Abstract
A series of the TeO2–GeO2–ZnO glass system was single and double doped with different Tb3+/Eu3+ ratios. Their luminescent and colorimetric properties were analyzed for possible use as phosphor materials in lighting devices. The characterization by X-ray diffraction and Raman spectroscopy verified the glassy nature of the fabricated samples. The luminescent properties of the doped glasses were analyzed by means of steady-state fluorescence and time-resolved spectroscopy. The Tb3+ excitation bands observed in the codoped samples while monitoring the 700 nm emission of Eu3+, as well as the shortening of Tb3+ lifetime in presence of europium indicated a Tb3+ → Eu3+ energy transfer, which main interaction type is electric dipole–dipole, according to Inokuti–Hirayama model. The Eu3+ → Tb3+ energy transfer also occurs in the samples but with lower efficiency. The CIE1931 chromaticity coordinates, upon different excitation wavelengths, show a multicolor tunning from green to orange-red due to the lanthanide concentration ratio and the Tb3+ → Eu3+ energy transfer process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Optoelectronic devices like lasers, fiber amplifiers, displays devices, and light-emitting diodes (LEDs), among others, have been promoting research towards materials with good physical, stability, and chemical properties adequate to host rare-earth ions for the mentioned applications [1,2,3,4,5,6]. In several trivalent lanthanide ions, the absorption bands due to 4f–4f or 5d–4f transitions are located in the range of InGaN, GaN, and AlGaN (350–400 nm) near-ultraviolet LEDs (NUV-LEDs) that are compatible with solid-state lighting devices [7,8,9]. Depending on the target application, a single ion or a combination of two or three rare-earth ions are introduced in oxide glasses to obtain multicolor or white light emission, following the red (R), green (G), and blue (B) system, tailoring the concentration of the lanthanide ions [9, 10]. For example, Sołtys et al. [11] used a combination of Dy3+ (blue, yellow), Tb3+ (green), and Eu3+ (red) ions in triply doped PbO-B2O3-Al2O3-WO3 glasses, varying their concentrations, resulting in warm white light emission upon direct excitation of 6H15/2 → 4K17/2 transition of Dy3+ (390 nm) with the Dy3+ → Tb3+ → Eu3+ energy transfer process. In this respect, Tb3+ ions with a 4f8 configuration and its dominant green emission from 5D4 → 7F5 transition, upon NUV excitation, have been demonstrated to be a suitable donor for acceptor ions like Sm3+ and Eu3+ [6, 12, 13]. Jha et al. [12] work on Tb3+/Sm3+-doped ZnO-P2O5-BaO-TiO2 glasses revealed that the single-doped glasses presented pure green and orange emission. In contrast, the emission of the codoped glasses is possibly tuned from yellowish green to warm white through the energy transfer mechanism from Tb3+ to Sm3+. Similarly, the combination of trivalent terbium and europium ions has also been studied in several host materials due to the capability to tune the emission over a wide range of visible light, because of the energy transfer process that occurs from terbium to europium ions [14,15,16]. Even though the emission tunability is a desirable phosphor property for light-emitting diodes or display applications, in the last decade, few reports evaluated the luminescent and colorimetric properties of Tb3+/Eu3+ double-doped glasses variating europium concentration. Among the glass host materials where the effect of europium content have been studied are the aluminum-zinc-phosphates [14], fluorophosphate [16], borate [15, 17], fluorozirconate [17], germanate [18], multicomponent [19] glasses, and oxide and oxyfluoride glass–ceramics [20, 21]. Furthermore, the energy transfer mechanism has been exploited in these works to tune the global emission tonality. Given the amorphous nature of glasses, presenting short-range order, the spectroscopic characteristics of the trivalent lanthanide ions contained in them are determined by the local structure. According to Bocharova et al. [22], dopant ions in disordered solids can distribute randomly or with local concentration displacement. In this work, the authors found that the dopant distribution and the Tb3+ → Eu3+ energy transfer depend on the dopant concentration, as well as the glass structure and glass composition.
Gopi et al. [15] work on Tb3+/Eu3+-doped borate glasses showed that at small increments of Eu3+ dopant from 0.1 to 1.0 mol%, under a 464 nm excitation, can shift the emission from greenish-yellow to orange-red. Also, Loos et al. [17] reported that Tb3+/Eu3+ pair exhibit a similar chromatic behavior in borate and fluorozirconate glasses, upon excitation of 376 nm and 486 nm, confirming that the energy transfer process is enhanced as europium concentration increases from 0.1 to 0.5 mol%. Meanwhile, Wileńska et al. [19] studied Tb/Eu-doped multicomponent glasses with the composition AlF3-TiO2-40PbO-H3BO3-SiO2 obtaining green, yellow, and cold-white light emission at different xEu-(1 − x)Tb molar ratios while maintaining the total concentration of dopants below 1 mol%. de Queiroz et al. [16] increased the molar ratio of Tb3+/Eu3+ up to 1.5 mol% in fluorophosphate glasses and studied their luminescent and chromatic properties varying the excitation wavelength between 355 and 360 nm. They evidenced that the emission tonality of the glasses lies in a broad region of the visible light, in the red, green, and blue regions. Raju et al. [23] only studied the photoluminescent properties of Tb3+ and Eu3+ single-doped TeO2–B2O3–CdO–Li2O–AlF3 glasses up to concentrations of 1.25 mol%. It is worth mentioning that codoping tellurite glasses with Tb3+/Eu3+ has not been studied in-depth for high europium molar concentration, performing time-resolved photoluminescence spectroscopy analysis. Regarding oxide glasses, those based on tellurium oxide (TeO2) and germanium oxide (GeO2) are amorphous materials with high thermal and chemical stability, mechanical resistance, low melting temperature, mid-IR transparency, and low phonon energies of 700 and 880 cm−1, respectively, that are appropriate as hosts for photoluminescent trivalent rare-earth ions [1, 24,25,26]. GeO2 is a natural glass former, while TeO2 is a conditional glass former, which requires to be combined with other compounds like alkaline oxides, sulfides, fluorides or chlorides to form a stable glass [26,27,28]. GeO2 improves the mechanical resistance of the matrix, as well as their thermal and chemical stability because of the higher bond strength of Ge–O bonds than Te–O bonds [28, 29]. Lesniak et al. [30] reported that for TeO2–GeO2:Eu3+ glasses, GeO2 concentration influences the fluorescence intensity ratio of Eu3+ ions, the refractive index and phonon energy of the glasses. Zinc oxide is often used in combination with TeO2 because it contributes to stability, mechanical resistance, and high concentration acceptance (up to 40 mol% of ZnO) of the glass matrix. The addition of ZnO causes a disruption of the Te–O–Te bonds forming Te–O− terminal bonds, with non-bridging oxygens, depolymerizing the TeO2 network [31, 32]. The non-bridging oxygens, together with tellurium lone electron pairs, have been related to the capability of TeO2 glass to accept and accommodate modifier cations and their optical properties [33]. Thus, we report a comprehensive analysis of the luminescent, photometric, and colorimetric characteristics of TeO2–GeO2–ZnO (TGZ) glasses impurified with trivalent terbium, europium, and both lanthanides, with emphasis on the high molar concentration of europium, while evaluating the effect of the Tb3+ → Eu3+ energy transfer on the color tuning of the global emission of the double-doped TGZ glasses, covering a wide range of europium concentrations from 0.5 to 2 mol%.
2 Experimental details
TGZ glasses with a composition of (80-x–y)TeO2–10GeO2–10ZnO:xTb3+, yEu3+ (mol%) were fabricated according to the previously reported methodology [32], using reagent grade oxides powders, Eu(NO3)3·5H2O and Tb(CO3)3·xH2O that were acquired from Sigma-Aldrich. Table 1 presents mol% concentrations of the precursors and dopants used to synthesize the glasses. The obtained samples were cut and polished into 0.6 × 0.6 × 0.2 cm blocks. The vibrational features of the glasses were studied through Raman spectroscopy using a Horiba LabRAM HR spectrometer equipped with a He–Ne laser (λ = 632.8 nm). Photoluminescence spectra were registered by a Fluorolog fluorometer using a 450 W ozone-free Xe lamp. The fluorescence decay times of the emission originated from Tb3+ and Eu3+ were obtained through pulsed excitation using an Opolette HE 355 LD + UVDM (~ 10 ns, pulse energy = 1.0 mJ), and the resulting fluorescence signal was analyzed with a Jobin–Yvon Triax 550 monochromator coupled to a Horiba Jobin–Yvon i-Spectrum Two ICCD.
3 Results and discussion
3.1 X-ray diffraction and Raman spectroscopy
Figure 1a presents a representative diffractogram of the TGZ glasses. Three broad bands located around 2θ = 22, 27, and 50 degrees can be distinguished, which are related to short-range order. The absence of well-defined diffraction peaks, within the diffractometer resolution, verifies the glassy structure of the sample.
Raman spectra of the fabricated TGZ glasses are displayed in Fig. 1b, showing the characteristic features of TeO2-based glasses, which consist of two dominant bands. Band A extends from 300 to 600 cm−1, while band B covers the 600–1000 cm−1 range. Band C around 1550 cm−1, can be observed in the glasses containing europium ions, and its origin is related to the presence of interstitial O2 molecules due to the decomposition of the europium nitrate hydrate [26]. The band at short wavenumbers is composed of the following vibrational features: Zn–O–Zn and Te–O–Zn bonds (380 cm−1), O–Te–O in TeO4 units (440 cm−1), and Te–O–Te bridges (490 cm−1) associated with network connectivity [32, 34,35,36]. Also, around ~ 450 cm−1, a symmetric stretching vibration of Ge–O–Ge bonds in GeO4 4-membered rings can be found overlapped with the previously mentioned Te–O and Zn–O bond vibrations [37]. As for band B, three main vibrational features are present, which are the stretching modes of O–Te–O in TeO4 (660 cm−1), TeO3+1 and [TeO3]2− (750 cm−1), and short Te–O− terminal bonds (820 cm−1) with non-bonding oxygens (NBOs) [38]. Regarding germanium oxide, the anti-symmetric stretching vibrations of Ge–O–Ge bonds appear around 790 and 870 cm−1, corresponding to the transverse and longitudinal optical split characteristic of GeO2 [37]. It is worth mentioning that the TeO2 vibrational modes dominate the Raman spectra, as it was previously reported for the TeO2–GeO2 binary glasses with contents below 20%mol [28]. In addition, there is still no conclusive evidence of the formation of a Te–O–Ge bond that could demonstrate the formation of mixed polyhedral units [28,29,30, 36]. However, Kalampounias et al. [28] reported that GeO2 could act as a modifier, increasing the formation of TeO3 units. A more insightful analysis of the glass structure through Raman spectra deconvolution analysis of europium-doped TGZ glasses and the TGZ matrix structural details were previously reported [38]. However, it was not possible to deconvolute the Raman spectra of TGZ glasses to evidence the bands related to germanium oxide polyhedra because they are completely overlapped with those of TeO2.
3.2 Photoluminescence
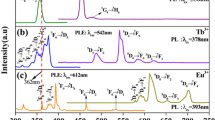
The excitation spectrum of europium single-doped glass is presented in Fig. 2, it was obtained by monitoring the emission at 612 nm, related to 5D0 → 7F2 transition. It consists of several bands associated to parity-forbidden intra-4f transitions of Eu3+: 7F0 → 5D4 (365 nm), 7F0 → 5L10 (367 nm), 7F0 → 5G2,3,4,5 (377 nm), 7F0 → 5L8 (383 nm), 7F0 → 5L7 (393 nm), 7F1 → 5L7 (400 nm), 7F0 → 5D3,5L6 (415 nm), 7F3 → 5D3 (449 nm) 7F0 → 5D2 (465 nm), 7F2 → 5D2 (487 nm), 7F0 → 5D1 (525 nm), 7F1 → 5D1 (532 nm), 7F2 → 5D1 (553 nm), 7F0 → 5D0 (578 nm), 7F1 → 5D0 (588 nm) [39,40,41].
The photoluminescence spectrum of TGZE glass, obtained upon excitation at 393 nm (Fig. 3), consists of multiple bands assigned to intraconfigurational 4f6 → 4f6 transitions of europium ions from 5D3, 5D2, 5D1, and 5D0 levels to the 7FJ manifold. The origin of these bands can be explained as follows: during the excitation, electrons are promoted from 7F0 to 5L6 level, from where they decay non-radiatively to the 5D3 emitting level, and subsequently, to the lower 5DJ = 2, 1, 0 emitting levels, followed by a radiative decay towards the 7FJ levels. The same figure presents the emission spectra of TGZTE0.5, TGZTE1, and TGZTE1.5 and TGZTE2 glasses upon the same excitation wavelength.
In all samples, the most intense emission bands are the typical orange-red bands associated to the hypersensitive electric dipole 5D0 → 7F2 transition, and the magnetic dipole 5D0 → 7F1 transition, which is insensitive to the surrounding environment [35, 42, 43]. It is noticeable that the intensity of the blue-green bands decreases as the content of europium ions increases, while the intensity of the red bands is enhanced [34, 42, 43]. This phenomenon is due to an Eu → Eu energy transfer, through cross-relaxation processes, given that the energy difference associated to 5D3 → 5D2 and 5D2 → 5D1 is resonant with 7F0 → 7F4 and 7F1 → 7F4 transitions, according to the following possible mechanisms:
Due to the energy difference between 5D1 and 5D0 levels is only 1747 cm−1, a multiphonon relaxation 5D1 → 5D0 occurs, causing that the emissions from 5D1 level to be negligible compared to those from the 5D0 level [34, 42, 44].
The excitation spectrum of terbium single-doped glass (TGZT) is shown in Fig. 4. It was obtained by monitoring the emission at 543 nm and consists of multiple bands related to transitions from 7F6 ground state to 5G2 + 5L7 + 5L8, 5L9 + 5G4, 5D2 + 5G5, 5L10, 5G6 + 5D3, and 5D4 excited levels of Tb3+, located at 342, 353, 358, 369, 378 and 485 nm, respectively, being the last one the most intense [45,46,47,48]. The emission of the TGZT glass, excited at 342 nm, is displayed in the same figure. It consists of four low intensity bands related to transitions from the 5D3 level to 7F6, 7F5 7F4, 7F3 levels, and four bands associated to transitions from 5D4 level to 7F6, 7F5, 7F4, and 7F3 levels. The most intense emission band is the one associated to 5D4 → 7F5 transition giving origin to the characteristic bright green emission of Tb3+ ions.
3.3 Tb3+ → Eu3+ energy transfer
The effect of Eu3+ content in the codoped glasses was evaluated in a series of samples prepared varying the europium concentration from 0.5 to 2 mol% (TGZTE0.5, TGZTE1, TGZTE1.5, TGZTE2). The emission spectra of this samples, upon excitation of 342 nm, are presented in Fig. 5. This wavelength was selected because it does not excite Eu3+ ions (see Fig. 2) and it is resonant with 7F6 → 5L7 + 5G2 + 5L8 transitions of terbium ion. The luminescence results in the typical blue-green–red bands of 4f8 intra-configurational transitions of Tb3+ ion, from 5D4 to 7FJ manifold, in addition to the characteristic orange-red emissions of Eu3+ ions, associated to 5D0 → 7FJ transitions.
On the other hand, Fig. 6 depicts the excitation spectra of TGZE, TGZTE0.5, TGZTE1, TGZTE1.5, and TGZTE2 glasses, acquired while monitoring the emission at 700 nm, 5D0 → 5F4 transition of europium ions, where no Tb3+ emission exists. Two noteworthy features are present in this figure: first, the excitation bands associated to Eu3+ ions are dominant; and second, the presence of additional excitation bands which are related to terbium ions with maxima around 342, 366, 378, and 486 nm. It is worth mentioning the overlap of the excitation bands of both lanthanide ions at 366 nm and 378 nm; however, the excitation bands around 342 nm and 486 nm only correspond to terbium ion. Also, a decrease in intensity of the terbium excitation bands is observed when the concentration of europium ions increases from 0.5 to 2 mol% in the codoped samples (Fig. 7). Considering the spectral overlap between Tb3+ emissions (5D4 → 7F5 + 5D4 → 7F4) and Eu3+ excitation transitions (7F1 → 5D1, 7F1 → 5D0) depicted in Fig. 8, and the results mentioned above, it is possible to suggest that a Tb3+ → Eu3+ energy transfer process occurs in the codoped glasses, which could take place through the following three mechanisms, which are illustrated in the partial energy level diagram shown in Fig. 9 [45, 49]:
-
(1)
5D3 [Tb3+] + 7F0 [Eu3+] → 7F6 [Tb3+] + 5G2,3,4,5,5L8 [Eu3+],
-
(2)
5D3 [Tb3+] + 7F0 [Eu3+] → 7F5 [Tb3+] + 5D3,5L6 [Eu3+] and
-
(3)
5D4 [Tb3+] + 7F1 [Eu3+] → 7F4 [Tb3+] + 5D1 [Eu3+].
To inquire further about the Tb3+ → Eu3+ energy transfer, time decay profiles of terbium 5D4 → 7F5 transition (543 nm) were measured in the single-doped and codoped glasses. Tb3+ lifetimes were estimated by fitting the experimental data to an exponential function (Fig. 10a). The incorporation of Eu3+ in the terbium-doped glasses causes a reduction of the lifetime of the 543 nm emission, and this reduction is greater as the concentration of europium ions increases. This effect suggests a non-radiative energy transfer from Tb3+ to Eu3+. The efficiency (η) and probability (P), of Tb3+ → Eu3+ energy transfer can be calculated by the following equations, respectively [50]:
where τTb and τTb–Eu are the Tb3+ lifetimes in the single-doped and codoped TGZ glasses, respectively. The decay times, efficiency, and probability values are enlisted in Table 2, showing that the efficiency of the process is improved, and its probability is bigger as the concentration of Eu3+ increases. The behavior of the efficiency of the energy transfer at different concentrations of Eu3+ is shown in Fig. 10b.
The Inokuti–Hirayama model (I–H model) [51] is appropriate to describe energy transfer processes where there is not fast energy transfer migration, and can be useful to determine the main mechanism involved in the Tb3+ → Eu3+ non-radiative energy transfer observed in the codoped glass samples. This model was applied to the temporal decay of the terbium emission 5D4 → 7F5 at 543 nm, considering multipolar interaction. The non-exponential curve of the temporal decay of the luminescence intensity, I(t), can be written as follows:
where I0 denotes the intensity at t = 0, τ0 is the lifetime value of the donors (Tb3+) in the absence of Eu3+, γs is the energy transfer parameter, while the S value can take values of 6 (electric dipole–dipole), 8 (dipole-quadrupole), and 10 (quadrupole–quadrupole), depending on the multipolar interaction between the luminescent centers involved in the energy transfer process. In this way, the temporal behavior of 5D4 → 7F5 transition of Tb3+ under excitation at 342 nm, was fitted using Eq. (3) with τ0 = 1.086 ms, assuming different types of multipolar interactions. For all samples, the best fitting of the experimental data were obtained for S = 6, which infers that the Tb3+ → Eu3+ energy transfer process is dominated mainly by dipole–dipole interactions (Fig. 10a). Result that is similar to those observed in other Tb3+/Eu3+ codoped glasses [11, 45, 52]. The estimated energy transfer parameter γ6 values obtained from the I–H model for TGZTE series of glasses are enlisted in Table 3. It is observed that the energy transfer parameter varies directly with Eu3+ concentration. The critical interaction distance (Rc) for the energy transfer between both lanthanide ions can be calculated using the γ6 parameter through the following equation [53]:
where ρEu is the Eu3+ (acceptor) concentration. For the TGZTE glasses the ρEu and Rc values are enlisted in Table 3.
The estimated critical interaction distances were found in 6.4–6.7 Å range for all TGZTE samples. Also, considering that ions are evenly distributed in the glass and under the assumption that they are confined in the interior of a sphere with radius R, the distance between a pair of randomly distributed ions (Drandom) can be calculated using Eq. 5 [53]:
where ρT is the total ion concentration (Tb3+ plus Eu3+). The estimated Drandom values are between 20 and 18 Å (see Table 3). Since the obtained Rc values are considerably shorter than the estimated interaction distance between a randomly distributed pair of ions, it is possible to suggest that the Tb3+ → Eu3+ energy transfer takes place inside clusters, instead of randomly located ions in TGZ glasses.
3.4 Eu3+→Tb3+ energy transfer
In the Tb3+ excitation spectra shown in Fig. 7, the presence of two small excitation bands can be distinguished around 393 nm and 465 nm, that are associated to 7F0 → 5L6 and 7F0 → 5D2 transitions of europium ion, respectively. This fact indicates that the Eu3+ → Tb3+ energy transfer also takes place. To get a better insight about this process, the decay times of the 700 nm emission of Eu3+ (5D0 → 5F4 transition) in the codoped glasses were compared to the lifetimes of a TGZ series glasses single doped with 0.5, 1.0, 1.5 and 2 mol% of Eu3+: 1.119 ms (TGZE0.5), 1.084 ms (TGZE1.0), 1.054 ms (TGZE1.5) and 1.056 ms (TGZE2.0). According to the estimated results, the Eu3+ → Tb3+ energy transfer in the codoped glasses occurs less efficiently, which is demonstrated by small variation of Eu3+ decay times, as well as their calculated efficiency and probability (Table 4). As can be noted, the presence of Tb3+ in the codoped glasses reduces the lifetime of the 700 nm emission, compared to the value obtained in the TGZE samples. However, this reduction may also be due to a cross-relaxation process between the europium ions, as described above.
3.5 Photometric analysis
The tellurite glasses studied in this paper can find application as multicolor phosphor materials, given its wide range of tunability, from green to orange-red, by modifying the lanthanide concentration ratio or the excitation wavelength. To evaluate this tuning property, the fabricated glasses were excited in the UV range using different wavelengths and the chromaticity coordinates of their emission were estimated and plotted in the CIE 1931 chromaticity diagram (Fig. 11). Additionally, the correlated color temperature was calculated by means of McCamy equation [54]:
where n = (x – 0.3320)/(y – 0.1858), being x and y the chromaticity coordinates. The global emission of the glasses can be modulated from green to red region, passing through the yellow and orange regions of the spectrum, by simply modifying the Eu3+/Tb3+ ratio or the excitation wavelength (342, 366, 378, and 393 nm). The calculated chromaticity coordinates, CCT and color purity (CP) values are summarized in Table 5.
When the codoped samples are excited upon 342 nm (where europium ions do not have excitation band), the corresponding emission arises from the bands associated with both Tb3+ and Eu3+ ions, because the Tb3+ → Eu3+ energy transfer process. Besides, the increase of europium concentration and the consequent decrease of terbium emissions causes a shift of the global emission tonality towards the orange region (Fig. 11a). In the case of the values shown in Fig. 11b, c, the tunability of the global emission arises from the direct excitation of both ions and the energy transfer process. Figure 12 shows photographs of the glasses under UV-LED light excitation of 365 nm, evidencing the emission color change of the single and codoped glasses from green to reddish-orange. Excitation wavelengths of 366 nm and 378 nm are resonant with 7F6 → 5L10, 5G6, 5D3 transitions of Tb3+ and 7F0 → 5D4, 7F0 → 5L10, 7F0 → 5G2,3,4,5 transitions of Eu3+. Then, the origin of europium emission bands is due, first, to direct excitation of the Eu3+ and second to the Tb3+ → Eu3+ energy transfer process. Finally, when the glasses are excited at 393 nm, the double-doped glasses emission tonality comes fundamentally from europium ions in the reddish region, as shown in Fig. 11d.
4 Conclusions
Tb3+, Eu3+ single-doped and Tb3+/Eu3+ codoped TeO2–GeO2–ZnO glasses were successfully prepared by melt quenching technique. The luminescent properties investigated upon UV excitation allowed to identify the presence of a Tb3+ → Eu3+ energy transfer process in the codoped samples. In such samples, the emission from terbium 5D4 level decays faster than in TGZT (Tb3+-doped glass), revealing that Tb3+ can sensitize Eu3+ through non-radiative energy transfer. The fitting of the Tb3+ luminescence decay profile using the Inokuti-Hirayama model, allow us to infer that the Tb3+ → Eu3+ energy transfer process can be dominated by an electric dipole–dipole interaction in all codoped glasses. Furthermore, an increase of the energy transfer efficiency was observed, coupled with an enhancement of the Eu3+ emission at the expense of reducing the characteristic emission of Tb3+, as the Eu3+ concentration increases. The photometric characterization of Tb3+–Eu3+-doped glasses was carried out at different excitation wavelengths by evaluating the CIE 1931 chromaticity coordinates, showing a multicolor tuning of the emission from green to the orange-red region as a function of europium concentration with high color purity. Thus, the glasses under study can be good candidates as multicolor phosphors for their emission color tuning capability for solid-state devices.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
K. Siva Rama Krishna Reddy, K. Swapnaa, Sk. Mahamuda, M. Venkateswarlu, A.S. Rao, G. Vijaya Prakash, Investigation on structural and luminescence features of Dy3+ ions doped alkaline-earth boro tellurite glasses for optoelectronic devices. Opt. Mater. 85, 200–210 (2018). https://doi.org/10.1016/j.optmat.2018.08.057
H. Cankaya, A.T. Gorgulu, A. Kurt, A. Speghini, M. Bettinelli, A. Sennaroglu, Comparative spectroscopic investigation of Tm3+: tellurite glasses for 2-μm lasing applications. Appl. Sci. 8, 333 (2018). https://doi.org/10.3390/app8030333
Y. Zhou, J. Chen, O.M. Bakr, H.T. Sun, Metal-doped lead halide perovskites: synthesis, properties, and optoelectronic applications. Chem. Mater. 30, 6589–6613 (2018). https://doi.org/10.1021/acs.chemmater.8b02989
J. Rajagukguk, J. Kaewkhao, M. Djamal, R. Hidayat, S.Y. Ruangtaweep, Structural and optical characteristics of Eu3+ ions in sodium-lead-zinc-lithium-borate glass system. J. Mol. Struct. 1121, 180–187 (2016). https://doi.org/10.1016/j.molstruc.2016.05.048
R. Priyanka, S. Arunkumar, R.M. Ch Basavapoornima, K.M. Mathelane, Structural and spectroscopic investigations on Eu3+ ions doped boro-phosphate glasses for optical display applications. J. Lumin. 220, 116964 (2020). https://doi.org/10.1016/j.jlumin.2019.116964
Y. Chen, G. Chen, X. Liu, J. Xu, T. Yang, C. Yuan, C. Zhou, Down-conversion luminescence and optical thermometric performance of Tb3+/Eu3+ doped phosphate glass. J. Non-Cryst. Solids 484, 111–117 (2018). https://doi.org/10.1016/j.jnoncrysol.2018.01.027
Y. Muramoto, M. Kimura, S. Nouda, Development and future of ultraviolet light-emitting diodes: UV-LED will replace the UV lamp. Semicond. Sci. Technol. 29, 4 (2014). https://doi.org/10.1088/0268-1242/29/8/084004
M. Kneissl, A brief review of III-nitride UV emitter technologies and their applications, in III-nitride ultraviolet emitters. ed. by M. Kneissl, J. Rass (Springer International Publishing, 2016), pp.1–7. https://doi.org/10.1007/978-3-319-24100-5_1
O. Kıbrıslı, A.E. Ersundu, M. Çelikbilek Ersundu, Dy3+ doped tellurite glasses for solid-state lighting: An investigation through physical, thermal, structural and optical spectroscopy studies. J. Non-Cryst. Solids 513, 125–136 (2019). https://doi.org/10.1016/j.jnoncrysol.2019.03.020
V.A.G. Rivera, F.A. Ferri, L.A.O. Nunes, E. Marega Jr., White light generation via up-conversion and blue tone in Er3+/Tm3+/Yb3+-doped zinc-tellurite glasses. Opt. Mater. 67, 25–31 (2017). https://doi.org/10.1016/j.optmat.2017.03.028
M. Sołtys, A. Górny, J. Pisarska, W.A. Pisarski, Lead borate glasses triply doped with Dy3+/Tb3+/Eu3+ ions for white emission. Opt. Mater. 82, 110–115 (2018). https://doi.org/10.1016/j.optmat.2018.05.043
K. Jha, A.K. Vishwakarma, M. Jayasimhadri, D. Haranath, Multicolor and white light emitting Tb3+/Sm3+ doped zinc phosphate barium titanate glasses via energy transfer for optoelectronic device applications. J. Alloys Compd. 719, 116–124 (2017). https://doi.org/10.1016/j.jallcom.2017.05.076
M. Jayasimhadri, K. Jha, B.V. Ratnam, H.J. Woo, K. Jang, A.S. Rao, D. Haranath, Single NUV band pumped PbO-GeO2-TeO2:Tb3+ yellowish green emitting glass material for tricolor white LEDs. J. Alloys Compd. 711, 395–399 (2017). https://doi.org/10.1016/j.jallcom.2017.03.252
H.I. Francisco-Rodriguez, A. Lira, O. Soriano-Romero, A.N. Meza-Rocha, S. Bordignon, A. Speghinie, R. Lozada-Morales, U. Caldiño, Lithium-aluminum-zinc phosphate glasses activated with Tb3+ and Tb3+/Eu3+ for green laser medium, reddish-orange and white phosphor applications. Opt. Mater. 79, 358–365 (2018). https://doi.org/10.1016/j.optmat.2018.04.004
S. Gopi, S.K. Jose, E. Sreeja, P. Manasa, N.V. Unnikrishnan, C. Joseph, P.R. Biju, Tunable green to red emission via Tb sensitized energy transfer in Tb/Eu codoped alkali fluoroborate glass. J. Lumin. 192, 1288–1294 (2017). https://doi.org/10.1016/j.jlumin.2017.09.009
T.B. de Queiroz, M.B.S. Botelho, T.S. Gonçalves, R. Dousti, A.S.S. de Camargo, New fluorophosphate glasses codoped with Eu3+ and Tb3+ as candidates for generating tunable visible light. J. Alloys Compd. 647, 315–321 (2015). https://doi.org/10.1016/j.jallcom.2015.06.066
S. Loos, M. Mungra, B. Ahrens, R.L. Leonard, A. Evans, J.A. Johnson, F. Steudel, S. Schweizer, Concentration-dependent luminescence and energy transfer in Tb3+/Eu3+ doped borate and fluorozirconate glasses. J. Lumin. 187, 298–303 (2017). https://doi.org/10.1016/j.jlumin.2017.03.030
B. Kukliński, D. Wileńska, S. Mahlik, K. Szczodrowski, M. Grinberg, A.M. Kłonkowski, Luminescent GeO2–Pb–Bi2O3 glasses co-doped with Tb3+1 and Eu3+: excitation energy transfer and color chromaticity. Opt. Mater. 36, 633–638 (2014). https://doi.org/10.1016/j.optmat.2013.10.042
D. Wileńska, K. Szczodrowski, S. Mahlik, B. Kukliński, M. Grinberg, A.M. Kłonkowski, White emitting phosphors based on glasses of the type 10AlF3–10TiO2–39PbO–30H3BO3–10SiO2–xEu2O3–(1–x)Tb2O3: an energy transfer study. J. Lumin. 166, 54–59 (2015). https://doi.org/10.1016/j.jlumin.2015.05.002
F. Hu, X. Wei, S. Jiang, S. Huang, Y. Qin, Y. Chen, C.K. Duan, M. Yin, Fabrication and luminescence properties of transparent glass-ceramics containing Eu3+-Doped TbPO4 nanocrystals. J. Am. Ceram. Soc. 98, 464–468 (2015). https://doi.org/10.1111/jace.13283
R. Wang, D. Zhou, J. Qiu, Y. Yang, C. Wang, Color-tunable luminescence in Eu3+/Tb3+ codoped oxyfluoride glass and transparent glass-ceramics. J. Alloys Compd. 629, 310–314 (2015). https://doi.org/10.1016/j.jallcom.2014.12.233
T.V. Bocharova, A.N. Vlasova, G.O. Karapetyan, V.G. Kuryavyi, A.M. Mironov, N.O. Tagil’tseva, Local environment of Eu3+ and Tb3+ ions in fluorophosphate glasses of the Ba(PO3)2–MgCaSrBaAl2F14 system. Glass Phys. Chem. 34, 683–692 (2008). https://doi.org/10.1134/S1087659608060059
K.V. Raju, S. Sailaja, C.N. Raju, B.S. Reddy, Optical characterization of Eu3+ and Tb3+ ions doped cadmium lithium alumino fluoro boro tellurite glasses. Spectrochim. Acta A Mol. Biomol. Spectrosc. 79, 87–91 (2011). https://doi.org/10.1016/j.saa.2011.02.009
D.A. Rodríguez-Carvajal, A.N. Meza-Rocha, U. Caldiño, R. Lozada-Morales, E. Alvarez, Ma.E. Zayas, Reddish-orange, neutral and warm white emissions in Eu3+, Dy3+ and Dy3+/Eu3+ doped CdO-GeO2-TeO2 glasses. Solid State Sci. 61, 70–76 (2016). https://doi.org/10.1016/j.solidstatesciences.2016.09.009
M.S. Sadeq, H.Y. Morshidy, Effect of mixed rare-earth ions on the structural and optical properties of some borate glasses. Ceram. Int. 45(15), 18327–18332 (2019). https://doi.org/10.1016/j.ceramint.2019.06.046
R. El-Mallawany, Some physical properties of tellurite glasses, in Tellurite glass smart materials. ed. by R. El-Mallawany (Springer Nature, 2018), pp.8–13. https://doi.org/10.1007/978-3-319-76568-6_1
G. Yankov, L. Dimowa, N. Petrova, M. Tarassov, K. Dimitrov, T. Petrov, B.L. Shivachev, Synthesis, structural and non-linear optical properties of TeO2–GeO2–Li2O glasses. Opt. Mater. 35, 248–251 (2012). https://doi.org/10.1016/j.optmat.2012.08.002
A.G. Kalampounias, N.K. Nasikas, G.N. Papatheodorou, Structural investigations of the xTeO2–(1–x)GeO2 (x=0, 0.2, 0.4, 0.6, 0.8 and 1) tellurite glasses: A composition dependent Raman spectroscopic study. J. Phys. Chem. Solids 72, 1052–1056 (2011). https://doi.org/10.1016/j.jpcs.2011.05.016
N. Ghribi, M. Dutreilh-Colas, J.-R. Duclère, F. Gouraud, T. Chotard, R. Karray, A. Kabadou, P. Thomas, Structural, mechanical and optical investigations in the TeO2-rich part of the TeO2-GeO2-ZnO ternary glass system. Solid State Sci. 40, 20–30 (2015). https://doi.org/10.1016/j.solidstatesciences.2014.12.009
M. Lesniak, J. Zeid, B. Starzyk, M. Kochanovicz, M. Kuwik, J. Zmojda, P. Miluski, A. Baranowska, J. Dorosz, W. Pisarski, J. Pisarska, D. Dorosz, Investigation of the TeO2/GeO2 ratio on the spectroscopic properties of Eu3+-doped oxide glasses for optical fiber application. Materials 15, 117 (2022). https://doi.org/10.3390/ma15010117
H.A.A. Sidek, S. Rosmawati, Z.A. Talib, M.K. Halimah, W.M. Daud, Synthesis and Optical Properties of ZnO-TeO2 Glass System. Am. J. Appl. Sci. 6, 1489–1494 (2009). https://doi.org/10.3844/ajassp.2009.1489.1494
N.S. Tagiara, D. Palles, E.D. Simandiras, V. Psycharis, A. Kyritsis, E.I. Kamitsos, Synthesis, thermal and structural properties of pure TeO2 glass and zinc-tellurite glasses. J. Non-Cryst. Solids 457, 116–125 (2017). https://doi.org/10.1016/j.jnoncrysol.2016.11.033
A. Gulenko, O. Masson, A. Berghout, D. Hamani, P. Thomas, Atomistic simulations of TeO2-based glasses: interatomic potentials and molecular dynamics. Phys. Chem. Chem. Phys. 16, 14150–14160 (2014). https://doi.org/10.1039/c4cp01273a
M.E. Alvarez-Ramos, R.C. Carrillo-Torres, R. Sánchez-Zeferino, U. Caldiño, J. Alvarado-Rivera, Co-emission and energy transfer of Sm3+ and/or Eu3+ activated zinc-germanate- tellurite glass as a potential tunable orange to reddish-orange phosphor. J. Non-Cryst. Solids 521, 119462 (2019). https://doi.org/10.1016/j.jnoncrysol.2019.119462
I.V. García-Amaya, Ma.E. Zayas, J. Alvarado-Rivera, M. Cortez-Valadez, M. Pérez-Tello, N. Cayetano-Castro, F. Martínez-Suárez, A. Mendoza-Córdova, Influence of Eu2O3 on phase crystallization and nanocrystals formation in tellurite glasses. J. Non-Cryst. Solids 499, 49–57 (2018). https://doi.org/10.1016/j.jnoncrysol.2018.07.018
G. Monteiro, L.F. Santos, J.C.G. Pereira, R.M. Almeida, Optical and spectroscopic properties of germanotellurite glasses. J. Non-Cryst. Solids 350, 2695–2701 (2011). https://doi.org/10.1016/j.jnoncrysol.2010.12.062
J. Alvarado-Rivera, D.A. Rodríguez-Carvajal, M.C. Acosta-Enriquez, M.B. Manzanares-Martínez, E. Álvarez, R. Lozada-Morales, G.C. Díaz, A. de León, Ma.E. Zayas, Effect of CeO2 on the glass structure of sodium germanate glasses. J. Am. Ceram. Soc. 97, 3494–3500 (2014). https://doi.org/10.1111/jace.13202
R.C. Carrillo-Torres, G. Saavedra-Rodríguez, J. Alvarado-Rivera, U. Caldiño, R. Sánchez-Zeferino, M.E. Alvarez-Ramos, Tunable emission and energy transfer in TeO2-GeO2-ZnO and TeO2-GeO2-MgCl2 glasses activated with Eu3+/Dy3+ for solid state lighting applications. J. Lumin. 212, 116–125 (2019). https://doi.org/10.1016/j.jlumin.2019.04.008
K. Binnemans, Interpretation of europium (III) spectra. Coord. Chem. Rev. 295, 1–45 (2015). https://doi.org/10.1016/j.ccr.2015.02.015
W. Stambouli, H. Elhouichet, B. Gelloz, M. Ferid, Optical and spectroscopic properties of Eu-doped tellurite glasses and glass ceramics. J. Lumin. 138, 201–208 (2013). https://doi.org/10.1016/j.jlumin.2013.01.019
K. Maheshvaran, P.K. Veeran, K. Marimuthu, Structural and optical studies on Eu3+ doped boro-tellurite glasses. Solid State Sci. 17, 54–62 (2013). https://doi.org/10.1016/j.solidstatesciences.2012.11.013
G. Blasse, B.C. Grabmaier, Luminescent Materials, 1st edn. (Springer-Verlag, Berlin, 1994), pp.71–107
V. Lavín, U.R. Rodríguez-Mendoza, I.R. Martín, V.D. Rodríguez, Optical spectroscopy analysis of the Eu3+ ions local structure in calcium diborate glasses. J. Non-Cryst. Solids 319, 200–216 (2003). https://doi.org/10.1016/S0022-3093(02)01914-2
N. Wada, K. Kojima, Glass composition dependence of Eu3+ ion red fluorescence. J. Lumin. 126, 53–62 (2007). https://doi.org/10.1016/j.jlumin.2006.05.002
U. Caldiño, E. Álvarez, A. Speghini, M. Bettinelli, New greenish-yellow and yellowish-green emitting glass phosphors: Tb3+/Eu3+ and Ce3+/Tb3+/Eu3+ in zinc phosphate glasses. J. Lumin. 135, 216–220 (2013). https://doi.org/10.1016/j.jlumin.2012.10.013
M.R. Dousti, R.J. Amjad, Spectroscopic properties of Tb3+-doped lead zinc phosphate glass for green solid state laser. J. Non-Cryst. Solids 420, 21–25 (2015). https://doi.org/10.1016/j.jnoncrysol.2015.04.002
A.-B.F.A. Mohammed, G. Lakshminarayana, S.O. Baki, Kh.A. Bashar, I.V. Kityk, M.A. Mahdi, Optical and dielectric studies for Tb3+/Sm3+ codoped borate glasses for solid-state lighting applications. Opt. Mater. 86, 387–393 (2018). https://doi.org/10.1016/j.optmat.2018.10.033
N. Deop, A.S. Rao, Spectroscopic studies of single near ultraviolet pumped Tb3+ doped lithium lead alumino borate glasses for green lasers and tricolour w-LEDs. J. Lumin. 194, 56–63 (2018). https://doi.org/10.1016/j.jlumin.2017.09.057
E. Álvarez, M.E. Zayas, J. Alvarado-Rivera, F. Félix-Domínguez, R.P. Duarte-Zamorano, U. Caldiño, New reddish-orange and greenish-yellow light emitting phosphors: Eu3+ and Tb3+/Eu3+ in sodium germanate glass. J. Lumin. 153, 198–202 (2014). https://doi.org/10.1016/j.jlumin.2014.03.031
M.E. Alvarez-Ramos, J. Alvarado-Rivera, M.E. Zayas, U. Caldiño, J. Hernández-Paredes, Yellow to orange-reddish glass phosphors: Sm3+, Tb3+ and Sm3+/Tb3+ in zinc tellurite-germanate glasses. Opt. Mater. 75, 88–93 (2018). https://doi.org/10.1016/j.optmat.2017.09.033
M. Inokuti, F. Hirayama, Influence of energy transfer by the exchange mechanism on donor luminescence. J. Chem. Phys. 43, 1978–1989 (1965). https://doi.org/10.1063/1.1697063
A.U. Trápala-Ramírez, J.L.N. Gálvez-Sandoval, A. Lira, I. Camarillo, M.E. Álvarez-Ramos, A.N. Meza-Rocha, U. Caldiño, Calcium-zinc phosphate glasses activated with Tb3+/Eu3+ for laser and white LED applications. J. Lumin. 215, 116621 (2019). https://doi.org/10.1016/j.jlumin.2019.116621
M.E. Álvarez-Ramos, Study of the optical properties and cross relaxation process of Dy3+ under simultaneous UV-IR excitation in tellurite glasses. J. Lumin. 233, 117874 (2021). https://doi.org/10.1016/j.jlumin.2020.117874
C.S. McCamy, Correlated color temperature as an explicit function chromaticity coordinates. Color. Res. Appl. 17, 142–144 (1992). https://doi.org/10.1002/col.5080170211
Acknowledgements
The authors thank to Consejo Nacional de Ciencia y Tecnología (CONACyT) for their support from project Cátedra-CONACyT 1959.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, analysis and editing were performed by JA-R, GS-R, RCC-T, FF-D and RS-Z. The first draft of the manuscript was written by MEA-R and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there is no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alvarez-Ramos, M.E., Alvarado-Rivera, J., Félix-Domínguez, F. et al. Multicolor green to orange-red emission of Tb3+ and Eu3+-codoped tellurite glasses: Eu3+ concentration and Tb3+ → Eu3+ energy transfer. Appl. Phys. A 129, 75 (2023). https://doi.org/10.1007/s00339-022-06347-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-06347-6