Abstract
Some physical and optical properties of xPbF2⋅(100–x)TeO2 (0 ≤ x ≤ 90 mol%) glasses and glass–ceramics have been studied. Density increases linearly with increasing PbF2 content up to 70 mol% PbO, then tends to be constant for 70 < PbF2 ≤ 90 mol%. The molar volume remains constant in the first region then increases for PbF2 > 70 mol%. The main factor which controls the molar volume is the change in free volume and packing density. There is a limited increase in conductivity with increasing PbF2 content then it decreases for PbF2 ≥ 50 mol%. Pb2+ ions are the main charge carriers. The band gap Eg and the linear refractive index n change in an opposite manner where Eg increases with increasing PbF2 content for PbF2 ≤ 50 mol%, then it decreases sharply for PbF2 > 70 mol%. For PbF2 ≤ 50 mol%, the Urbach energy EU decreases then seems to be constant for further additions. Metallization criterion M and molar refractivity Rm change in a similar manner to Eg and n, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Glasses containing oxide-fluoride systems have good optical properties and perfect thermal and chemical stability [1]. PbF2 was able to form stable glasses due to its dual role as a modifier and former [2]. PbF2 glasses can be believed as appropriate candidates for electrochemical applications [3], like power sources, particularly in the scope of solid-state batteries. Besides, they have prospective applications in IR fiber optics and laser windows [4].
The density of xPbO2⋅(100-x)TeO2 (13.6 ≤ x ≤ 21.8 mol%) and xPbF2⋅(100-x)TeO2 (13.7 ≤ x ≤ 26 mol%) glasses was measured [5]. It is found that it increases with increasing PbO and PbF2 content, respectively. Also, the electrical conductivity of xPbF2⋅(100-x)(PbO:TeO2) (0 ≤ x ≤ 60 mol%) glasses was studied by El Damrawi [6], it is stated that replacing PbO and TeO2 by PbF2 decreases the activation energy for conduction and increases the conductivity. The increase in conductivity is due to the increase in both the concentration and mobility of charge carriers. Transport of fluorine ions in those glasses is explained by the random site model.
The UV spectra of TeO2-PbF2 glasses were measured by Shiqing et al. [7]. It is found that with adding PbF2 into tellurite glasses, the excitation energy of the absorption band decreases. This is because the polarizability of O2– is higher than that of F–. In addition, F– ions can break oxygens of the network (making them NBOs) and tighten the mobility gap.
In this work, we aim to study the density, electrical conductivity and optical properties of xPbF2⋅(100–x)TeO2 glasses and glass–ceramics. Also, explore the role of F– and Pb2+ ions in conductivity. This is an extended work of a previous study on the structure of these glasses [8].
2 Experimental
As start materials with high purity (99%, Sigma-Aldrich), reagent grades of PbF2 and TeO2 were used to prepare the investigated glasses and glass–ceramics. Batches were melted in silica crucibles for 25 min, at 780–830 °C, depending on their respective compositions. The crucible was swirled repeatedly until the melt became visually homogeneous. Glass disks were obtained at room temperature after the melt was dropped on a steel plate and compressed by another one.
The density (D) was determined by applying the standard Archimedes method at room temperature for four samples of each glass. The used immersion liquid was Xylene. Density values are accurate to ± 2%.
The dc conductivity was determined by measuring the resistance in the range of 103–1013 Ω for samples with a thickness ranging between 1.5–3 mm. About ± 0.04 eV and ± 5% are considered as experimental errors for the activation energy and conductivity, respectively.
To determine the optical transition the optical properties were measured by UV–Visible–NIR Spectrophotometer (JASCO model V770) in the range 190–2000 nm through absorption spectra.
3 Results and discussion
3.1 Density and molar volume
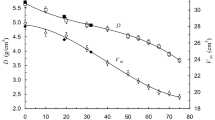
Figure 1 shows the change of density D and the molar volume (Vm) with PbF2 content. It is clear that D increases linearly with increasing PbF2 content up to 70 mol% then it seems to have steady values with further additions of PbF2. The increase in D with increasing PbF2 content may be due to that the molecular mass of PbF2 (245.1968 g/mol) is larger than that of TeO2 (159.5988 g/mol). Nevertheless, the constancy of D for PbF2 ≥ 70 mol% indicates that the latter assumption is not the sole reason for increasing density. In xPbF2⋅(1–x)B2O3 glasses where (30 ≤ x ≤ 80), Doweidar et al. [9] mentioned that the density of the glasses increases with increasing PbF2 content. Also, the overall density of the structural units formed with addition of PbF2 in the matrix (\({\text{Pb}}_{1/2}^{2 + } \left[ {{\text{TeO}}_{3 + 1} } \right]^{ - }\), TeO3/2F and PbF2) [8] is larger than that of TeO4 ones and this leads to an increase in density. Vogel et al. [5] mentioned that in PbF2–TeO2 glasses, density increases with increasing PbF2 content.
Figure 1 represents the dependence of molar volume (Vm) on PbF2 content. The Vm can be estimated from the experimental density data and the molecular mass (M) of glass by the following relation.
There is no change in Vm for compositions with PbF2 ≤ 70 mol%, then it increases for PbF2 > 70 mol%. The constancy of Vm for PbF2 ≤ 70 mol% points out to the dependence of D on the molecular mass. The change of Vm with PbF2 content (Fig. 1) might be correlated with the change in packing density (Pd) and free volume (Vf). The free volume (Vf) can be given as
where mi is the number of ions (i) and Vi is the volume of such ion of type (i) whereas the later can be expressed as
ri denotes the radius of ion of type (i) [10] and i refers to Te4+, O2−, Pb2+ and F− ions. Vi includes the volume of atoms and/or ions inside the unit and its surrounding space in the glass matrix. The packing density of the oxides can be given as [11]
Figure 2 shows the change of free volume and packing density with PbF2 content. It is obvious from Fig. 2 that Vf has almost constant values for PbF2 ≤ 50 mol% and increases gradually for PbF2 > 50. However, Pd behaves in a different manner at higher concentrations where it decreases gradually for PbF2 > 50 mol%, while constant for PbF2 ≤ 50 mol%. The constancy of Vf and Pd in the composition range 0 ≤ PbF2 ≤ 50 might be the reason for the constancy in Vm. In addition, the volumes of the structural units related to PbF2 and those for TeO2 have convergent values. Also, for PbF2 > 50 mol% the increase in Vf and the decrease in Pd may be responsible for the increase in Vm of the glass and the constancy of density D for PbF2 > 70 mol%.
3.2 Electric conduction
Figure 3 shows the variation of the natural logarithm of the direct current electrical conductivity (logσ) with the reciprocal of absolute temperature (1000/T) in PbF2–TeO2 glasses and glass–ceramics. The change of logσ with 1000/T is linear which reveals that the conduction process is ionic in nature according to Arrhenius equation
Here σo is a constant. T, k and E are absolute temperature, activation energy for the conduction process and Boltzmann’s constant, respectively.
Figure 4 shows the change of logσ 473σlog conductivity at 473 K) and E with PbF2 content. There are two regions in the change of logσ473and E. The first region is for PbF2 ≤ 50 mol% where the conductivity increases by about 1.3 orders with increasing PbF2 content. However, in this region E decreases linearly with increasing PbF2 content. The second region is for PbF2 ≥ 50 wherewith increasing PbF2 content logσ473 decreases steadily up to 90 mol% PbF2. In this region, E seems to be nearly constant.
The conductivity [12] is given by the following relation
where q, c and μ are, respectively, the ionic charge, the concentration of mobile ions and the mobility of charge carrier. It is understood from Eq. (6) that σ depends on c and/or μ. El Agammy et al. [8] showed that in the studied glasses N4 (the fraction of four coordinated tellurium atoms) decreases for PbF2 ≤ 30 mol% and the majority of F– ions enters the glass structure as terminal ones to convert TeO4 units to (\({\text{Pb}}_{1/2}^{2 + } \left[ {{\text{TeO}}_{3 + 1} } \right]^{ - }\) and TeO3/2F) units. A similar behavior of N4 was shown in NaF–TeO2 glasses and glass–ceramics [13]. As the increase in conductivity for PbF2 ≤ 50 mol% is only 1.3 orders of magnitude and the electronegativity of F atom (3.98) is greater than that of O atom (3.44), we can infer that the bonding energy of Te–O is smaller than that of Te–F [14], it is presumed that the main charge carriers are Pb2+ ions and the transport of these ions is responsible for the conduction process. Considering that the conductivity depends on c and/or μ, where the mobility represents the possibility of ease in movement of the ions under an external electric field. When PbF2 was modified up to 50 mol%, σ increased by just 1.3 orders. This might be due to the ionic radius of Pb2+ ions (1.19 Å) and the dependence of the free volume on PbF2 content.
As shown in Fig. 2, Vf is nearly constant up to 50 mol% PbF2. In this region, the number of charge carriers increases (Pb2+ ions) by adding PbF2. It is then assumed that the constancy in Vf might limit the increase in the conductivity. Doweidar et al. [15] found a confined increase in conductivity in xCaF2⋅(100–x)B2O3 glasses. They stated that a decrease in Vf with increasing CaF2 content leads to a decrease in the mean mobility. Replacing TeO2 by PbF2 increases the charge carriers and might decrease the activation energy in this region.
In the second region (PbF2 > 50 mol%), there is a gradual decrease in logσ473 (Fig. 4) and E is nearly constant. For PbF2 > 50, it is confirmed by X-ray diffraction and transmission electron microscopy [8] that amorphous and crystalline PbF2 are the major phases in these glasses and glass–ceramics. Also, SEM [8] shows agglomerates of these phases as clusters of different sizes. Despite the increase of Vf and c for PbF2 ≥ 50 mol%, there is a decrease in log473 and E is nearly constant. This may be due to that the majority of PbF2 that enters the matrix tends to form its own crystalline and amorphous matrix as clusters which are not connected to each other’s [8]. Also, the matrix of PbF2 might be the dominant in the glass in this region. This separation makes the continuous migration of Pb2+ in continuous pathways of charge carriers more difficult and limits the conductivity. The bonding energy of Te–F bond is ~ 683.7 eV [14] and that of Pb–F is about 684.1 eV [16], which are close to each other’s. This leads to the assumption that F– ions do not contribute in the conduction process and the association of lead and fluorine ions may hinder the diffusion of the Pb2+ ions which might limit the conductivity.
3.3 Optical properties
3.3.1 Optical absorption spectra, optical band gap and refractive index
The optical absorption spectra of xPbF2⋅(100-x)TeO2 glasses and glass–ceramics (10 ≤ x ≤ 90 mol%) plotted as a function of the wavelength in the range of 190–2000 nm as shown in Fig. 5. The fundamental absorption peak is centered at ~ 280 nm and then with increasing PbF2 content, especially for PbF2 > 70 mol%, it shifts to higher wavelengths. The optical absorbance A is related to the absorption coefficient α through the equation [17],
where d is the sample thickness. In Davis and Mott equation [18],
where B is a constant, m is an indicator that locates the optical transition type, h, Eg and υ are Planck’s constant, optical band gap and photon’s frequency. The optical transition type must take the following values m = 1/2 and 2 for direct and indirect transition, respectively. Moreover, it is stated by many others [19,20,21,22,23] that indirect allowed transitions (m = 2) are valid for oxide glasses.
Depending on that, Tauc plots were used to estimate the optical band gap. Figure 6 represents Tauc plots for the variation of (αhυ)1/2 versus (hυ). Figure 7 shows the method of estimating Eg [24,25,26]. Figure 8 shows the variation of the band gap on the content of PbF2. There are three regions for the variation. The first is for PbF2 ≤ 50 mol% where Eg increases with increasing PbF2 content. The second is for 50 ≤ PbF2 ≤ 70 mol% where Eg is nearly constant. The last is for PbF2 > 70 mol% where Eg decreases sharply then becomes constant. In the first region, Eg value varies from 2.7 to 3.03 eV. Previously, El Agammy et al. [8] deduced that in the studied glasses, PbF2 completely modifies the structure for PbF2 ≤ 10 mol% and plays the dual role (former and modifier) for PbF2 > 10 mol%. In addition, the modifier PbF2 converts TeO4 units to \({\text{Pb}}_{1/2}^{2 + } \left[ {{\text{TeO}}_{3 + 1} } \right]^{ - }\) and TeO3/2F units, while former PbF2 builds its own matrix. The increase of Eg in the first region might be due to that the rate of increase in CPb(f) (former part of PbF2 (mol%)) is high [8], in contrary CPb(m) (modifier part of PbF2 (mol%)) seems to decrease with low rate through this region. When CPb(m) increases it is expected that the concentration of NBO bonds increases in the matrix [8]. In this case, the increase in CPb(f) and decrease in CPb(m) might decrease the rate of forming NBO, and as a result Eg increases [23, 27, 28]. The constancy and the decrease in the second and last regions might be due to that the PbF2 matrix becomes the dominant one, rather than the TeO2 matrix. In addition, the sudden decrease that occurred for PbF2 > 70 mol% might be due to the formation of more ordered structure and the noticed existence of PbF2 crystalline phases in this region [8]. Also, the main matrix seems to be mainly saturated with PbF2. In addition, Fig. 2 shows that Vf has almost constant values for PbF2 ≤ 50 mol% and increases gradually for further additions of PbF2. However, Pd behaves in a different manner at higher concentrations. The increase in Vf and decrease in Pd for PbF2 > 50 mol% may be responsible for increasing the molar volume of the glass and decreasing Eg in this range.
Table 1 presents the values of the band gap, linear refractive index, molar volume, molar refractivity, metallization criterion and Urbach energy. Figure 8 shows the variation of the linear refractive index n on PbF2 content. The refractive index n for glasses has been correlated to the band gap as follows [29],
where the value 20 in this relation has dimension eV according to Duffy [30]. There are three regions for the variation of n with PbF2 content. The first is for PbF2 ≤ 50 mol%. In this region, n decreases with increasing the content of PbF2. The second is for 50 ≤ PbF2 ≤ 70 mol% where n is constant. The last one is that for PbF2 > 70 mol% where n increases with increasing PbF2 content. The n value varies from 2.39 to 2.62. These variations are due to the same structural changes that affect the variation of Eg. Further, because energy gap is inversely proportional to refractive index according to Eq. (9), the structural changes and the variation of both CPb(f) and CPb(m) with PbF2 [8] are responsible for the change of n. It is assumed that in the first region, the increase and decrease of CPb(f) and CPb(m), respectively, limit the NBO formation and hence n decreases.
3.3.2 Molar refractivity and metallization criterion
The molar refraction (Rm) of the studied glasses and glass–ceramics was estimated using the Lorentz-Lorentz equation,
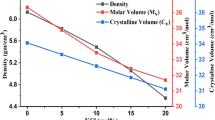
Figure 9 shows that the molar refraction is nearly constant for PbF2 ≤ 70 mol% and then increases from 17.11 to 19.9 cm3 for further additions from PbF2. This behavior is similar to that of the molar volume and the refractive index with PbF2 content as shown in Figs. 1 and 8, respectively. This behavior is due to the dependence of the molar refraction on the refractive index and molar volume.
The following equation is used to estimate the metallization criterion (M) for PbF2–TeO2 glasses and glass–ceramics [29],
M anticipates the way of behaving of glass materials to metallization or insulation depending on the values of Rm and Vm [31]. Values of M change between 0.338 and 0.389 (Table 1), whereas Fig. 9 shows that M increases gradually for PbF2 ≤ 70 mol% and then decreases suddenly for further additions of PbF2. This behavior is similar to that of Eg (Fig. 8). These trends reveal that the glass material is closer to metallization behavior than insulation for PbF2 ≤ 70 mol%. On contrary, for PbF2 > 70 mol% the insulating behavior is the dominant one. These results are consistent with the electrical conductivity results as shown in Fig. 3 where logσ 473increases with increasing PbF2 content for PbF2 ≤ 50 mol% then decreases steadily up to 90 mol% PbF2.
3.3.3 Urbach energy
Urbach energy EU characterizes the range of the exponential tail of the absorption edge. The relation between Urbach energy and the absorption tails is given by,
where EU is the Urbach energy and αo is a constant. Figure 10 shows plots for ln(α) against photon energy, E = hυ, for determination of the Urbach energy EU. Figure 11 represents an example for estimating EU [32].
Figure 12 shows the change of EU with PbF2 content. The values of EU lie between 0.32 and 0.54 eV which are in the range of amorphous semiconductors [27, 33]. EU decreases with increasing PbF2 up to 50 mol% and seems to be constant for higher PbF2 contents. This variation might be due to the formation of crystalline phases such as Te2O3F2 and PbF2 [8] which reinforces the possibility of formation of a matrix with a long-range order. This order in the glass contributes to a reduction of EU. Analogous behavior of EU was noticed with TiO2 [27].
4 Conclusion
In PbF2–TeO2 glasses and glass–ceramics, D increases due to that the molecular mass of PbF2 is larger than that of TeO2. The change in Vf and Pd is responsible for the change in Vm. It is considered that Pb2+ ions are the main charge carriers. For PbF2 ≤ 50, the constancy of Vf causes a limited increase in conductivity. Whereas, for PbF2 > 50 mol% the conductivity decreases because PbF2 tends to form its own crystalline and amorphous matrix in the form of clusters. Eg increases with increasing PbF2 content for PbF2 ≤ 50 mol%, then it decreases sharply then becomes constant for PbF2 > 70 mol%. n changes in an opposite manner to that of Eg. EU decreases with increasing PbF2 up to 50 mol%. Then for further additions of PbF2, it is nearly constant. These trends might be related to the structural variations that take place with modifying the network by PbF2.
References
E. Kashchieva, Y. Ivanova, Electron microscopic investigations of microheterogeneous structure in glasses from the GeO2-PbO-PbF2 oxide-halide system. J. Mater. Sci. Lett. 10, 1356–1358 (1991)
W. Akshatha, Y. Raviprakash, S.D. Kamath, Dielectric properties and relaxation dynamics in PbF2-TeO2-B2O3-Eu2O3 glasses. Trans. Nonferrous Met. Soc. China 25, 2637–2645 (2015)
J.M. Reau, M. Poulain, Ionic conductivity in fluorine-containing glasses. Mater. Chem. Phys. 23, 189–209 (1989). https://doi.org/10.1016/0254-0584(89)90024-2
H. Nasu, T. Uchigaki, K. Kamiya, H. Kanbara, K. Kubodera, Nonresonant-type third-order nonlinearity of (PbO, Nb2O5)-TiO2-TeO2 glass measured by third-harmonic generation. Jpn. J. Appl. Phys. 31, 3899–3900 (1992). https://doi.org/10.1143/jjap.31.3899
W. Vogel, H. Burger, G. Zerge, B. Muller, K. Forkel, G. Winterstein, A. Boxberger, H. Romhild, Halogenid-und sulfathaltige telluritglaser. Silikattechnelk 25, 207 (1974)
G. El-Damrawi, Transport behavior of PbO-PbF2-TeO2 glasses. Phys. Status Solidi 177, 385–392 (2000)
S. Xu, G. Wang, J. Zhang, S. Dai, L. Hu, Z. Jiang, Composition dependent upconversion of Er3+-doped PbF2-TeO2 glasses. J. Non. Cryst. Solids 336, 230–233 (2004)
E.F. El Agammy, H. Doweidar, K. El-Egili, R. Ramadan, Structure of PbF2–TeO2 glasses and glass-ceramics. J. Mater. Res. Technol. 9, 4016–4024 (2020). https://doi.org/10.1016/j.jmrt.2020.02.028
H. Doweidar, G. El-Damrawi, M. Abdelghany, Structure-properties correlations in PbF2-B2O3 glasses. Phys. Chem. Glass. J. Glass Sci. Technol. Part B 55, 121–129 (2014)
K. Barbalace, Periodic table of elements. Environ. Chem. Com., 4–14 (2007)
M. Burgess, D. McClarnon, M. Affatigato, S. Feller, Packing as a probe of structure in alkaline earth glass systems. J. Non. Cryst. Solids 354, 3491–3502 (2008)
C. Kittel, P. McEuen, P. McEuen, Introduction to Solid State Physics (Wiley, New York, 1996)
E.F. El Agammy, H. Doweidar, K. El-Egili, R. Ramadan, M. Jaremko, A.H. Emwas, Structure of NaF–TeO2 glasses and glass-ceramics. Ceram. Int. 46, 18551–18561 (2020). https://doi.org/10.1016/j.ceramint.2020.04.161
Y. Jia, J. Lin, W. Zhang, C. Li, J. Ren, L. Rong, Structure and infrared emission of Ho3+/Yb3+ Codoped TeO2-ZnO-ZnX2 (X=F, Cl, Br) glasses. J. Chin. Ceram. Soc. 42, 545–556 (2014)
H. Doweidar, G. El-Damrawi, M. Abdelghany, Structure and properties of CaF2-B2O3 glasses. J. Mater. Sci. 47, 4028–4035 (2012)
A. Osaka, Y. Miura, T. Tsugaru, Bonding state of fluorine in lead-tin oxyfluorophosphate glasses. J. Non. Cryst. Solids 125, 87–92 (1990)
W.S. AbuShanab, E.B. Moustafa, A.H. Hammad, R.M. Ramadan, A.R. Wassel, Enhancement the structural, optical and nonlinear optical properties of cadmium phosphate glasses by nickel ions. J. Mater. Sci. Mater. Electron. 30, 18058–18064 (2019)
E.A. Davis, Nf. Mott, Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 22, 903–922 (1970)
E.F. El Agammy, A.M.A. Mostafa, M. Al-Zaibani, H.O. Tekin, R. Ramadan, A. Essawy, S.A.M. Issa, Tailoring the structuralism in xBaO·(30-x) Li2O·70B2O3 glasses for highly efficient shields of Gamma radiation and neutrons attenuators. Phys. Scr. (2021)
M.M. Hivrekar, D.B. Sable, M.B. Solunke, K.M. Jadhav, Different property studies with network improvement of CdO doped alkali borate glass. J. Non. Cryst. Solids 491, 14–23 (2018)
A.H. Hammad, A.M. Abdelghany, Optical and structural investigations of zinc phosphate glasses containing vanadium ions. J. Non. Cryst. Solids 433, 14–19 (2016)
S.Y. Marzouk, R. Seoudi, D.A. Said, M.S. Mabrouk, Linear and non-linear optics and FTIR characteristics of borosilicate glasses doped with gadolinium ions. Opt. Mater. (Amst) 35, 2077–2084 (2013)
M.R. Sahar, N. Noordin, Oxychloride glasses based on the TeO2-ZnO-ZnCl2 system. J. Non Cryst. Solids 184, 137–140 (1995)
A.M.A. Mostafa, E.F. El Agammy, M. Al-Zaibani, R. Ramadan, S.A.M. Issa, H.O. Tekin, Characterization of synthesized xBaO-(40-x)Li2O-60B2O3 glass system: a multi-dimensional research on optical and physical properties. J. Mater. Sci. Mater. Electron., 1–19 (2021)
A.M.A. Mostafa, H.M. Zakaly, S.A. Al-Ghamdi, S.A. Issa, M. Al-Zaibani, R.M. Ramadan, E.F. El Agammy, PbO–Sb2O3–B2O3–CuO glassy system: evaluation of optical, gamma and neutron shielding properties. Mater. Chem. Phys. 258, 123937 (2021). https://doi.org/10.1016/J.MATCHEMPHYS.2020.123937
E.F. El Agammy, H. Doweidar, K. El-Egili, R.M. Ramadan, Physical and optical properties of NaF–TeO2 glasses and glass–ceramics. Appl. Phys. A. 127, 42 (2021). https://doi.org/10.1007/s00339-020-04153-6
W. Stambouli, H. Elhouichet, M. Ferid, Study of thermal, structural and optical properties of tellurite glass with different TiO2 composition. J. Mol. Struct. 1028, 39–43 (2012)
M.S. Malik, C.A. Hogarth, The effect of chloride ions on the optical properties of TeO2-CuO-CuCl2 glasses. J. Mater. Sci. 25, 116–120 (1990)
V. Dimitrov, S. Sakka, Electronic oxide polarizability and optical basicity of simple oxides. Int. J. Appl. Phys. 79, 1736–1740 (1996)
J.A. Duffy, Chemical bonding in the oxides of the elements: a new appraisal. J. Solid State Chem. 62, 145–157 (1986)
S.L.S. Rao, G. Ramadevudu, M. Shareefuddin, A. Hameed, M.N. Chary, M.L. Rao, Optical properties of alkaline earth borate glasses. Int. J. Eng. Sci. Technol. 4, 25–35 (2012)
L. Skuja, K. Kajihara, Y. Ikuta, M. Hirano, H. Hosono, Urbach absorption edge of silica: reduction of glassy disorder by fluorine doping. J. Non. Cryst. Solids 345, 328–331 (2004)
E.A. Davis, N.F. Mott, Electronic Processes in Non-Crystalline Materials (Clarendon Press, Oxford, 1971)
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El Agammy, E.F., Doweidar, H., El-Egili, K. et al. PbF2–TeO2 glasses and glass–ceramics: a study of physical and optical properties. Appl. Phys. A 127, 962 (2021). https://doi.org/10.1007/s00339-021-05069-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-021-05069-5