Abstract
Senescence is a vital aspect of fruit life cycles, and directly affects fruit quality and resistance to pathogens. Reactive oxygen species (ROS), as the primary mediators of oxidative damage in plants, are involved in senescence. Mitochondria are the main ROS and free radical source. Oxidative damage to mitochondrial proteins caused by ROS is implicated in the process of senescence, and a number of senescence-related disorders in a variety of organisms. However, the specific sites of ROS generation in mitochondria remain largely unknown. Recent discoveries have ascertained that fruit senescence is greatly related to ROS and incidental oxidative damage of mitochondrial protein. Special mitochondrial proteins involved in fruit senescence have been identified as the targets of ROS. We focus in discussion on our recent advances in exploring the mechanisms of how ROS regulate fruit senescence and fungal pathogenicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fruit senescence is a developmentally programmed degeneration process and regulated by the various internal and external factors such as genetic factors, developmental signals, hormones, light and temperature (Adams-Phillips et al. 2004; Giovannoni 2004; Vrebalov et al. 2009; Karlova et al. 2011; Klee and Giovannoni 2011; Qin et al. 2012). Senescence greatly impacts fruit postharvest quality and resistance to pathogen attack and environmental stress (Tian et al. 2004). In general, senescent fruit are easily attacked by fungal pathogens and the diseases caused by fungal pathogens can greatly accelerate fruit senescence after harvest (Tian et al. 2007). It has been well known that ethylene plays a crucial role in regulating climacteric fruit ripening and senescence (Picton et al. 1993; Alba et al. 2005; Lee et al. 2012), whereas non-climacteric fruits do not require increased ethylene biosynthesis in the ripening and senescence process (Alexander and Grierson 2002; Causier et al. 2002). Certain common mechanism shared by all types of fruit may exist as the regulator of fruit senescence.
ROS contribute to aging and diseases causing lipid oxidation, protein oxidation, DNA strand break and base modification, and modulation of gene expression (Ames et al. 1993; Simon et al. 2000; Stadtman 2000; Spiteller 2001). Since mitochondria are the primary site of generation of ROS such as superoxide anion (O2 −), hydrogen peroxide (H2O2), hydroxyl radicals, and singlet oxygen (Turrens 2003), ROS accumulation can cause oxidative damage to mitochondrial proteins, resulting in dysfunction of various mitochondrial components and finally accelerating aging (Chan 2006). The important role of ROS in fruit senescence and fungal pathogenic ability has been recognized (Brennan and Frenkel 1977; Lacan and Baccou 1998; Rogiers et al. 1998; Jimenez et al. 2002; Tian et al. 2006; Chan et al. 2007; Qin et al. 2009b). Characterization of ROS roles that regulate fruit senescence is also crucial to understand the biology underlying the senescence phenomenon itself and the plant life cycle, and exploring the molecular and biochemical basis of fruit senescence and defense response to pathogen attack.
The role of ROS in regulating fruit senescence
Previous studies indicate that the steady-state amounts of the products of oxygen free radicals that attack macromolecules tend to increase with age, especially in long-lived, post-mitotic cells (Yan and Sohal 1998). Oxidative damage caused by intracellular ROS to biological macromolecules, including proteins, DNA, and lipids, contributes to the irreversible, deleterious changes of biological systems (Genova et al. 2004; Kujoth et al. 2005). The generation of ROS and the degree to which they cause oxidative damage play important roles in the progression of senescence and various senescence-associated disorders (Stadtman 1992). A variety of studies have shown that ROS, as highly reactive molecules, are primarily generated by mitochondria, the complex organelles within the eukaryotic cell that play critical roles in multiple cellular processes, such as adenosine triphosphate (ATP) synthesis, β-oxidation, calcium homeostasis, apoptosis and cell signaling (Brown 1992; Møller 2001; Turrens 2003). Fruit ripening, as an oxidative phenomenon, requires the removal of ROS such as H2O2, and there is a balance between the production of ROS and their removal by antioxidant systems (Jimenez et al. 2002). In general, during ripening or senescence, antioxidant enzymes eliminate O2 − and H2O2 and thus inhibit the rate of postharvest senescence (Lacan and Baccou 1998; Lester 2003).
The accumulation of ROS accelerating fruit senescence
Fruit senescence is an oxidative phenomenon accompanied by a pronounced increase in ROS, particularly H2O2 and O2 − accumulation (Brennan and Frenkel 1977; Frenkel and Eskin 1977; Warm and Laties 1982). Recent results indicate that H2O2 contents are greatly enhanced in senescing peach fruit (Qin et al. 2009b), muskmelon (Lacan and Baccou 1998) and tomato (Jimenez et al. 2002). Since manganese superoxide dismutase (MnSOD) scavenges O2 − produced in the mitochondria, decreasing catalytic activity of MnSOD usually results in the accumulation of O2 −. MnSOD activity in senescing apple fruit was higher than that of young fruit, leading to higher levels of O2 − (Qin et al. 2009a). However, lower ROS content by lowering oxygen concentration (2–5 %) in storage environment can effectively retard fruit senescence, whereas treatment of fruit with H2O2 enhances senescence (Tian et al. 2004; Qin et al. 2007). High oxygen treatment can increase the production of H2O2 in mitochondria (Turrens et al. 1982) and affect the activity of antioxidant enzymes in fruit and accelerate fruit senescence (Wang et al. 2005). These results demonstrate that oxidative stress causes ROS accumulation and is implicated in the process of fruit senescence.
Mitochondrial proteins in response to fruit senescence
Mitochondria are ubiquitous organelles within eukaryotic cells that perform a variety of biochemical functions (Chan 2006). As the center of energy metabolism in cell, almost all oxidative and reductive reactions are carried out in mitochondria (Brown 1992). The primary biochemical functions of mitochondria are the oxidation of organic acids through the tricarboxylic acid (TCA) cycle and the synthesis of ATP to meet cellular energy demands (Heazlewood et al. 2004). Mitochondria consist of an outer membrane, inner membrane and inter-membrane space. The inner mitochondrial membrane is the major intracellular site of generation of ROS (Yan and Sohal 1998). Oxidative modification of mitochondria components have been implicated in various pathophysiological states associated with oxidative stress and senescent changes (Kraytsberg et al. 2006; Toroser et al. 2007).
Proteomics has been a powerful tool for screening the specific proteins that are differentially expressed in response to various stresses in the last few years (Ali and Komatsu 2006; Qin et al. 2007; Aghaei et al. 2008). Changes in proteins in response to fruit ripening and resistance have been identified based on redox proteomics, including antioxidant proteins and pathogenesis related-proteins (Chan et al. 2007), heat shock proteins and dehydrogenases (Chan et al. 2008), as well as 1-aminocyclopropane-1-carboxylic acid synthase, CBS domain-containing protein and alcohol dehydrogenase 1 (Wang et al. 2009). Moreover, some mitochondrial proteins, which are responsible for oxidative stress, are differentially expressed in the process of fruit senescence (Fig. 1a, b). The sites of specific proteins in mitochondria have also been defined, including two porin proteins located in outer membrane, six proteins related to electron transport chain and carbon metabolism in inner membrane, three proteins belong to citric acid cycle, and other four proteins (Mn-SOD, heat shock protein, Beta-cyanoalanine synthase 1 and formate dehydrogenase) in inner space (Fig. 1c). These results provide evidence that ROS may regulate fruit senescence by changing expression profiles of specific mitochondrial proteins and impairing the biological function of these proteins.
Hierarchical clustering analysis of the changes in mitochondrial protein expression between young and senescent fruit (derived from Qin et al. 2009a). a Spots were clustered into two clusters (I and II) according to their percentage of volume using the Pearson clustering algorithm. Each row in the color heat map indicates a single protein, and each column represents proteins from young and senescent fruit. A bright red color indicates a high protein expression value for a specific protein spot, and a bright green color represents a low protein expression value. For each protein, the spot number and the functional annotation are shown. b Functional classification of proteins in each cluster. c Localization of identified proteins in the mitochondria
Oxidative damage resulting in mitochondria dysfunction
Mitochondria, as a primary generator of endogenous ROS, are particularly vulnerable to oxidative damage (Sweetlove et al. 2002). Mitochondrial DNA is highly susceptible to oxidative damage because it is located close to the inner mitochondrial membrane, where the ROS are generated (Kujoth et al. 2005). Accumulating evidence suggests that mitochondrial protein oxidation is directly related to their biochemical characteristics such as enzyme activities, structural functions, and susceptibility to proteolysis (Bulteau et al. 2006). Oxidative damage to mitochondria caused by ROS has been implicated in the process of senescence, as well as a number of senescence-related disorders in a variety of organisms (Balaban et al. 2005; Nyström 2005; Scheckhuber et al. 2007). The impairments of mitochondrial function caused by ROS include oxidative damage of respiratory chain, mtDNA deletion and lipid peroxidation (Yan et al. 1997; Das et al. 2001), which cause redox signaling, mitochondrial dysfunction and apoptosis and result in diseases and aging (Balaban et al. 2005; Kujoth et al. 2005). The oxidative modification of specific mitochondrial proteins was reported to be related to senescence process in plants (Møller and Kristensen 2006).
Carbonylated proteins can be formed by direct oxidation of amino acid side chains or via indirect reactions with lipid peroxidation products (Nyström 2005). The advent of proteomics and mass spectrometry make it possible to identify the specific proteins that are susceptible to oxidative modifications (England et al. 2004). Among a variety of methods for assessing protein oxidative damage, protein carbonylation has been used extensively. Using two-dimensional gel electrophoresis coupled with immunoblotting to determine protein carbonylation (damaged proteins) in mitochondria of peach fruit during the senescence process, 28 proteins that ranged in molecular mass from 30 to 60 kDa and exhibited obvious carbonylation were identified by Qin et al. (2009b). Damaged proteins included outer mitochondrial membrane transporter, TCA cycle enzymes and antioxidant proteins (Fig. 2). Based on the analysis of the mitochondrial permeability transition, Qin et al. (2009a) suggested that oxidative modification is attributed to the alteration of mitochondrial function and permeability transition. Mitochondrial dysfunction is a major cause of senescence in a variety of organisms (Nyström 2005; Toroser et al. 2007). The undesirable accumulation of ROS causes oxidative damage of mitochondrial proteins, resulting in the collapse of mitochondrial membrane potential and cellular dysfunctions or cell death (Genova et al. 2004). Oxidative damage of specific mitochondrial proteins would result in impairment of mitochondrial function, thereby, leading to fruit senescence.
Identification of oxidatively damaged protein from peach mitochondria during fruit senescence (derived from Qin et al. 2009b). a Two-dimensional (2D) immunoblots of carbonylated mitochondrial proteins during fruit senescence. Mitochondrial proteins isolated from young and senescent peach fruit were separated by 2D gel electrophoresis using 13 cm Immobiline Drystrip with a pH 3–10 nonlinear gradient. After electrophoresis, proteins were transferred to PVDF membrane for immunoblot analysis with anti-dinitrophenyl-group antibodies. b Protein classification of identified proteins. c Proteins that have been identified by mass spectrometry
The function of ROS in regulating fungal pathogenicity
Plants have intra- and intercellular signaling mechanisms to generate both local and systemic responses to pathogen infection (Dietrich et al. 1994). Responses to pathogens are triggered by recognition of pathogen-encoded molecules, subsequent signal transduction, and biosynthesis or molecules acting to halt pathogen growth (Dixon and Lamb 1990). When fungal pathogens attack fruits, they often encounter the defense strategies of the host, including the accumulation of barrier-forming substances and the production of antimicrobial compounds that act directly to prevent pathogen invasion (Tian et al. 2006). Cellular environmental factors within the host, such as constitutive and induced toxic molecules, represent a challenge to an invading fungus. An oxidative burst, during which large quantities of ROS are generated by different host enzyme systems of a plant, is one of the earliest host responses after pathogen attack (Mellersh et al. 2002). In general, pathogens can develop several defense responses, including increased activity of antioxidant enzymes and nonenzymatic protective molecules to protect their cells from ROS damage (Moradas-Ferreira et al. 1996).
Antioxidant enzymes related to fruit defense response
Antioxidant enzymes play an important role in oxidative stress resistance of fungal pathogens. Catalase (CAT), the enzyme that catalyzes the degradation of H2O2 into water and oxygen, is considered to be one of the major H2O2 scavenging enzymes in all aerobic organisms (Yang and Poovaiah 2002). The enzyme is present from lower to higher organisms and its activity is associated with lower rates of cancer and diabetes, and slower aging in mammalian systems (Melov et al. 2000; Preston et al. 2001). Similarly, glutathione S-transferase (GST), the enzyme that detoxifies hazardous compounds such as fatty acid peroxides by conjugating glutathione to these toxic compounds, is also important in protecting cells from oxidative stress (Veal et al. 2002). Recent reports indicate that antioxidant proteins (CAT, GST) are up-regulated in peach and sweet cherry fruit treated by salicylic acid (SA), suggesting that the antioxidant enzymes are involved in defense responses of fruit (Chan et al. 2007, 2008).
ROS level affecting fungal pathogenic ability
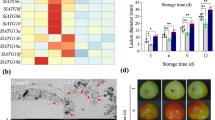
Many exogenous factors that inhibit oxidative enzyme activity result in higher ROS level of fugal pathogens and suppression of fungal growth and pathogenicity (Qin et al. 2007). Borate treatment induced higher ROS levels in cells of Penicillium expansum (Fig. 3b), resulting in stronger inhibitory effects on spore germination of the pathogen in vitro (Fig. 3a) and on its pathogenic ability in the apple fruit (Fig. 3c). CAT and GST exhibited reduced expression levels under the borate stress, suggesting that the two antioxidant enzymes act as ROS scavenger in P. expansum (Qin et al. 2007). Borate also stimulated ROS generation in Colletotrichum gloeosporioides causing anthracnose in mango fruits, and leading to mitochondria degradation of the fungal pathogen (Shi et al. 2012). Borate-treated pathogen showed slower spore germination rate in vitro and lower pathogenic ability in mango fruit (Shi et al. 2012).
Effects of borate on growth, ROS production, and pathogenicity of Penicillium expansum. a Microscopic observation of pathogen growth after 17 h of incubation at 25 °C. s, m, and agt represent spore, mycelium, and abnormal germ tube, respectively (Qin et al. 2007). b Generation of ROS in spores of P. expansum. Spores were cultured at 25 °C in potato dextrose broth medium supplemented with 0 and 0.1 % potassium tetraborate. After incubation for 4 h, spores were stained with the oxidant-sensitive probe DCHF-DA and observed with a fluorescence microscope (Qin et al. 2007). c Effects of borate on decay caused P. expansum in apple fruit
Also nitric oxide (NO) in high concentration has been reported to induce the generation of intracellular ROS of P. expansum, which subsequently causes severe oxidative damage to proteins crucial to the process of spore germination, leads to suppression of spore germinability (Lai et al. 2011). Application of exogenous superoxide dismutase (SOD) and ascorbic acid (Vc) to maintain ROS at basal level can repair cellular damage caused by ROS and reduce the detrimental effects of NO on P. expansum (Lai et al. 2011). Oxidative stress caused by NO may be attributed to: (1) the increase of ROS production and the decrease of ROS detoxifying ability (Mur et al. 2006), because NO has high-affinity binding activity to Cu2+-B center of cytochrome oxidases (complex I II, IV), the final electron acceptor, which limits the flow of electrons from NADH to the ubiquinol pool and markedly increased the O2 − yield (Carreras et al. 2004; Giulivi et al. 2006); and (2) NO can bind to the heme moiety of CAT to form a ferric nitrosyl and Fe–NO adduct, preventing the binding of H2O2 to the metal ion, thus inhibiting CAT activity (Moradas-Ferreira et al. 1996). Therefore, antioxidant defense systems are important to mediate ROS levels. The suppression of expression of antioxidant proteins and genes in fungal spores under the exogenous factor stresses can impair the ability of scavenging ROS, leading to the accumulation of ROS, greater growth and pathogenic ability of fungal pathogens.
Molecular targets for the ROS attack in mitochondria
Plant hosts release high levels of ROS, mostly O2 − and H2O2 upon recognizing a pathogen (Bolwell et al. 1995). This rapid production of ROS, called the oxidative burst, provides an extremely hostile environment for pathogen (Hamann et al. 2008). Mitochondria are important organelles for infection process of fungal pathogen and play a crucial role in the survival of fungal pathogen under oxidative stress of H2O2 (Ingavale et al. 2008). Being adjacent to the site of ROS generation, mitochondrial components such as proteins are particularly vulnerable to oxidative damage (Yan and Sohal 1998). Exogenous H2O2 can cause the accumulation of ROS within the cell of fungal pathogens (Das et al. 2001). H2O2 exposure causes a concentration-dependent loss of cell viability in P. expansum, and cell death of the pathogen is companied by the severe decrease of ΔΨm, a crucial parameter of mitochondrial function (Qin et al. 2011). The results suggest that mitochondria serve as the major intracellular target of exogenous H2O2.
Mitochondrial membrane proteins serving as ROS targets
The integrity of the plasma membrane is related to whether oxygen radicals can lead to rapid disintegration of biological membranes. Damage to plasma membrane can result in loss of osmotic balance and influx of fluids and ions, as well as loss of proteins and ribonucleic acids, eventually leading to the onset of cell death (Qin et al. 2010). Sources of cellular ROS include leakage from the mitochondrial electron transport chain as well as a number of ROS-generating plasma membrane and cytosolic enzymes (Thannickal and Fanburg 2000; Yagoda et al. 2007). Upon exposure to half lethal dose of H2O2, P. expansum showed a limited loss of plasma membrane integrity, indicating that membrane damage was not the main reason for H2O2-induced cell death and that an intracellular target might be involved in this process (Qin et al. 2011). Two-dimensional gel electrophoresis coupled with immunoblotting has revealed that mitochondrial proteins including outer membrane transporters (porin), antioxidant proteins (MnSOD) and TCA cycle enzymes (malate dehydrogenase and aconitase) of peach fruit experienced an oxidative damage during the senescence process (Qin et al. 2009b).
Outer membrane transporters are channel-forming proteins that form the major pathway in mitochondria and control metabolite flux across the membrane (Lemasters and Holmuhamedov 2006). Small metabolites up to 5,000 daltons can be permitted to transported across these membrane-spanning channels in its open configuration (Harris and Thompson 2000). The change in the specific modification state of outer membrane in mitochondria was considered to be involved in mitochondrial dysfunction (Fukada et al. 2004). Outer membrane transporters are linked to the mitochondrial permeability transition pore (PTP), which consists of several proteins, including the adenine nucleotide translocator, the cyclophilin-D, and sites between the mitochondrial inner and outer membranes (Shimizu et al. 2001). Opening of PTP would result in the swelling of the mitochondrial matrix space and rupture of the outer mitochondrial membrane (Madesh and Hajnóczky 2001). ROS regulate fruit senescence via changing expression profiles of specific mitochondrial proteins, particularly outer membrane transporters, and impairing their biological function, which serve as target attacked by ROS in mitochondria.
Mitochondrial complex III is responsible for ROS production
There are subunits in mitochondrial respiratory chain. The mitochondrial complex I has a central role in the regulation of longevity and regulates aging through at least two mechanisms: (1) an ROS-dependent mechanism that leads to mitochondrial DNA damage; and (2) an ROS-independent mechanism through the control of the NAD+ to NADH ratio (Stefanatos and Sanz 2011). The mitochondrial complex II of the electron transport chain contributes to localized mROS that regulates plant stress and defense responses (Gleason et al. 2011). The mitochondrial complex III is thought to contribute to hypoxia-induced ROS production in animals and yeasts (Guzy et al. 2005, 2007).
Mitochondrial complexes I, III and V have been identified in P. expansum by our lab (Qin et al. 2011). We monitored the change in intracellular ROS levels by using DCHF-DA, a cell-permeable ROS indicator that penetrates live cells but does not fluoresce unless oxidized by ROS, and observed that more cells were stained with DCHF-DA under H2O2 stress, implying that more ROS were generated (Fig. 4a). Based on the analysis showing that fluorescent signals of DCHF-DA can be co-localized with those of Mitotracker (a fluorescent dye that stains mitochondria), the mitochondria are proven to be responsible for H2O2-induced ROS production (Fig. 4b). After myxothiazol was applied, the mitochondrial complex III showed a depletion of DCHF-DA signal, which represented the reduction in the production of ROS (Qin et al. 2011). These data suggest that exogenous H2O2 can cause the accumulation of ROS in cell of the fungi by targeting mitochondrial complex III. Also the mitochondrial complex III is the major site for ROS production in P. expansum under oxidative stress of H2O2.
Determination of ROS production and cellular location of ROS formation in Penicillium expansum under oxidative stress (Qin et al. 2011). a Production of ROS in P. expansum after H2O2 treatment assessed by the oxidant-sensitive probe 2′,7′-dichlorodihydrofluorescein diacetate (DCHF-DA). b Co-localization of the Mitotracker orange (a fluorescent dye that stains mitochondria) and DCHF-DA staining inside the germlings. Before fluorescent staining, the fungal spores were cultured in potato dextrose broth medium until germination, and then treated with indicated concentrations of H2O2 for 60 min at 25 °C. Scale bar, 20 μm
Concluding remarks
A model describing the involvement of ROS in regulation of fruit senescence and fungal pathogenicity is presented (Fig. 5). When fruit age, ROS accumulation in cells causes oxidative damage and changes biochemical characteristics of the mitochondrial proteins, including outer membrane transporter, TCA cycle enzymes, and some antioxidant proteins, which serve as the targets attacked by ROS in mitochondria. The oxidative damage of specific mitochondrial proteins caused by ROS is responsible for impairment of protein targets, which in turn facilitates further release of ROS and enhances oxidative damage to mitochondrial protein, eventually leading to the mitochondrial dysfunction and fruit senescence. Moreover, H2O2 stress can induce ROS accumulation in mitochondria of fungal pathogens, resulting in oxidative damage of specific mitochondrial proteins, such as respiratory chain complexes I and III, F1F0 ATP synthase and mitochondrial phosphate carrier protein. Among of them, the mitochondrial complex III is proven to be molecular target attacked by ROS. The oxidative damage of mitochondrial proteins can destroy mitochondrial structure and cause mitochondrial dysfunction, finally leading to reduce fungal pathogenic ability to fruit host. Therefore, exploring the site of ROS generation and their molecular targets is not only beneficial for understanding the mechanisms that ROS regulate fruit senescence and fungal pathogenicity, but also for providing a basis for future development of novel control technologies of harvested fruit quality and antifungal agents.
References
Adams-Phillips L, Barry C, Giovannoni J (2004) Signal transduction systems regulating fruit ripening. Trends Plant Sci 9:331–338
Aghaei K, Ehsanpour AA, Komatsu S (2008) Proteome analysis of potato under salt stress. J Proteome Res 7:4858–4868
Alba R, Payton P, Fei Z et al (2005) Transcriptome and selected metabolite analyses reveal multiple points of ethylene control during tomato fruit development. Plant Cell 17:2954–2965
Alexander L, Grierson D (2002) Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot 53:2039–2055
Ali GM, Komatsu S (2006) Proteomic analysis of rice leaf sheath during drought stress. J Proteome Res 5:396–403
Ames BN, Shigenaga MK, Hagen TM (1993) Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci 90:7915–7922
Balaban RS, Nemoto S, Finkel T (2005) Mitochondria, oxidants, and aging. Cell 120:483–495
Bolwell GP, Butt VS, Davies DR, Zimmerlin A (1995) The origin of the oxidative burst in plants. Free Radic Res 23:517–532
Brennan T, Frenkel C (1977) Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol 59:411–416
Brown GC (1992) Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem J 284:1–13
Bulteau A, Szweda LI, Friguet B (2006) Mitochondrial protein oxidation and degradation in response to oxidative stress and aging. Exp Gerontol 41:653–657
Carreras MC, Franco MC, Peralta JG et al (2004) Nitric oxide, complex I, and the modulation of mitochondrial reactive species in biology and disease. Mol Aspects Med 25:125–139
Causier B, Kieffer M, Davies B (2002) MADS-box genes reach maturity. Science 296:275–276
Chan DC (2006) Mitochondria: dynamic organelles in disease, ageing, and development. Cell 125:1241–1252
Chan ZL, Qin GZ, Xu XB, Li BQ, Tian SP (2007) Proteome approach to characterize proteins induced by antagonist yeast and salicylic acid in peach fruit. J Proteome Res 6(5):1677–1688
Chan ZL, Wang Q, Xu XB et al (2008) Functions of defense-related proteins and dehydrogenases in resistance response induced by salicylic acid in sweet cherry fruit at different maturity stages. Proteomics 8(22):4791–4807
Das N, Levine RL, Orr WC, Sohal RS (2001) Selectivity of protein oxidative damage during aging in Drosophila melanogaster. Biochem J 360:209–216
Dietrich RA, Delaney TP, Uknes SJ et al (1994) Arabidopsis mutants simulating disease resistance response. Cell 77:565–577
Dixon RA, Lamb CJ (1990) Molecular communication in interactions between plants and microbial pathogens. Annu Rev Plant Physiol Plant Mol Biol 47:339–367
England K, O’Driscoll C, Cotter TG (2004) Carbonylation of glycolytic proteins is a key response to drug-induced oxidative stress and apoptosis. Cell Death Differ 11:252–260
Frenkel C, Eskin M (1977) Ethylene evolution as related to changes in hydroperoxides in ripening tomato fruit. HortScience 12:552–553
Fukada K, Zhang F, Vien A, Cashman NR, Zhu H (2004) Mitochondrial proteomic analysis of a cell line model of familial amyotrophic lateral sclerosis. Mol Cell Proteomics 3:1211–1223
Genova ML, Pich MM, Bernacchia A (2004) The mitochondrial production of reactive oxygen species in relation to aging and pathology. Ann NY Acad Sci 4:86–100
Giovannoni JJ (2004) Genetic regulation of fruit development and ripening. Plant Cell 16(Suppl):S170–S180
Giulivi C, Kato K, Cooper CE (2006) Nitric oxice regulation of mitochondrial oxygen consumption I: cellular physiology. Am J Physiol Cell Physiol 291:1225–1231
Gleason C, Huang SB, Thatcher LF, Foley RC, Anderson CR, Carroll AJ, Millar AH, Singh KB (2011) Mitochondrial complex II has a key role in mitochondrial-derived reactive oxygen species influence on plant stress gene regulation and defense. Proc Natl Acad Sci USA 26:10768–10773
Guzy RD, Hoyos B, Robin E, Chen H, Liu L et al (2005) Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 1:401–408
Guzy RD, Mack MM, Schumacker PT (2007) Mitochondrial complex III is required for hypoxia-induced ROS production and gene transcription in yeast. Antioxid Redox Signal 9:1317–1328
Hamann A, Brust D, Osiewacz HD (2008) Apoptosis pathways in fungal growth, development and ageing. Trends Microbiol 16:276–283
Harris MH, Thompson CB (2000) The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ 7:1182–1191
Heazlewood JL, Tonti-Filippini JS, Gout AM et al (2004) Experimental analysis of the Arabidopsis mitochondrial proteome highlights signaling and regulatory components, provides assessment of targeting prediction programs, and indicates plant-specific mitochondrial proteins. Plant Cell 16:241–256
Ingavale SS, Chang YC, Lee H (2008) Importance of mitochondria in survival of Cryptococcus neoformans under low oxygen conditions and tolerance to cobalt chloride. PLoS Pathog 4:e1000155
Jimenez A, Creissen G, Kular B et al (2002) Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta 214:751–758
Karlova R, Rosin FM, Busscher-Lange J et al (2011) Transcriptome and metabolite profiling show that APETALA2a is a major regulator of tomato fruit ripening. Plant Cell 23:923–941
Klee HJ, Giovannoni JJ (2011) Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet 45:41–59
Kraytsberg Y, Kudryavtseva E, McKee AC et al (2006) Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet 38:518–520
Kujoth GC, Hiona A, Pugh TD et al (2005) Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian ageing. Science 309:481–484
Lacan D, Baccou JC (1998) High levels of antioxidant enzymes correlate with delayed senescence in nonnetted muskmelon fruits. Planta 204:377–382
Lai TT, Li BQ, Qin GZ, Tian SP (2011) Oxidative damage involves in the inhibitory effect of nitric oxide on spore germination of Penicillium expansum. Current Microbiol 62:229–234
Lee JM, Joung JG, McQuinn R et al (2012) Combined transcriptome, genetic diversity and metabolite profiling in tomato fruit reveals that the ethylene response factor SlERF6 plays an important role in ripening and carotenoid accumulation. Plant J 70:191–204
Lemasters JJ, Holmuhamedov E (2006) Voltage-dependent anion channel (VDAC) as mitochondrial governator—thinking outside the box. Biochim Biophys Acta 1762:181–190
Lester GE (2003) Oxidative stress affecting fruit senescence. In: Hodges DM (ed) Postharvest oxidative stress in horticultural crops. Food Products Press, New York, pp 113–129
Madesh M, Hajnóczky G (2001) VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J Cell Biol 155:1003–1015
Mellersh DG, Foulds IV, Higgins VJ, Heath MC (2002) H2O2 plays different roles in determining penetration failure in three diverse plant-fungal interactions. Plant J 29:257–268
Melov S, Ravenscroft J, Malik S et al (2000) Extension of life-span with superoxide dismutase/catalase mimetics. Science 289:1567–1569
Møller IM (2001) Plant mitochondria and oxidative stress: electron transport, NADPH turnover and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol 52:561–591
Møller IM, Kristensen BK (2006) Protein oxidation in plant mitochondria detected as oxidized tryptophan. Free Radic Biol Med 40:430–435
Moradas-Ferreira P, Costa V, Piper P, Mager W (1996) The molecular defenses against reactive oxygen species in yeast. Mol Microbiol 19:651–658
Mur LAJ, Carver TLW, Prats E (2006) NO way to live; the various role of nitric oxide in plant-pathogen interactions. J Exp Bot 57:489–502
Nyström T (2005) Role of oxidative carbonylation in protein quality control and senescence. EMBO J 24:1311–1317
Picton S, Barton SL, Bouzayen M, Hamilton AJ, Grierson D (1993) Altered fruit ripening and leaf senescence in tomatoes expressing an antisense ethylene-forming enzyme transgene. Plant J 3:469–481
Preston TJ, Muller WJ, Singh G (2001) Scavenging of extracellular H2O2 by catalase inhibits the proliferation of HER-2/Neu transformed rat-1 fibroblasts through the induction of a stress response. J Biol Chem 276:9558–9564
Qin GZ, Tian SP, Chan ZL, Li BQ (2007) Crucial role of antioxidant proteins and hydrolytic enzymes in pathogenicity of Penicillium expansum: analysis based on proteomic approach. Mole Cell Proteomics 6:425–438
Qin G, Meng X, Wang Q, Tian S (2009a) Oxidative damage of mitochondrial proteins contributes to fruit senescence: a redox proteomics analysis. J Proteome Res 8:2449–2462
Qin GZ, Wang Q, Meng XH et al (2009b) Proteomic analysis of changes in mitochondrial protein expression during fruit senescence. Proteomics 9:4241–4253
Qin GZ, Zong YY, Chen QL, Hua DL, Tian SP (2010) Inhibitory effect of boron against Botrytis cinerea on table grapes and its possible mechanisms of action. Int J Food Microbiol 138:145–150
Qin GZ, Liu J, Li BQ, Cao BH, Tian SP (2011) Hydrogen peroxide acts on specific mitochondrial proteins to induce cell death of fungal pathogen revealed by proteomic analysis. PLoS One 6:e21945
Qin G, Wang Y, Cao B, Wang W, Tian S (2012) Unraveling the regulatory network of the MADS box transcription factor RIN in fruit ripening. Plant J 70:243–255
Rogiers SY, Kumar GNM, Knowles NR (1998) Maturation and ripening of fruit of Amelanchier alnifolia Nutt. are accompanied by increasing oxidative stress. Ann Bot 81:203–211
Scheckhuber CQ, Erjavec N, Tinazli A et al (2007) Reducing mitochondrial fission results in increased life span and fitness of two fungal ageing models. Nat Cell Biol 9:99–105
Shi XQ, Li BQ, Qin GZ, Tian SP (2012) Mechanism of antifungal action of borate against Colletotrichum gloeosporioides related to mitochondrial degradation in spores. Postharvest Biol Technol 67:138–143
Shimizu S, Matsuoka Y, Shinohara Y, Yoneda Y, Tsujimoto Y (2001) Essential role of voltage-dependent anion channel in various forms of apoptosis in mammalian cells. J Cell Biol 152:237–250
Simon HU, Haj-Yehia A, Levi-Schaffer F (2000) Role of reactive oxygen species (ROS) in the apoptosis induction. Apoptosis 5:415–418
Spiteller G (2001) Lipid oxidation in aging and age-dependent disease. Exp Gerontol 36:1425–1457
Stadtman ER (1992) Protein oxidation and aging. Science 257:1220–1224
Stadtman ER (2000) Protein oxidation in aging and age-related diseases. Ann NY Acad Sci 928:22–38
Stefanatos R, Sanz A (2011) Mitochondrial complex I: a central regulator of the aging process. Cell Cycle 10:1528–1532
Sweetlove LJ, Heazlewood JL, Herald V et al (2002) The impact of oxidative stress on Arabidopsis mitochondria. Plant J 32:891–904
Thannickal VJ, Fanburg BL (2000) Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279:1005–1028
Tian SP, Jiang AL, Xu Y, Wang YS (2004) Responses of physiology and quality of sweet cherry fruit to different atmospheres in storage. Food Chem 87:43–49
Tian SP, Wan YK, Qin GZ, Xu Y (2006) Induction of defense responses against Alternaria rot by different elicitors in harvested pear fruit. Appl Microbiol Biotechnol 70:729–734
Tian SP, Qin GZ, Li BQ, Wang Q, Meng XH (2007) Effects of salicylic acid on disease resistance and postharvest decay control of fruit. Stewart Postharvest Rev 6:1–7
Toroser D, Orr WC, Sohal RS (2007) Carbonylation of mitochondrial proteins in Drosophila melanogaster during aging. Biochem Biophys Res Commun 363:418–424
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552:335–344
Turrens JF, Freeman BA, Crapo JD (1982) Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Arch Biochem Biophys 217:411–421
Veal EA, Toone WM, Jones N, Morgan BA (2002) Distinct roles for glutathione S-transferases in the oxidative stress response in Schizosaccharomyces pombe. J Biol Chem 277:35523–35531
Vrebalov J, Pan IL, Arroyo AJ et al (2009) Fleshy fruit expansion and ripening are regulated by the Tomato SHATTERPROOF gene TAGL1. Plant Cell 21:3041–3062
Wang YS, Tian SP, Xu Y (2005) Effects of high oxygen concentration on pro- and anti-oxidant enzymes in peach fruits during postharvest stages. Food Chem 91:99–104
Wang Q, Qin GZ, Lai TF, Tian SP (2009) Response of jujube fruit to exogenous oxalic acid treatment based on proteomic analysis. Plant Cell Physiol 50:230–242
Warm E, Laties GG (1982) Quantification of hydrogen peroxide in plant extracts by the chemiluminescence reaction with luminal. Phytochemistry 21:827–831
Yagoda N, von Rechenberg M, Zaganjor E et al (2007) RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447:864–868
Yan LJ, Sohal RS (1998) Mitochondrial adenine nucleotide translocase is modified oxidatively during aging. Proc Natl Acad Sci USA 95:12896–12901
Yan LJ, Levine RL, Sohal RS (1997) Oxidative damage during aging targets mitochondrial aconitase. Proc Natl Acad Sci USA 94:11168–11172
Yang T, Poovaiah BW (2002) Hydrogen peroxide homeostasis: activation of plant catalase by calcium/calmodulin. Proc Natl Acad Sci USA 99:4097–4102
Acknowledgments
We thank the National Natural Science Foundation of China (grant no. 31030051), the National Basic Research Program of China (973 Program, grant no. 2011CB100604) and the CAS/SAFEA International Partnership Program for Creative Research Teams (grant no. 20090491019) for the support of the research projects related to this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tian, S., Qin, G. & Li, B. Reactive oxygen species involved in regulating fruit senescence and fungal pathogenicity. Plant Mol Biol 82, 593–602 (2013). https://doi.org/10.1007/s11103-013-0035-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-013-0035-2