinensis L. Front Plant Sci. https://doi.org/10.3389/fpls.2018.01016
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 25:402–408
Marcos R, Izquierdo Y, Vellosillo T, Kulasekaran S, Cascon T, Hamberg M, Castresana C (2015) 9-Lipoxygenase-derived oxylipins activate brassinosteroid signaling to promote cell wall-based defense and limit pathogen. Infect Plant Physiol 169:2324–2334. https://doi.org/10.1104/pp.15.00992
Nahar K, Kyndt T, Hause B, Hofte M, Gheysen G (2013) Brassinosteroids suppress rice defense against root-knot nematodes through antagonism with the jasmonate pathway. Mol Plant-Microbe Interact 26:106–115. https://doi.org/10.1094/mpmi-05-12-0108-fi
Novo M, Silvar C, Merino F, Martinez-Cortes T, Lu F, Ralph J, Pomar F (2017) Deciphering the role of the phenylpropanoid metabolism in the tolerance of Capsicum annuum L. to Verticillium dahliae Kleb. Plant Sci 258:12–20. https://doi.org/10.1016/j.plantsci.2017.01.014
Saha D, Kumar R, Ghosh S, Kumari M, Saha A (2012) Control of foliar diseases of tea with Xanthium strumarium leaf extract. Ind Crop Prod 37:376–382. https://doi.org/10.1016/j.indcrop.2011.12.030
Song LX et al (2018) Brassinosteroids act as a positive regulator for resistance against root-knot nematode involving RESPIRATORY BURST OXIDASE HOMOLOG-dependent activation of MAPKs in tomato. Plant Cell Environ 41:1113–1125. https://doi.org/10.1111/pce.12952
Wei Y, Zhou D, Peng J, Pan L, Tu K (2017) Hot air treatment induces disease resistance through activating the phenylpropanoid metabolism in cherry tomato fruit. J Agric Food Chem 65:8003–8010. https://doi.org/10.1021/acs.jafc.7b02599
Willekens H et al (1997) Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J 16:4806–4816. https://doi.org/10.1093/emboj/16.16.4806
Xia XJ et al (2009) Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol 150:801–814. https://doi.org/10.1104/pp.109.138230
Xia XJ, Zhou YH, Ding J, Shi K, Asami T, Chen Z, Yu JQ (2011) Induction of systemic stress tolerance by brassinosteroid in Cucumis sativus. New Phytol 191:706–720. https://doi.org/10.1111/j.1469-8137.2011.03745.x
Xinzhang (2012) Physiological mechanism of resistance to anthracnose of different Camellia varieties. Afr J Biotechnol. https://doi.org/10.5897/ajb11.1099
Yang Y, Ahammed GJ, Wu C, Fan S, Zhou Y (2014) Crosstalk among jasmonate, salicylate and ethylene signaling pathways in plant disease and immune responses. Curr Protein Pept Sci 16:450–461
Yogendra KN et al (2015) Transcription factor StWRKY1 regulates phenylpropanoid metabolites conferring late blight resistance in potato. J Exp Bot 66:7377–7389. https://doi.org/10.1093/jxb/erv434
Zhang H, Tsao R (2016) Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci 8:33–42. https://doi.org/10.1016/j.cofs.2016.02.002
Zheng HZ, Cui CL, Zhang YT, Wang D, Jing Y, Kim KY (2005) Active changes of lignification-related enzymes in pepper response to Glomus intraradices and/or Phytophthora capsici. J Zhejiang Univ Sci B 6:778–786. https://doi.org/10.1631/jzus.2005.B0778
Acknowledgements
This work was supported by the National Key R&D Program of China (2017YFE0107500), the Zhejiang Provincial Natural Science Foundation of China (Y19C160031), the Open Fund of State Key Laboratory of Tea Plant Biology and Utilization (SKLTOF20170106), the Science and Technology Innovation Project of the Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2014-TRICAAS), the Henan University of Science and Technology Research Start-up Fund for New Faculty (13480058), the Henan Natural Science Foundation (182300410046), the Science and Technology Innovation Talents Support Plan of Henan Province (19HASTIT009), the Programs for Science and Technology Development of Henan province (172102410050), and the Key Laboratory of Horticultural Crop Growth and Quality Control in Protected Environment of Luoyang City.
Author information
Authors and Affiliations
Contributions
XL and WYH conceived and designed the research; LZ, GJA, JPW, YL, PY, LPZ, and XL performed the experiments and analyzed the data; XL and WYH discussed the data; GJA and XL wrote the manuscript with the contributions from the other authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, L., Ahammed, G.J., Li, X. et al. Exogenous Brassinosteroid Enhances Plant Defense Against Colletotrichum gloeosporioides by Activating Phenylpropanoid Pathway in Camellia sinensis L.. J Plant Growth Regul 37, 1235–1243 (2018). https://doi.org/10.1007/s00344-018-9857-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-018-9857-0