Abstract
FTIR spectroscopy has been employed to investigate the structure of CaF2–B2O3 glasses. It is proposed that CaF2 partially modifies the borate network forming \( {\text{Ca}}_{ 1 / 2}^{ 2+ } [{\text{BO}}_{ 3 / 2} {\text{F]}}^{ - } \)units. The rest of CaF2 is assumed to build an amorphous network formed of CaF4 tetrahedra. Analysis of density and molar volume revealed that the volume of CaF4 tetrahedron in the studied glasses is slightly greater than that in the crystalline form. Data of density, molar volume, and electric conductivity have been correlated with the glass structure. As far as the authors know, CaF2–B2O3 glasses are investigated for the first time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since publishing of the pioneer work of Gressler and Shelby on PbF2–B2O3 [1] and PbO–PbF2–B2O3 glasses [2], the structure and properties of borate glasses containing F− ions have been the subject of growing interest. These types of glass show a wide variety of physical properties and are characterized with their anionic conduction. They are classified among the well-known fast ionic conductors. However, there is a main controversy about the role of F− ions.
X-ray photoelectron spectroscopy (XPS) of F (1s) in (70−x)PbO·xPbF2·30B2O3 glasses [3] showed two peaks. A peak is assigned to F− ions in Pb–F–Pb clusters (free F−) and the other is related to non-bridging fluorine atoms in the borate network (B–F−…Pb2+). Gopalakrishnan et al. [4] indicated that in xPbF2·(1−x)B2O3 glasses fluorine is incorporated predominantly as B–O–F at high B2O3 content, whereas it forms Pb–O–F bonds at high PbF2 content.
Chowdari and Rong [5] indicated that upon increasing the fluorine content in fluorinated lithium borate glasses, the number of four-coordinated boron atoms increases and the asymmetric BO3 groups with non-bridging oxygen atoms decreases. These results are assumed as an evidence that fluorine participates in the boron–oxygen network. X-ray photoelectron spectra of F (1s) show two peaks. One of them is related to fluoride ions associated with lithium ions forming Li–F bond and the other is assigned to non-bridging fluorine atoms (B–F…Li+). The latter may contribute to the formation of BO3F and BO2F2 units with non-bridging bonds. Formation of these structural units was confirmed by NMR investigations on NaF–Na2O–B2O3 glasses [6, 7] with an estimation of the highest probability for the formation of BO3F units. Suresh and Chandramouli [8] discussed structural variations in (30−x) Na2O·xNaF·50B2O3·20Bi2O3 glasses, with increasing the NaF content, on the basis of the formation of BO3F and BO2F2 units. Both structural units are also considered to be formed in Li2O–SrO–SrF2–B2O3 glasses [9].
Alkali fluoroborate glasses with alkali fluoride content up to 45 mol% LiF or 55 mol% NaF or KF showed composition-properties trends similar to that observed for alkali borate glasses. To explain the differences in behavior between the alkali fluoroborate glasses and the corresponding alkali borate glasses Shelby and Baker [10] proposed a structural model based on the replacement of BO4 tetrahedra by BO3F tetrahedra. The model is based on NMR investigations in sodium fluoroborate glasses [11, 12].
Formation of some different types of fluoroborate units like BO2F (or BOF2) is assumed in B2O3–xLi2O–yLiX (X = F, Cl, Br, and I) [13]. The B–F in these units is a terminal one and the bond can be described as a non-bridging bond.
Changes in the electric conductivity with fluorine content in MeF2–Na2B4O7 glasses (Me = Mg, Ca, Sr, and Ba) have been attributed to formation of a number of structural units. Sokolov et al. [14] assumed the structure of those glasses is built up of nonpolar BO3/2 units and various polar groupings such as Na+[BO4/2]−, Na+[F−BO3/2]−, Me1/2[BO4/2]−, Me1/2[F−BO3/2]−, [MeF4/2], and [MeF6/3]. Among these units, Na+[BO4/2]− and Na+[F−BO3/2]− are the most probably formed species [15, 16]. The negative charge on the regular [BO4/2] tetrahedron has a uniform distribution, whereas it is localized at F− ion in the distorted [F−BO3/2] tetrahedron.
Another type of structural units is proposed by Sokolov et al. [17]. They proposed formation of Na+[F−BO4/2]− oxyfluoride structural units in NaF–Na2O–B2O3 and NaF–B2O3 glasses. Although these units are electrically unbalanced, it has been indicated that fluorine ions are not consumed only in these units but they can also be bound to Na+ ions in F−…Na+[F−BO4/2] structural units.
Hager and El-Hofy [18] used IR spectroscopy to study the structure of (70−x)B2O3·30BaF2·xLiX glasses, where X = F, Cl and Br. They concluded that these glasses mainly contain BO3, BO2F triangles and BO4, and BOF3 tetrahedra. Formation of BO2F triangles is, however, ruled out in NaF–Na2O–B2O3 glasses [7, 19].
Multi nuclei 7Li, 11B, 19F, and 207Pb single- and double-resonance NMR experiments indicated that units such as BO4/2 −, BO3/2F− are expected to form in 50B2O3–(50−x)PbO–xLiF glasses (10 ≤ x ≤ 40 mol%) [20]. Furthermore, formation of BO2/2F units is also possible. The 19F–7Li dipole–dipole coupling of 50B2O3–10PbO–40LiF glass is weaker than that of crystalline LiF. This result stands against considering formation of large LiF-like domain in these glasses. At the same time, it agrees well with the assumption that LiF contributes in the formation of the above structural units. The authors also assumed a random distribution of F− ions with respect to Pb2+ ions without preferential formation of PbF2-like domains.
Ayta et al. [21] investigated Li2O–B2O3–Al2O3 glasses containing CaF2. The compositions they investigated are [(50Li2O–45B2O3–5Al2O3) (mol%) + xCaF2 (wt%)], with 0 ≤ x ≤ 50. Thermoluminescence glow curves presented an evidence for CaF2-crystal formation in these glasses.
The above literature survey reveals that a little work has been carried out on alkaline earth oxyfluoro borate glasses. In addition, as far as the authors know, no work has yet been done on binary alkaline earth fluoroborate glasses. In this article, the structure of CaF2–B2O3 glasses would be explored by means of infrared spectroscopy. Density and electric conduction parameters would be correlated with the glass structure.
Experimental
Glasses having the formula xCaF2–(100−x)B2O3 (30 ≤ x ≤ 45 mol%) were prepared from reagent grade chemicals. Boric acid H3BO3 and calcium fluoride CaF2 were used as sources for the corresponding oxides. The glasses were prepared under normal atmospheric conditions by melting the mixture of raw materials in porcelain crucibles in an electric furnace for about 30 min at a temperature ranged between 800 and 900 °C depending on the composition. To get a homogeneous glass the melt was swirled from time to time. After the melt becomes homogeneous, it was poured on steel plates and then pressed by a ceramic plate to obtain glass disks at room temperature. The disks were used for the measurement of the electrical conductivity. Samples prepared by pouring the melt onto steel plates without pressing were used for the density measurement. All the obtained samples were visually homogeneous and transparent. The glass samples were kept in desiccators until required.
The amorphous nature of the samples was confirmed by X-ray diffraction investigations. A Bruker D8 Advance powder XRD was used. It is fitted with a Vantech Super Speed position sensitive detector and a Cu K α X-ray tube with a Gobel Mirror. Measurements were made over the range 4°–130° in 2-theta. X-ray diffraction patterns of all the studied glasses show only broad humps typical of amorphous materials.
The density (D) of glasses was determined at room temperature using the Archimedes method with xylene as an immersion fluid. At least, three samples of each glass were used to determine the density. Density values are precise to ±0.02 g/cm3.
For measuring the dc resistivity, polished disks with a thickness of about 1 mm were coated with silver paste to serve as electrodes. The resistance was measured using a type TM14 insulation tester (Levell Electronics Ltd, UK) with a range of 103–1013 Ω. Three samples of each glass were used to measure the resistance. The experimental error in determining the activation energy for conduction is estimated to be less than 0.025 eV, whereas the relative error in the conductivity is expected to be ±5%.
The infrared spectra of the glasses were recorded at room temperature using the KBr disk technique. A Mattson 5000 FTIR spectrometer was used to obtain the spectra in the wavenumber range between 400 and 2000 cm−1 with a resolution of 2 cm−1. At least two spectra for each sample were recorded. Infrared spectra were corrected for the dark current noises and background using the two-point baseline correction. After correction, the IR spectra were analyzed using the deconvolution method. More details about this method are reported elsewhere [22, 23].
Results
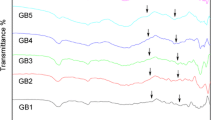
Figure 1 shows normalized IR spectra of the investigated xCaF2·(100−x)B2O3 glasses (30 ≤ x ≤ 45 mol%). There are two main strong absorption bands in the regions 800–1200 and 1200–1800 cm−1. In addition, a small absorption band appears around 700 cm−1 in all the spectra. There is mostly no change of band centers with composition of glass.
Figure 2 shows the dependence of density (D) and molar volume (V m) on the CaF2 content in CaF2–B2O3 glasses. There is a linear increase in the density, whereas a linear decrease is observed in V m. The increase in D reveals that the developed structural units, by replacing CaF2 for B2O3, are denser than the BO3 units. On the other hand, the decrease in V m indicates that the resultant volume of the formed structural units is smaller than that of the start glass (30CaF2·70B2O3).
Dependence of the density D and molar volume V m of the studied glasses on the CaF2 content. Open symbols represent the experimental values and the closed ones are calculated. Eq. 7 and the experimental D data were used to get experimental V m values. Estimated error limit in the latter is about 0.9%. Eqs. 6 and 8 are used to get the calculated D and V m values. Lines are fitting plots of the data
All investigated glasses show a linear dependence of the logarithm of conductivity (log σ) on the reciprocal of absolute temperature (1/T), Fig. 3. This a feature of ionic conduction process that can be described by the Arrhenius relation
In this relation σ 0 is a constant that depends on the glass composition and its thermal history, E is the activation energy for the conduction process, k is the Boltzmann’s constant and T is the absolute temperature.
Discussion
Infrared spectra
The IR spectra shown in Fig. 1 can be described based on the assignment of those of borate glasses modified with oxides. The absorption of borate glasses in the region ~850–1100 cm−1 is related to B–O bond stretching vibration of BO4 tetrahedra and the absorption peaks in the region ~1150–1600 cm−1 are attributed to stretches of B–O in BO3 units [24–28]. Formation of BO4 tetrahedra in oxide glasses results from modifying the borate matrix with the modifier oxide. In this study, where there is no modifier oxide, B2O3 is modified only with CaF2. The presented literature survey on the structure of fluorborate glasses reveals that, among various structural units that may form in such glasses, [BO3/2F]− distorted tetrahedra are suggested to be the single [10–12] or the most probably formed [6, 7, 15, 20] oxyfluoro borate species. In this study, four-coordinated boron atoms are assumed to be in the form of BO3F (B4 units) and the absorption in the region ~800–1200 cm−1 might be correlated with their vibrations.
IR spectra of various glasses [27–29] could be quantitatively analyzed by deconvoluting the absorption peaks into their individual component bands. Figure 4 shows, as an example, the deconvolution of IR spectrum of the glass 45CaF2·65B2O3.
Figure 5 shows a slight increase in the fraction N 4 of four-coordinated boron atoms with increasing the CaF2 content. The fraction N 4 of four-coordinated boron atoms can be calculated from the area under the component bands since the area is related to the concentration of the borate groups originating it. N 4 is defined as the ratio of [concentration of B4 units/concentration of (BO3 + B4) units]. The increase in N 4 indicates that BO3 units convert successively into B4 units. The values obtained of N 4 (Fig. 5) agree quite well with those found for CaO–B2O3 glasses by analyzing the IR spectra [30] and pulsed neutron diffraction and molecular dynamics studies [31]. N 4 values are nearly the same at similar concentration of CaF2 and CaO. Because of the comparable masses and nearly equal radii of fluorine and oxygen, drastic changes are not expected to be observed in the mid-IR spectra when introducing fluorine at the expanse of oxygen [32].
N 4 values obtained from the equation of fitting line in Fig. 5
can be employed to obtain the concentration of various structural units in the studied glasses. The quantity (B2O3)4 (mol%) of B2O3 converted into B4 units, is given as
where (B2O3) is the concentration of B2O3 in the glass (mol%). The quantity (B2O3)4 equals to the quantity, CaF2(m), of CaF2 that modifies B2O3 to B4 units at a rate of two units per CaF2 molecule. The rest of B2O3 that remains as BO3 units (B2O3)3, can be given by
In addition, the quantity CaF2(f), of CaF2 that enters the structure as network former is given as
In crystalline CaF2, Ca is tetrahedrally coordinated with F− ions [33]. It is, therefore assumed that CaF2(f) may be present in the structure in the form of CaF4 tetrahedra. Figure 6 shows X-ray diffraction patterns of crystalline CaF2 and CaF2–B2O3 glasses having 30, 40, and 45 mol% CaF2. There is coincidence of the 2θ values of the sharp peaks in the spectrum of crystalline CaF2 with the centers of the broad humps in the diffraction patterns of the glasses. This suggests that CaF2 is present in its amorphous form in the studied glasses. In Fig. 7 is shown the dependence on CaF2 content of (B2O3)4, (B2O3)3, and CaF2(f), as obtained from Eqs. 3–5, respectively. There is a linear decrease in both (B2O3)3 and (B2O3)4 while CaF2(f) increases linearly overall the CaF2 content. The decrease in both of (B2O3)3 and (B2O3)4 can mainly be attributed to the low rate of N 4 increase (Fig. 5) and partially to the decrease in the content of B2O3 when increasing that of CaF2. The change in the content of (B2O3)4 and CaF2(f) indicates that CaF2 tends preferentially to form its own matrix rather than modifying the borate network.
Density and molar volume
The information obtained from the IR spectra can be used to calculate D and V m. As previously indicated [34], the density of a glass can be expressed as
where n u is the number of the structural unit u per mole of glass. M u and V u are, respectively, the mass and volume of the structural unit.
As revealed from the IR spectra, the investigated glasses contain different structural units. These are symmetric BO3 units, B4 units and the network former units of CaF2. On the basis of Eq. 6 the density can then be expressed as
Here n 4, n 3 and n f represent, respectively, the number (per mole of glass) of B4, BO3, and the network former units of CaF2 (CaF4 tetrahedra). M f and V f are, respectively, the mass and volume of the former units, CaF2(f), of CaF2. The masses are taken as M 4 = (B + 1.5O + F + 0.5Ca), M 3 = (B + 1.5O) and M f = (Ca + 2F). To calculate n 4, n 3 and n f Eq. 1 would be used, where x is the CaF2 content in the glass expressed in mol%. N 4 values obtained from Eq. 1 can be used in Eqs. 3–5 to get n 3, n 4, and n f as
and
where N A is Avogadro’s number. The factor 2 appears in Eqs. 3a and 4a because each CaF2 converts two BO3 units into two B4 units and each B2O3 molecule produces two BO3 units.
The density of vitreous B2O3 is 1.838 g/cm3 [35] and thus a value of V 3 = 3.14 × 10−23 cm3 could be estimated. This value of V 3 has been found mostly the same in various borate glasses, such as PbO–B2O3 [36], Al2O3–PbO–B2O3 [29] and Na2O–Al2O3–B2O3 [37]. In the light of these findings, it is assumed that V 3 has the same value in CaF2–B2O3 glasses.
To get the volumes V 4 and V f (of B4 and the network former units of CaF2, respectively) it is adequate to start by analyzing the molar volume. The latter is given as
where M is the relative molecular mass of glass. In terms of the concentration and volume of structural units it can be expressed as
Taking V 3 = 3.14 × 10−23 cm3, Eq. 9 can be solved simultaneously for two V m values close to each other to get V 4 and V f. In the seek of getting reliable values, the equation of fitting line for the experimental data of V m in Fig. 2 would be used. This equation gives the molar volume as a function of the concentration x of CaF2 (mol%) as
Figure 8 shows the dependence of the volume of B4 and CaF2(f) on the CaF2 content. It is shown that V 4 is mostly constant whereas V f decreases linearly with increasing the content of CaF2. The decrease in V f reaches about 7% at 44 mol% CaF2. The average values of V 4 and V f are 3.10 × 10−23 and 4.32 × 10−23 cm3, respectively. These values, together with that of V 3, can be used, respectively, in Eqs. 7 and 9 to calculate both D and V m. Reasonable agreement between calculated and experimental data could be obtained. However, a better agreement (Fig. 2) could be attained by means of V 3 = 3.12 × 10−23 , V 4 = 3.1 × 10−23 and V f = 4.25 × 10−23 cm3. It is worthy to indicate that the density of CaF2 is 3.18 g/cm3 [38] that gives V f = 4.077 × 10−23 cm3. This reveals that the mean value of V f in the amorphous form is about 1.04 times that in the crystalline form. This result is consistent with the conclusions of Ayta et al. [21]. Thermoluminescence glow curves presented an evidence for CaF2-crystal formation in [(50Li2O–45B2O3–5Al2O3) (mol%) + xCaF2 (wt%)] glasses, with 0 ≤ x ≤50. The decrease in V f with increasing CaF2 content (Fig. 8) can be attributed to competition of the former CaF2(f) units to occupy space among other units in the glass matrix. The decrease in V f with increasing the CaF2 content (Fig. 8) reveals that CaF4 units tend to reach their volume in the pure crystalline form.
The difference observed between the volume of CaF4 units in the investigated glasses and in crystalline CaF2 is consistent with various studies. Watanabe et al. [39] investigated CaO–CaF2–SiO2 glasses (5 ≤ CaF2 ≤ 25 mol%) using 19F NMR. They reported that Ca–F clusters are formed and the cluster size, as well as the Ca–F distance, increase with increasing the CaF2 content. Brauer et al. [40] used 19F and 29Si MAS NMR to investigate SiO2–P2O5–Na2O–CaO–CaF2 glasses (0 ≤ CaF2 ≤ 32.7 mol%). It is deduced that fluorine does not form Si–F bonds. Instead, fluorine forms mixed sodium–calcium species. However, by increasing the CaF2 content, fluorine preferentially complexes Ca2+ rather than Na+ ions. They attributed the higher chemical shift of F–Ca(n), compared with F–Ca(4) or F–Ca(3) in crystalline species, to the slightly longer Ca–F distance in the amorphous form [41]. Recently, an investigation on the density of SiO2–P2O5–Na2O–CaO–CaF2 glasses (0 ≤ CaF2 ≤ 25.54 mol%) revealed that CaF2 enters the structure as CaF4 tetrahedra [42]. The mean volume found for CaF4 unit (4.142 × 10−23 cm3) is close of that in the present work.
The density of the studied glasses can be calculated by using the predicted volumes in Eq. 7. Figure 2 shows that the calculated density and molar volume agree well with the experimental data, which supports the structural view of the studied glasses.
Electric conduction
Figure 9 shows that there is a small change in both E and logσ 523 (log conductivity at 523 K) when changing the CaF2 content. Between 30 and 45 mol% CaF2 there is an increase of about one order in logσ 523. These features are greatly different from those of PbF2–B2O3 glasses [1], where a fast increase in logσ 473 (about six orders) takes place between 35 and 60 mol% PbF2. The marked change in the conductivity of PbF2–B2O3 glasses is attributed to the ease of transport of F− ions as charge carriers [1]. The small increase in logσ 523 and the activation energy (Fig. 9) can be taken as a basis to rule out the role of F− ions as the major type of charge carriers in the studied CaF2–B2O3 glasses. It is therefore to assume that the electric conduction in these glasses is mainly due to transport of Ca2+ ions rather than F− ions.
In Fig. 7 is shown that the content of CaF2(f) (the former CaF2) increases whereas that of B4 (the modifier CaF2) slightly decreases with increasing the concentration of CaF2. It can be indicated that the concentration of these components expressed as number/cm3 changes in the same manner. The increase in conductivity with increasing CaF2 content (Fig. 9) leads likely to consider Ca2+ ions in CaF2(f) being the main charge carriers in the studied CaF2–B2O3 glasses. Because of the small increase in conductivity it can be deduced that the mobility has a pronounced effect. The conductivity is generally given by [43] as
where n is the concentration of mobile ions expressed as (number/cm3), q is the ionic charge, and μ is the mobility of the charge carrier. It must be noted that the concentration n of mobile ions is just a fraction of ionic species that is assigned as charge carriers. This fraction is usually unknown and therefore it is not possible to determine the mobility of charge carriers from Eq. 11. To get an idea about the change of mobility with composition of glass it can be assumed that all Ca2+ ions in CaF2(f) are mobile ions. Thus, Eq. 11 can be used to calculate the mean mobility (\( \overline{\mu } \)) of Ca2+ ions. Figure 10 shows that, in spite of the marked increase (>four times) in the total concentration n Ca of Ca2+ ions in CaF2(f), the logarithm of \( \overline{\mu } \) decreases with increasing the CaF2 content. The decrease in \( \overline{\mu } \) can be correlated with the decrease in the molar volume (Fig. 2) and thus the free volume of glass when the CaF2 content increases (Fig. 11). The decrease in free volume can also be the reason that E does not change when changing the CaF2 content (Fig. 9).
In addition to the effect of decreasing free volume, another factor may contribute to the low conductivity and its limited increase between 30 and 45 mol% CaF2. This is the nature of binding of Ca2+ ions in the studied glasses. These ions are bound to F− ions either in [F−BO3/2], where the negative charge is localized at F− ion in the distorted [F−BO3/2] tetrahedron [15, 16], or in CaF4 tetrahedra. A reduction in the mobility of Na+ ions in xNa2O–y(NaF)2–0.64B2O3 and y(NaF)2–(1−y)B2O3 glasses were reported upon increasing the concentration of fluoride ions. The effect is attributed to a stronger interaction between Na+ ions and F− ions, compared to that with O2− ions [32]. Similar results were obtained by Jain et al. [44] upon replacing 1% of total oxygen concentration by fluorine in 0.35(Li, Na)2O·0.65B2O3 glasses. In this study, an increase in the CaF2 content means an increase F/O ratio and this leads to a decrease in the mobility of Ca2+ ions.
Conclusion
IR absorption in the region ~850–1200 cm−1 is assumed to be related to stretching vibrations of \( {\text{Ca}}_{1 /2}^{2 + } [{\text{BO}}_{3 /2} {\text{F]}}^{ - } \) units. A part of CaF2 is incorporated in the borate network to form these units. The rest of CaF2 forms its own matrix, presumably in the form of CaF4 tetrahedra. Calculated density agrees well with the experimental density. Calculated density has been obtained by considering that the studied CaF2–B2O3 glasses may contain BO3, \( {\text{Ca}}_{ 1 / 2}^{ 2+ } [{\text{BO}}_{ 3 / 2} {\text{F]}}^{ - } \)and CaF2(f) units. Calculated volume of the latter has mostly the same value as in the crystalline CaF2. Ca2+ ions are the main charge carriers in the studied glasses. A limited increase in conductivity is related to the decrease in the mean mobility, in spite of the increase in the total concentration of Ca2+ ions. The decrease in the mean mobility is attributed to a decrease in the free volume with increasing the CaF2 content.
References
Gressler CA, Shelby JE (1988) J Appl Phys 64:4450
Gressler CA, Shelby JE (1989) J Appl Phys 66:1127
Wang Y, Osaka A, Miura Y (1989) J Mat Sci Lett 8:421
Gopalakrishnan R, Tan KL, Chowdari BVR, Vijay AR (1994) J Phys D Appl Phys 27:2612
Chowdari BVR, Rong Z (1995) Solid State Ionics 78:133
Kline D, Bray PJ (1966) Phys Chem Glasses 7:41
Jager Chr, Haubenreisser U (1985) Phys Chem Glasses 26:152
Suresh S, Chandramouli V (2004) Indian J Pure Appl Phys 42:560
Huang Y, Jang K, Wang X, Jiang C (2008) J Rare Earths 26:490
Shelby JE, Baker LD (1998) Phys Chem Glasses 39:23
Shelby JE, Downiel K (1989) Phys Chem Glasses 30:151
Shelby JE, Ortolanor L (1990) Phys Chem Glasses 31:25
Souto S, Massot M, Balkanski M, Royer D (1999) Mater Sci Eng B 64:33
Sokolov IA, Naraev VN, Nosakin AN, Pronkin AA (2000) Glass Phys Chem 26:383
Pronkin AA, Naraev VN, Eliseev SYu (1988) Fiz Khim Stekla 14:926 cited in [14]
Pronkin AA, Naraev VN, Tsoi TB, Eliseev SYu (1992) Sov J Glass Phys Chem Engl Transl 18:304 cited in [14]
Sokolov IA, Naraev VN, Nosakin AN, Pronkin AA (2000) Glass Phys Chem 26:584
Hager IZ, ElHofy M (2003) Phys Stat Sol A 198:7
Maya L (1977) J Am Ceram Soc 60:323
Cattaneo AS, Lima RP, Tambelli CE, Magon CJ, Mastelaro VR, Garcia A, de Souza JE, de Camargo ASS, de Araujo CC, Schneider JF, Donoso JP, Eckert H (2008) J Phys Chem C 112:10462
Ayta WEF, Silva VA, Dantas NO (2010) J Lumin 130:1032
Moustafa YM, Doweidar H, El-Damrawi G (1994) Phys Chem Glasses 35:104
Moustafa YM, El-Egili K (1998) J Non Cryst Solids 240:144
Kamitsos EI, Patsis AP, Karakassides MA, Chryssikos GD (1990) J Non Cryst Solids 126:52
Kamitsos EI, Karakassides MA, Chryssikos GD (1987) J Phys Chem 91:1073
Kamitsos EI, Karakassides MA, Chryssikos GD (1987) Phys Chem Glasses 28:203
Doweidar H, Saddeek YB (2009) J Non Cryst Solids 355:348
Doweidar H, Saddeek YB (2010) J Non Cryst Solids 356:1452
Doweidar H, El-Egili K, El-Damrawi G, Ramadan RM (2008) Phys Chem Glasses 4(9):271
Yiannopoulos YD, Chryssikos GD, Kamitsos EI (2001) Phys Chem Glasses 42:164
Ohtori N, Takase K, Akiyama I, Suzuki Y, Handa K, Sakai I, Iwadate Y, Funukaga T, Umesaki N (2001) J Non Cryst Solids 293–295:136
Kamitsos EI, Karakassides MA (1988) Solid State Ionics 28–30:783
Web Elements Periodic Table of Elements. http://www.webelements.com
Doweidar H (2009) J Non Cryst Solids 355:577
Shaw RR, Uhlmann DR (1969) J Non Cryst Solids 1:474
Doweidar H, Oraby AH (1997) Phys Chem Glasses 38:69
Doweidar H, Moustafa YM, Abd El-Maksoud S, Silim H (2001) Mater Sci Eng A 301:207
Leide DR (ed) (2005) CRC Handbook of Chemistry and Physics, 85th edn. CRC Press, Boca Raton
Watanabe T, Hayashi M, Hayashi S, Fukuyama H, Nagata K (2004) VII International Conference on Molten Slags Fluxes and Salts, The South African Institute of Mining and Metallurgy, 699
Brauer DS, Karpukhina N, Lawb RV, Hill RG (2009) J Mater Chem 19:5629
Boden N, Kahol PK, Mee A, Mortimer M, Peterson GN (1983) J Magn Reson 54:419
Brauer DS, Al-Noaman A, Hill RG, Doweidar H (2011) Mater Chem Phys 130:121
Kittel C (1996) Introduction to Solid State Physics, 7th edn. Wiley, New York
Jain H, Downing HL, Peterson NL (1984) J Non Cryst Solids 64:335
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doweidar, H., El-Damrawi, G. & Abdelghany, M. Structure and properties of CaF2–B2O3 glasses. J Mater Sci 47, 4028–4035 (2012). https://doi.org/10.1007/s10853-012-6256-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-012-6256-y