Abstract
This paper presents a laser-based absorption technique for measuring temperature and CO concentration in high-pressure shock tubes. Two fundamental vibrations of CO (v" = 0, P8, 4.73 µm and v" = 1, R21, 4.56 µm) were selected for high-temperature sensitivity with a reduced influence from pressure broadening compared to previous work. Single-pass absorption (80 mm path length) was measured with two quantum-cascade lasers. The technique was demonstrated by measuring time-resolved temperature for non-reactive mixtures at 1100–1960 K and 1.2–9.7 bar. During partial oxidation of n-heptane, temperature and CO concentrations were measured with 4 µs time resolution at 1360–1670 K and 5.8–8.2 bar. Interference from broadband CO2 absorption was quantified and subtracted. Measured data in the burnout state are in excellent agreement with predictions from kinetics mechanisms (Mehl et al. Proc Combust Inst 33:193, 2011; Zhang et al. Combust Flame 172:116, 2016) over the entire range of operating conditions, which validates the performance of the current laser-absorption technique in reactive-mixture measurements. Additionally, time-resolved temperature and CO-concentration measurements agree well with predictions based on the Mehl et al. mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
High-pressure shock-tube experiments provide valuable information for practical combustion applications such as the determination of ignition delay times for isolated compounds as well as multi-component surrogates and practical fuels [1, 2]. The knowledge of the temperature and species concentration as a function of time is prerequisite to assess individual reaction pathways and investigate chemical kinetics. For a system without heat exchange, ideal shock-wave theory predicts temporally invariant constant and spatially homogeneous temperature for a few milliseconds after the passage of the reflected shock wave. Time-resolved determination of temperature becomes critical when thermochemical [3] or gas-dynamic processes [4] cause temperature and pressure variations during the test time. Under high-pressure conditions, the boundary layer growth caused by gas viscous effects leads to additional pressure (and thus, temperature) disparities between experiments and predictions based on ideal assumptions behind the reflected shock waves. Therefore, in such systems, time-resolved temperature measurements are important for the evaluation of kinetics data.
Laser-based absorption spectroscopy (LAS) has been applied in many laboratories and industrial applications [5] to measure the temperature and concentration of species such as H2O, CO, and CO2 [6,7,8]. CO is the main product during partial oxidation of hydrocarbons or during pyrolysis of oxygenated hydrocarbons and a key intermediate in fuel-lean and stoichiometric combustion. Time-resolved CO-concentration measurements give insight into fuel oxidation to further check the accuracy of kinetics mechanisms and can be also used for process control [9] in industrial applications. Ratio-based thermometry measured on two ro-vibrational lines at fixed wavelengths has become a mature technique in shock tubes at low pressures (typically 1–2 bar) [3, 7, 10], where the molecular absorption lines can be well separated, interference free, and thus enable the selective assessment of the population of specific quantum states of the target species. Also, time-resolved temperature measurements in temperature-variable environments help accurately convert absorbance time profiles into species-concentration time profiles.
At elevated pressures, the spectrally resolved absorption technique becomes more challenging because of line broadening and potential line overlap with interfering species [11,12,13]. For accurate high-temperature measurements, several criteria have to be fulfilled: the absorption features must exhibit sufficient absorption strength and the ratio of the absorption at the selected lines must provide sufficient variation with temperature in the temperature range of interest. For high-pressure measurements, the chosen lines should be ideally free from interference from neighboring lines and absorption from other species in the gas mixture. Alternatively, methods are required to account for these contributions like, for example, in the work of He et al. [14] where the background interference of CO2 in the R-branch of CO near 4.56 µm (R21 line, v" = 1) was quantified. Careful matching of experimental conditions with line selection has enabled some notable successes. For example, Farooq et al. [8] measured the temperature in a shock tube up to 12 bar for non-reactive mixtures at moderate temperatures (up to 1150 K) with CO2 as target species using 2f/1f wavelength modulation (WMS) with combination band transitions near 2.7 μm. The method provided successful measurements of CO2 concentration histories during the combustion of n-heptane [8]. Nasir and Farooq [15] applied a two-line thermometry using two quantum-cascade lasers (QCLs) operated in pulsed mode near 4.55 and 4.89 µm with moderate temperature sensitivity for the CO absorption line pair in a rapid-compression machine at moderate temperatures (745–827 K) and high pressures (9.2–15.3 bar). Unfortunately, the interfering CO2 absorption was not considered. Mulvihill and Petersen [16] showed that under specific conditions, broadband absorption of CO2 near 4.56 µm can be significant and corrections must be applied for quantitative CO measurements at this wavelength. Sun et al. [17] demonstrated the ability of WMS to reject interference from the neighboring lines at high pressures for non-reactive mixture. While the method would also apply for the suppression of CO2 broadband absorption, this approach was not discussed in their work. Recently, Bendana et al. demonstrated two-line CO concentration and temperature measurements in the very fuel-rich harsh environment of liquid-fueled rocket combustion at pressures to 50 bar [18].
In this work, we demonstrate the possibility to use direct absorption spectroscopy in a shock tube at high pressures and high temperatures for time-resolved measurements of temperature and CO concentration in non-reactive mixtures and during partial oxidation of n-heptane. Two-line absorption thermometry was applied with transitions in the fundamental band of CO, P8 (v" = 0) and R21 (v" = 1) at 4.73 and 4.56 µm, respectively (for simplicity, these transitions are referred to as P8 and R21 in the following). Collisional broadening of these lines was measured (P8 in Ar and R21 in He/Ar for T5 between 1000 and 2000 K). Successful CO measurements were extracted when broadband interference absorption from CO2 was accounted. The strategy was then applied to time-dependent measurements of temperature of shock-heated non-reactive CO/Ar mixtures at 1100–1960 K and 1.2–9.7 bar. The effect of temperature variation due to non-ideal gas-dynamic effects was characterized and compared to temperature simulations assuming isentropic compression. The strategy was then applied to the fuel-rich (ϕ = 2.0) n-heptane/O2 reaction system since n-heptane chemistry is well understood. The kinetics mechanism of n-heptane has been developed and updated by many researchers [19,20,21,22,23,24] based on experimental data like ignition delay times and laminar flame speeds over a large range of conditions. Species concentrations (n-heptane, C2H4, OH, CO2 and H2O) were also measured to further constrain different kinetics models. Recently, we investigated the oxidation process of methane in the presence of dimethyl ether or n-heptane as additive using shock tube and flow reactor at high and low temperature, respectively [7]. The aim was to mimic the partial oxidation of methane/additive used for the polygeneration processes at fuel-rich conditions, where mechanistic details are not yet intensively explored [25]. It was found that the addition of DME or n-heptane decreases the inlet temperature and the peak temperature in engines operated in HCCI mode [7, 26]. This work provides additional validation data for the fuel-rich methane/n-heptane oxidation by examining fuel-rich n-heptane oxidation at high pressures with CO absorption and two-line CO thermometry measurements.
To measure the temperature and CO concentrations, the CO2 absorption that interferes with measurements of the CO P8 line was experimentally determined and parameterized and then subtracted with the iterative approach introduced in Ref [14] enabling time-resolved temperature and CO concentration at 1360–1670 K and 5.8–8.2 bar (CO2 background interference centered at the CO R21 line was also subtracted based on the results in Ref [14]). The strategies used for the interference correction are an important part of this work as such interference correction enables the use of a much wider selection of CO transitions (as the fundamental absorption band of CO is strongly overlapped with the fundamental absorption bands of CO2. The results were compared with simulations based on two detailed kinetic mechanisms (Mehl et al. [23] and Zhang et al. [24]).
2 Absorption spectroscopy
2.1 Theory
Although laser absorption is a well-established diagnostics method [5], a few fundamentals are presented here to define terms and establish units. When a narrow-bandwidth laser beam traverses a uniform gas medium, the attenuation of the incident beam intensity I0 follows the Beer–Lambert law according to the equation below:
with the transmitted beam intensity It, the pressure p, the absorption line strength S(T) at a given temperature T, the optical pathlength L, the mole fraction of the target species xi, the Voigt lineshape function φ(\(\nu\)), the absorbance \(\alpha \left(\nu \right)\), the absorption cross-sectional σ(T, p), and the mole concentration ni. The full-width at half maximum (FWHM) of collisional broadening, \(\Delta {\nu }_{c}\), is described as
where \(2{\gamma }_{ji}\) is the collisional broadening coefficient between the collisional partner j and the target species i at a given temperature; xj is the mole fraction of the collision partner j. Note that γ depends on the collision partner. Therefore, it is necessary to determine the correct collisional information for specific collisional partner. The collisional broadening coefficient γ can be described as
where n is the temperature exponent and T0 is a reference temperature, 296 K.
2.2 Line selection for CO two-line thermometry
Two continuous-wavelength, mid-IR quantum-cascade lasers from AdTech Optics and Hamamatsu Photonics were available to access the P-branch near 4.73 μm and R-branch near 4.56 μm of CO. Within the tuning range of these lasers, we chose two transitions in the fundamental band of CO P8 (v" = 0, E"/hc = 138.38 cm–1) and R21 (v" = 1, E"/hc = 3022.09 cm–1) centered at 2111.54 cm–1 and 2191.50 cm–1, respectively, for time-resolved two-line thermometry and CO concentration measurements. In contrast to the previously used P20/R21 line pair [3, 7], P8 line used in this work is free from interference from neighboring transitions improving the high-pressure performance. Figure 1 presents the line strength comparison for the two pairs based on HITEMP parameters [27]. In the 1100–3000 K temperature range, although P20 is stronger than P8, the temperature sensitivity of the ratiometric method, defined as the ratio of the change in line strength ratio of the two lines to the change in temperature, is much higher than unity and higher as for the previous line pair (Fig. 1b). The detection limit of the P8 line at room temperature and atmospheric pressure is ~ 3.5 ppm for 1-m path length in the present work and that of line P20 is ~ 33 ppm.
Figure 2 shows simulated spectra of the two line pairs based on HITEMP [27, 28] along with potentially interfering absorption from H2O and CO2. In the wavenumber range of ± 1.5 cm–1, P20 overlaps with the P14 line centered at 2060.33 cm–1 (v" = 1, E"/hc = 2543.06 cm–1). R21 has some interferences from the adjacent R12 line near 2190.02 cm–1 (v" = 0, E"/hc = 299.77 cm–1) and R13 line at 2193.36 cm–1 (v" = 0, E"/hc = 349.70 cm–1) lines. Thus, correct line information of R12 and R13 (line strength, collisional broadening parameters in Ar or He as bath gas) is necessary to subtract their contribution while measuring temperature and CO concentration using R21. The new line pair used in this work is nearly interference free from H2O absorption but affected by broadband interference from CO2 at 1700 K, in particular for R21 (Fig. 2). In addition, there are no interferences from CH4, C2H2, C2H4 and C2H6 based on simulations from the HITRAN 2016 database [29] for the new line pair. The laser signals did not show any absorption signals by n-heptane at room temperature and at elevated temperatures behind incident and reflected shock waves. The CO2 background centered at the CO P8 line position was measured separately as described in Ref. [14]. The CO2 interference is stronger at higher temperature and the strength of the interference at 1700 K illustrated in Fig. 2 decreases by approximately a factor of 2 when the temperature is reduced to 1200 K. As seen in Fig. 3, for the partial oxidation of n-heptane investigated here, the CO/CO2 concentration ratio is approximately 0.18, when the combustion approaches stoichiometric or fuel-lean conditions, the CO/CO2 ratio decreases and the interference increases. Thus, this absorption strategy is best suited for fuel-rich conditions or pyrolysis (for oxygenated fuels or temperature measurement with CO addition to the initial mixture).
Simulated CO and CO2 concentration during partial oxidation of n-heptane based on the Mehl et al. mechanism [23]. Conditions: T = 1700 K, p = 5 bar, mixture 6
3 Experiment
3.1 Shock tube
Time-resolved temperature and CO concentration measurements were performed in a shock tube with an inner diameter as 80 mm, a driver section of 3.6 m and a driven section of 7.3 m in length. Four pressure transducers in the sidewall were used to measure the incident shock-wave velocity. Temperature (T5) and pressure (p5) behind the reflected shock wave were determined based on 1D shock-wave equations and the initial temperature (T1) and pressure (p1) in the driven section. Typical uncertainties in T5 and p5 are around 1.0% and 1.5%, respectively. One pressure transducer (PCB112A03) was installed 20 mm from the endwall and used for time-resolved measurements of the pressure as input for data processing.
Table 1 shows the initial composition of all gas mixtures and experimental conditions in this work. Mixtures 1 and 2 were used to determine collisional broadening parameters of P8 in Ar and R21 in He/Ar. Here 1.5% CO was prepared in mixture 2 to improve the signal-to-noise ratio in absorbance signals considering the weak line strength of R21 as shown in Fig. 1a. Mixtures 3 and 4 were used for temperature and CO concentration measurements in the non-reactive mixture highly diluted in Ar or He/Ar. Mixture 5 was used to measure the temperature-dependent absorption cross section of CO2 centered at the CO P8 line. Mixture 6 was prepared by double dilution and used for time-resolved temperature and CO concentration measurements during the partial oxidation of n-heptane/O2/Ar (ϕ = 2.0). Here 4.0% H2 or 20.0% He was used to accelerate vibrational relaxation of CO. We found in previous work [7] that helium does not influence the kinetics of CO formation (rising process and final concentration). This is also confirmed in Ref. [30].
3.2 Laser-absorption setup
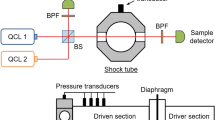
Figure 4 shows the schematics of the laser-absorption experiment. Compared to previous work [7], the beams of the two QCLs were now focused on a single-mode fiber (InF3, Thorlabs) by a 90° off-axis parabolic mirror to ensure probing the same volume in the shock tube. The fiber has a core diameter of 9 ± 0.5 μm and a numerical aperture as 0.25 at 4.5 µm. The coupling efficiency is below 40.0%. Additionally, the current arrangement became more robust because the optical fiber mechanically isolates the laser table from the absorption optics mounted at the shock tube. Two irises were fixed in front of the P8 laser (AdTech Optics) to shield it from back reflection from the off-axis parabolic mirror or from the beam splitter. The R21 laser (Hamamatsu) is coupled into the beam path with a single beam splitter and is less sensitive to retro-reflection. The alignment in the following part becomes more convenient by connecting the fiber with a visible laser.
Before passing through the shock tube, the laser beams were separated into four paths by three beam splitters: one passes through a 25-cm single-pass cell to adjust the absorption peak of P8; the second passes through a 20.25-m white cell to obtain the peak position of R21 despite its weak absorption at room temperature. Both cells were filled with CO highly diluted in argon. The third beam is coupled to a germanium etalon (FSR = 0.016 cm–1) to transform the signals from the time domain to the frequency domain when tuning the laser. The fourth beam was used as reference signal to account for variation in laser intensity. After passing through the shock tube, two irises were fixed to avoid the thermal emission. We checked that there was no significant thermal emission (less than 0.1% of the unattenuated laser signal) by operating the shock tube with both lasers switched off. After traversing the shock tube, the laser beam was separated by a beam splitter and collected by two detectors (Vigo, PVI-3TE-5, bandwidth 20 MHz). Narrow bandpass filters (Spectrogon, NB-4560–135 nm and NB-4720–100 nm) in front of either detector ensured the selective detection of signals from the respective laser only. The sampling rate for all measurements in this work is 5 MHz. During n-heptane partial-oxidation measurements, we did not observe significant attenuation in laser signals ever after several experiments over a wide range of temperatures without window cleaning. Therefore, signal losses due to window fouling are considered negligible.
For measuring collisional broadening coefficients (e.g., P8 in Ar or R21 in He/Ar) or determining temperature-dependent CO2 absorption cross sections, a function generator (HAMEG Instruments) generating a linear current ramp with a frequency of 5.0 kHz was connected to the laser controller (Arroyo Instruments, combined temperature and current elements) for current tuning of the laser wavelength (by ± 0.25 cm–1). The reference measurements in the calibration cells were used to determine the exact wavelength position of the CO P8 line while measuring the CO2 absorption cross section. This procedure is similar to the CO2 absorption cross-sectional measurements at the CO R21 line position in Ref. [14].
4 Data processing
The contribution of broadband CO2 absorption to both CO lines was subtracted during data evaluation. The contribution was calculated from time-dependent temperatures and CO2 concentrations simulated [24], and the parameterized temperature-dependent CO2 absorption cross sections. Temperatures and CO concentration profiles were then calculated in an iterative way as described in Ref. [14]. In this work, Chemical Workbench [31] was used for simulations. Since temperatures and pressures are not constant after ignition, the real state in the test section cannot be described by assuming either constant volume or constant pressure. Thus, the simulations were based on a “predefined pressure profile” reactor model (QP reactor) measured and smoothed pressure traces as input.
This work compared the measured temperature and CO concentration time-history during n-heptane partial oxidation with predictions from two state-of-the-art kinetic mechanisms, Mehl et al. [23] and Zhang et al. [24]. The Mehl et al. mechanism (n-heptane, detailed mechanism, version 3.1, LLNL) contains 654 species and 2827 reactions while the Zhang et al. mechanism contains 1268 species and 5336 reactions.
5 Results and discussion
5.1 Collisional line-broadening coefficients
During data analysis, pressure- and temperature-dependent line-broadening and line strengths must be considered. The latter were taken from HITEMP database [27]. Line-broadening parameters for CO R12 and R13 in Ar were taken from Ren et al. [10]; those of P8, R12, and R13 in He were from Predoi-Cross et al. [32]. Suitable information of line broadening and temperature coefficients for P8 in Ar (mixture 1) and for R21 in He/Ar (mixture 2) did not exist and thus was measured. Figure 5 shows the measured raw data in one shock-tube run when scanning the Adtech laser over the CO line at P8. From the scanned line, the absorbance was determined and fitted to a Voigt profile (Fig. S1(a) in supplemental material S1) considering the individual temperature for each experiment yielding the FWHM of collision broadening for specific temperatures and pressures. These state-specific collisional broadening coefficients are calculated based on Eq. 2 and fitted to an exponential function with temperature (Eq. 3, Fig. 6). The Voigt fitting profiles for both lines are shown in Fig. S1 in supplemental material S1. The results depicted in Fig. 6 are for CO P8 in Ar: 2γ (T0) = 0.095 ± 0.009 cm–1 bar–1, n = 0.665 ± 0.062; for R21 in He/Ar: 2γ (T0) = 0.070 ± 0.005 cm–1 bar–1, n = 0.543 ± 0.046.
5.2 Determination of the absorption cross section of CO2
The CO2 absorption cross section at the wavelength position centered at the CO P8 line was measured at 1250–2470 K and 1.1–5.2 bar. It strongly depends on temperature. Due to the broadband nature of the absorption spectrum, no significant pressure dependence is found in the investigated range (Fig. 7).
The experimental data were fitted with a third-order polynomial:
The maximum deviation between measured data and fitting data is less than 4.0%. For the CO P8 line, there is very weak CO2 interference when the temperature is below 1600 K for realistic CO/CO2 concentration ratios at fuel-rich conditions. Above 1600 K, the absorption cross section of CO2 strongly increases with temperature. The absorbance of CO2 is for instance less than 15% of the total absorbance signal at 1660 K and 8 bar but achieves a value of 21% at 2200 K at the fuel-rich conditions used in this work.
5.3 Temperature and CO-concentration measurements in non-reactive mixtures
5.3.1 Interferences from neighboring lines
High-pressure absorption measurements usually face challenges caused by interference from neighboring lines due to line broadening. For the line pair used in this paper (Fig. 2), the R21 line suffers from interference from the R12 and R13 lines. An evaluation without interference subtraction and the derivation of the resulting errors is provided here, as shown in Fig. 8. At pressures above ~ 5 bar, the neighboring lines (R12 and R13) become influential on R21 and need to be taken into account. Ignoring this interference results in an overestimation of temperature and CO concentration for the measured data by 1.2 and 2.0%, respectively, at T5 = 1215 K, p5 = 5.3 bar. When considering the interference, the temperature derived from the laser absorption fits well with the temperature determined by shock wave equations T5,0 considering a pressure increase with time due to gas-dynamic effects (temperature increase calculated with an adiabatic isentropic compression based on the measured pressure profile). The measured CO concentrations are also in agreement with the known initial concentration. It is predictable that with pressure increase, the deviations will be more evident.
5.3.2 Results in non-reactive mixtures
The pressure and temperature profiles at post-shock conditions were compared and analyzed for a range of pressures. Figure 9 shows the measured temperature and CO concentration profiles at 1100–1960 K and 1.2–9.7 bar. The comparison between measured data (T and xCO) and the known variables (T5 and xCO = 1.0%) shows that the maximum deviation between measured and calculated temperatures and CO concentrations is below 1.0 and 3.0%, respectively.
In the ideal case, the thermodynamic state behind the reflected shock wave is invariant in time and space providing a suitable environment for high-temperature kinetics investigations. The pressure and temperature, however, are not constant in practical cases. For the low-pressure case (Fig. 10a, T5 = 1745 K, p5 = 3.2 bar), the pressure gradually decreases at the beginning and then increases after 0.2 ms. The deviation from the ideal case (pink line) is about 23.1% after 2.8 ms. The temperature measured by laser absorption reflects the same behavior. For this experiment, the temperature first decreased by ~ 50 K at 0.2 ms and then increased by ~ 170 K at 2.8 ms. In contrast, for the high-pressure case (Fig. 10b, T5 = 1738 K, p5 = 9.4 bar), the measured pressure shows a steady increase only. The measured temperature increases by ~ 160 K at 2.8 ms (~ 10% compared to the frozen temperature) with a pressure rise dp/dt of 9.8%/ms.
For the low-pressure case, the slight decrease immediately after the passage of the reflected shock wave is related to gas expansion (relaxation) of the hot core region into the cold boundary layer; this effect is explained in Ref. [33]. With proceeding time, the temperature increase follows the isentropic compression caused by non-uniformities behind reflected shock waves induced by boundary layer growth and incident shock wave attenuation. For the high-pressure case, the non-uniformities are large for the measured test time. The temperature change is above 150 K, which can have strong effects on the kinetics. In addition, the temperature behavior at different set pressures is inconsistent with the admitted description like linear pressure increase dp/dt that impacts kinetics measurements. Therefore, care must be taken when evaluating kinetics parameters at high pressures. Details are shown in Sect. 5.4.2. For the two experiments, through addition of H2 the thermalization via vibrational relaxation is rapidly established after the passage of the reflected shock wave. The temperature change due to 4% H2 in the initial concentration was also considered when using 1D shock-wave equation to calculate T5 and p5 behind reflected shock waves.
For the two experiments shown in Fig. 10, the measured temperature (red line) agrees well with the temperature (black line) calculated based on isentropic assumption from the measured pressure which gives confidence in the use of the isentropic relationship and measured pressure profile to estimate changes in temperature behind reflected shock waves.
5.4 n-Heptane partial oxidation
5.4.1 Measured results
The measurement strategy described above is applied to experiments of partial oxidation of shock-heated fuel-rich (ϕ = 2) n-heptane/O2/Ar mixtures. In the mixtures, 20.0% He was used to accelerate the vibrational relaxation of CO and thus the collisional broadening coefficients of CO by He was taken into account for all lines used in this work.
To check the accuracy of the new approach also in He/Ar as bath gas, experiments were carried out in shock-heated CO diluted in He/Ar (mixture 4) in the 1230–2010 K and 1.8–5.4 bar range. The measured temperature and CO concentration agreed well with the known variables (T5 and xCO = 1.0) (as showing in supplemental material S2).
Figures 11 and 12 present time-resolved temperature and CO-concentration profiles for the 1360–1670 K and 5.8–8.2 bar range combined with the results of numerical simulations based on the detailed reaction mechanisms of Zhang et al. [24] and Mehl et al. [23]. In the initial phase, temperatures cannot be determined due to the poor signal-to-noise ratio before formation of sufficiently high CO concentrations. The measured temperatures quickly increase after the arrival of the reflected shock wave as a result of the heat release during the initial oxidation of n-heptane. A further gradual increase caused by compression induced by the boundary layer growth and incident shock wave attenuation then further increases the temperature in the plateau region. The CO concentrations also show a fast initial increase and then remain nearly constant. The measured temperatures agree well with the simulations from both mechanisms, indicating that using simulated temperatures for the CO2 background correction works reliably. Neglecting the CO2 contribution leads to significant errors (~ 8% in temperature and ~ 15% in CO concentration), as shown in Supplemental Material S3.
The measured CO concentrations are in between the predictions from both mechanisms for most experiments and the measured temperature and CO rise agrees best with the simulations based on the Mehl et al. mechanism. In contrast, the Zhang et al. mechanism predicts too slow increase in temperature and CO concentration, especially in the low-temperature region. The proposed detection strategy demonstrates its reliability to simultaneously determine temperature and CO concentration at high pressure/temperature and thus provide data useful for the validation of chemical reaction mechanisms.
5.4.2 Influence of gas-dynamic effects on the ignition of n-heptane/O2/Ar mixtures
The influence of gas-dynamic effects on the ignition of the n-heptane/O2/Ar mixture under high-pressure conditions is additionally illustrated in Fig. 13. As discussed in Sect.5.3, under high-pressure conditions, there is a gradual increase of pressure behind the reflected shock wave due to gas dynamics. If this is not considered, significant deviations in temperature and CO concentration between experimental data and numerical simulations (constant volume or constant pressure), especially during CO formation are observed. Therefore, it is necessary to use measured pressure profiles in the simulation of CO and temperature profiles (constrained-pressure assumption model).
Measured and simulated temperature and CO concentration. Experimental conditions: T5 = 1364 K, p5 = 8.2 bar, mixture 6. Black solid line: measured data; black dashed line: Numerical simulation based on Constrain P method [14]; red dashed line: numerical simulation based on constant energy and constant volume; green dashed line: numerical simulation based on constant enthalpy and constant pressure
5.5 Uncertainty analysis
This part describes the estimation of uncertainties for the measured quantities in this work. For more details, we additionally refer to Ref. [34]. The uncertainties in collisional line-broadening coefficients (2γ, Sect. 5.1) of both lines are 5.1% considering 1.0% uncertainty from measured pressure and 5.0% uncertainty from Voigt fitting. This work conservatively assumes the maximum value of the residual during the fitting process as uncertainty value, a similar assumption was used in Ref. [34]. The uncertainties in the measured CO2 absorption cross sections (Sect. 5.2) are ~ 4.5% considering 1.6% uncertainty from calculating isentropic temperatures based on the measured pressure profile, 1.0% uncertainty from measured pressure, and 4.0% uncertainty from peak-to-peak noise level in measured absorbance (Eq. (1)). The uncertainties in measured temperature (\(\vartriangle T/T\), Sect. 5.3.2) were estimated based on the following equation:
where R is the measured absorbance ratio of the line pair selected (\(R = {\upalpha }_{P8} /{\upalpha }_{R21}\)). S(T0) is room-temperature line strength. h is the Planck constant. c is the speed of light. kB is the Boltzmann constant. \(E''\) is the lower state energy of the transition. The uncertainties in room-temperature linestrengths of both lines are 2–5% in HITEMP database [29] and the median value, 3.5%, was used here. For the case with T5 = 1738 K and p5 = 9.4 bar, the uncertainty of R, \(\vartriangle R/R\), is estimated to be 8.0% considering 4.0% uncertainty from peak-to-peak noise level in measured absorbances for both lines, and 4.0% uncertainty from potential errors (e.g. collisional narrowing, details are shown in supplemental material S4) induced by improper Voigt fitting for both lines especially in high-pressure case. In addition, we considered 2% uncertainty induced by thermal boundary layer (an example was given by Nasir and Farooq [15] for uncertainty induced by thermal boundary layer in rapid compression machine). The total uncertainties in measured temperature are ~ 3.4% based on Eq. (5) for this case. The uncertainties in CO concentration are estimated based on Eq. (6). The uncertainties in measured absorbance are estimated as 4.0% considering the peak-to-peak noise level. The uncertainties in measured pressure are 1.0%. The uncertainties in high-temperature line strength are ~ 3.7% with 3.5% uncertainty in room-temperature line strength and 1.3% uncertainty induced by temperature. The uncertainties in lineshape function are estimated as 5.0%. The total uncertainties in measured CO concentrations are ~ 7.5% based on the following equation for this case:
For reactive cases (Sect. 5.4.1), the uncertainties are 4.4% for temperature and 7.8% for CO concentrations considering the uncertainties that arise from CO2 background correction.
6 Conclusions
This work reports about the implementation of a new two-line laser-absorption measurement strategy with fixed wavelength spectroscopy for time-resolved temperature and CO-concentration measurements behind reflected shock waves at pressures up to 10 bar. Two fundamental vibrational transitions of CO (v" = 0, P8 and v" = 1, R21) were selected and the collisional broadening parameters (P8 in Ar and R21 in He/Ar) were measured. The strategy was applied for non-reactive mixtures at 1100–1960 K and 1.2–9.7 bar. The measured temperatures show a gradual increase after the arrival of the reflected shock wave as a result of gas-dynamic effects. Deviations of up to 10% compared to the ideal assumption were found. The measured temperature was found to agree well with the temperature from isentropic calculation.
Experiments with partial oxidation of fuel-rich (ϕ = 2) n-heptane/O2/Ar mixtures were performed at 1360–1670 K and 5.8–8.2 bar considering the availability of well-developed n-heptane mechanisms. Under these conditions, subtracting the interference from broadband CO2 absorption is essential. For this purpose, the absorption cross-section of CO2 centered at the P8 line of CO was measured and fitted as a cubic function of temperature. The measured data of temperature and CO concentration were compared with simulations based on the Zhang et al. and Mehl et al. mechanism. The simulation of using the Mehl et al. mechanism was in excellent agreement with the measured data whereas the Zhang et al. mechanism predicts a too fast temperature and CO-concentration rise. In summary, the two-color absorption strategy works well for measurements in high-pressure environment.
References
R.K. Hanson, D.F. Davidson, Prog. Energy Combust. Sci. 44, 103 (2014)
S.M. Sarathy, A. Farooq, G.T. Kalghatgi, Prog. Energy Combust. Sci. 65, 67 (2018)
D. Nativel, B. Shu, J. Herzler, M. Fikri, C. Schulz, Proc. Combust. Inst. 37, 197 (2019)
G.A. Pang, D.F. Davidson, R.K. Hanson, Proc. Combust. Inst. 32, 181 (2009)
C.S. Goldenstein, R.M. Spearrin, J.B. Jeffries, R.K. Hanson, Prog. Energy Combust. Sci. 60, 132 (2017)
Z. Hong, D.F. Davidson, E.A. Barbour, R.K. Hanson, Proc. Combust. Inst. 33, 309 (2011)
F. Sen, B. Shu, T. Kasper, J. Herzler, O. Welz, M. Fikri, B. Atakan, C. Schulz, Combust. Flame 169, 307 (2016)
A. Farooq, J.B. Jeffries, R.K. Hanson, Appl. Opt. 48, 6740 (2009)
K. Banke, R. Hegner, D. Schröder, C. Schulz, B. Atakan, S.A. Kaiser, Fuel 243, 97 (2019)
W. Ren, A. Farooq, D.F. Davidson, R.K. Hanson, Appl. Phys. B 107, 849 (2012)
K. Sun, R. Sur, X. Chao, J.B. Jeffries, R.K. Hanson, R.J. Pummill, K.J. Whitty, Proc. Combust. Inst. 34, 3593 (2013)
R.M. Spearrin, C.S. Goldenstein, J.B. Jeffries, R.K. Hanson, Appl. Opt. 53, 1938 (2014)
W. Wei, W.Y. Peng, Y. Wang, R. Choudhary, S. Wang, J. Shao, R.K. Hanson, Appl. Phys. B 125, 9 (2018)
D. He, L. Shi, D. Nativel, J. Herzler, M. Fikri, C. Schulz, Combust. Flame 216, 194 (2020)
E.F. Nasir, A. Farooq, Proc. Combust. Inst. 36, 4453 (2017)
C.R. Mulvihill, E.L. Petersen, Appl. Phys. B 123, 255 (2017)
K. Sun, X. Chao, R. Sur, J.B. Jeffries, R.K. Hanson, Appl. Phys. B-Lasers O. 110, 497 (2013)
F.A. Bendana, D.D. Lee, S.A. Schumaker, S.A. Danczy, R.M. Spearrin, Appl. Phys. B 125, 204 (2019)
C.K. Westbrook, J. Warnatz, W.J. Pitz: Symposium (International) on Combustion 22, 893 (1989)
E. Ranzi, P. Gaffuri, T. Faravelli, P. Dagaut, Combust. Flame 103, 91 (1995)
H.J. Curran, P. Gaffuri, W.J. Pitz, C.K. Westbrook, Combust. Flame 114, 149 (1998)
E.D. B. Sirjean, D.A. Sheen, X.-Q. You, C. Sung, A.T. Holley, F.N. Egolfopoulos, H. Wang, S.S. Vasu, D.F. Davidson, R.K. Hanson, H. Pitsch, C.T. Bowman, A. Kelley, C.K. Law, W.Tsang, N.P. Cernansky, D.L. Miller, A. Violi, R.P. Lindstedt: JetSurF version 1.0 (Sep 15, 2009) https://web.stanford.edu/group/haiwanglab/JetSurF/JetSurF1.0/index.html
M. Mehl, W.J. Pitz, C.K. Westbrook, H.J. Curran, Proc. Combust. Inst. 33, 193 (2011)
K. Zhang, C. Banyon, J. Bugler, H.J. Curran, A. Rodriguez, O. Herbinet, F. Battin-Leclerc, C. B'Chir, K.A. Heufer, Combust. Flame 172, 116 (2016)
B. Atakan, A.K. Kaiser, J. Herzler, S. Porras, O. Deutschmann, T. Kasper, M. Fikri, R. Schießl, D. Schröder, C. Rudolph, D. Kaczmarek, H. Gossler, S. Drost, V. Bykov, U. Maas, C. Schulz: Renew. Sustain. Energ. Rev., in review (2020)
J. Herzler, Y. Sakai, M. Fikri, C. Schulz, Proc. Combust. Inst. 37, 5705 (2019)
L.S. Rothman, I.E. Gordon, R.J. Barber, H. Dothe, R.R. Gamache, A. Goldman, V.I. Perevalov, S.A. Tashkun, J. Tennyson, J. Quant. Spectrosc. Radiat. Transf. 111, 2139 (2010)
C.S. Goldenstein, V.A. Miller, R. Mitchell Spearrin, C.L. Strand: J. Quant. Spectrosc. Radiat. Transf. 200, 249 (2017)
I.E. Gordon, L.S. Rothman, C. Hill, R.V. Kochanov, Y. Tan, P.F. Bernath, M. Birk, V. Boudon, A. Campargue, K.V. Chance, B.J. Drouin, J.M. Flaud, R.R. Gamache, J.T. Hodges, D. Jacquemart, V.I. Perevalov, A. Perrin, K.P. Shine, M.A.H. Smith, J. Tennyson, G.C. Toon, H. Tran, V.G. Tyuterev, A. Barbe, A.G. Császár, V.M. Devi, T. Furtenbacher, J.J. Harrison, J.M. Hartmann, A. Jolly, T.J. Johnson, T. Karman, I. Kleiner, A.A. Kyuberis, J. Loos, O.M. Lyulin, S.T. Massie, S.N. Mikhailenko, N. Moazzen-Ahmadi, H.S.P. Müller, O.V. Naumenko, A.V. Nikitin, O.L. Polyansky, M. Rey, M. Rotger, S.W. Sharpe, K. Sung, E. Starikova, S.A. Tashkun, J.V. Auwera, G. Wagner, J. Wilzewski, P. Wcisło, S. Yu, E.J. Zak, J. Quant. Spectrosc. Radiat. Transf. 203, 3 (2017)
O. Mathieu, C.R. Mulvihill, E.L. Petersen, Fuel 236, 1164 (2019)
M. Deminsky, V. Chorkov, G. Belov, I. Cheshigin, A. Knizhnik, E. Shulakova, M. Shulakov, I. Iskandarova, V. Alexandrov, A. Petrusev, I. Kirillov, M. Strelkova, S. Umanski, B. Potapkin, Comput. Mater. Sci. 28, 169 (2003)
A. Predoi-Cross, K. Esteki, H. Rozario, H. Naseri, S. Latif, F. Thibault, V. Malathy Devi, M.A.H. Smith, A.W. Mantz,: J. Quant. Spectrosc. Radiat. Transf. 184, 322 (2016)
J.V. Michael, J.W. Sutherland, Int. J. Chem. Kinet. 18, 409 (1986)
D.I. Pineda, F.A. Bendana, K.K. Schwarm, R.M. Spearrin, Combust. Flame 207, 379 (2019)
Acknowledgements
Financial support of this work by the German Research Foundation within the DFG project 279056804 and 229633504. JBJ acknowledges support through the DFG Mercator Fellowship program. DH acknowledges support from Dr. Zhiming Peng, Tsinghua University and the state-sponsored postgraduate program for building high-level universities by China Scholarship Council (No. 201806210220).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, D., Nativel, D., Herzler, J. et al. Laser-based CO concentration and temperature measurements in high-pressure shock-tube studies of n-heptane partial oxidation. Appl. Phys. B 126, 142 (2020). https://doi.org/10.1007/s00340-020-07492-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-020-07492-7