Abstract
The effect of Na substitution on the formation and superconductivity of Tl-1212-type phase (Tl0.85Cr0.15)Sr2−xNaxCaCu2O7 (x = 0–0.30) is reported. The samples were prepared via the solid-state reaction method. X-ray diffraction patterns showed the presence of Tl-1212-type phase or Ca0.3Sr0.7CuO2 (CSCO) as a major or minor phase in the samples. The resistance versus temperature curves showed onset transition temperature, Tc-onset between 97 and 106 K and zero-resistance temperature, Tc-zero between 88 and 99 K. AC susceptibility measurement showed superconducting transition \({T_{{\text{c}}{\chi ^\prime }}}\) between 89 and 98 K. The peak temperature, Tp, of the imaginary part of the susceptibility, χ″, was the highest for x = 0.02 sample (Tp = 96 K). The inter-grain critical current density, Jc, at Tp for x = 0 sample was Jc (Tp = 68 K) = 14 A cm−2 and for x = 0.02, 0.20 and 0.30 Jc (Tp) at 96, 96, and 88 K, respectively, is 19 A cm−2. Na substitution increased the grain size of the Tl-1212 samples. All Na-substituted samples showed higher transition temperature, enhanced critical current density, and improved inter-grain coupling compared with the non-substituted sample. This work showed that other than rare-earth elements, monovalent alkali metal such as Na could also be used to enhance the superconducting properties of the Tl-1212 phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Since the discovery of the Tl-based high-temperature superconductor by Sheng and Hermann [1], many studies have been done to optimize the transition temperature. Many new superconducting phases have been reported including TlSr2CaCu2O7. The Tl-1212-type phase such as TlBa2CaCu2O7 and TlSr2CaCu2O7 has a similar structure with YBa2Cu3O7 [2]. TlSr2CaCu2O7 is superconducting at 80 K [3]. Several improvements have been made to enhance the superconducting properties of the Tl-1212-type phase. Substitution of rare earth with trivalent ions in TlSr2(R,Sr)Cu2O7 (R = rare earths) improved the transition temperature, Tc, to 90 K [4]. Cr-substituted TlSr2CaCu2O7 shows Tc over 100 K and Cr was reported to reside at the Tl site. The composition (Tl0.85Cr0.15)Sr2CaCu2O7 is one of the highest Tc of the Tl-1212 phase [5]. The Tl-1212 type phase is of interest due to its simple preparation, high quality, and sharp superconducting transition [5].
Elemental substitutions have been performed to stabilize the superconducting phase of copper-oxide-based materials. The effects of metals such as Zn and Mn [6] and rare-earth elements [7] on (Tl0.85Cr0.15)Sr2CaCu2O7 have been reported. There is also a study on the co-precipitation method to prepare ultrafine (Tl0.85Cr0.15)Sr2CaCu2O7 powders [8]. The past years have seen a growing effort to improve Tc of the Tl-1212-type phase by substitution of trivalent ions particularly the rare-earth elements [4, 8]. Alkali metals substituted TlSr2CaCu2O7 without Cr does not show superconductivity [9]. CdTe semiconductor has also been used to improve the Tl2Ba2CaCu2O8 (Tl-2212) superconductor [10].
Substitution of monovalent alkali metals such as Na into Tl-based high-temperature superconductors has not been reported extensively. Recent research has been carried out on alkali metal substitution into Tl-1223 [11,12,13] and Tl-1212 [14]-type phase. Na has one electron in its outer shell, which it can simply lose to get a stable energy and become reactive. Most studies of Na substitution/addition have only focused on Bi-based superconductors [15,16,17,18,19,20,21,22,23]. Na substitution/addition into Bi2Sr2CaCu2O8 (Bi-2212) superconductors increased the Tc, critical current density, Jc, and grain size. Na-doped (Cu0.5Tl0.25Na0.25)Ba2CaMCu3O10-δ (M = Be, Mg) superconductors increased Tc up to 88 K and enhanced the superconducting properties [24]. The ionic radius of Na1+ with coordination number (CN) 4–12 is 0.99–1.39 Å. This radius overlaps with Sr2+, whose ionic radius with CN 6–12 is 1.18–1.44 Å [25].
The objective of this study was to investigate the effect of alkali metal, Na on the formation and superconductivity of (Tl0.85Cr0.15)Sr2−xNaxCaCu2O7 for x = 0–0.30. Powder X-ray diffraction and DC electrical resistance versus temperature measurements together with Field-Emission Scanning Electron Microscope (FESEM) results are presented. AC susceptibility measurements were also performed to determine the transition temperature, \({T_{{\text{c}}{\chi ^\prime }}}\), and inter-grain critical current density. This study provides insights into the formation and superconductivity of Tl-1212-type phase by substitution of an alkali metal, Na. We found that Na improved the transition temperature and inter-grain critical current density.
2 Experimental details
Samples with nominal composition (Tl0.85Cr0.15)Sr2−xNaxCaCu2O7 (x = 0, 0.02, 0.05, 0.07, 0.10, 0.15, 0.20, and 0.30) were prepared using the solid-state reaction method. Appropriate amounts of high-purity (99.9+%) powders of SrCO3 (or SrCO), CaO (or CaCO3), CuO, and Na2CO3 were mixed and ground in an agate mortar. The powders were heated for 48 h at 800 °C with several intermittent grindings to obtain a homogenous powder. Appropriate amounts of Tl2O3 and Cr2O3 were then added to the precursor and completely mixed. The powders were then pressed into pellets with 13 mm diameter and 2 mm thickness. The pellets were then placed in an alumina boat and heated in a tube furnace at 1000 °C in flowing oxygen for 4 min followed by furnace cooling to room temperature.
The powder X-ray diffraction method was used to determine the resultant phases. A Bruker model D8 Advance diffractometer with CuKα \({\text{CuK}}_{{\upalpha }}\) source was used. The lattice parameters were calculated by employing at least ten diffraction peaks. The percentage of the volume fraction for Tl-1212 and Ca0.3Sr0.7CuO2 (CSCO) phase has been estimated using the formula:
where ∑I is the sum of intensity of each phase.
Field-Emission Scanning Electron Microscope (FESEM) micrographs were recorded using a Merlin Gemini scanning electron microscope. The chemical composition of the samples was analyzed by Energy-Dispersive X-Ray Analysis (EDX) using an Oxford instrument analyzer.
The DC electrical resistance versus temperature measurements were carried out using four-probe method with silver paste contacts in conjunction with a closed cycle refrigerator from CTI Cryogenics Model 22 and a temperature controller from Lake Shore Model 330. A constant current source between 1 and 100 mA was used throughout the measurements.
The AC susceptibility measurements were performed using an AC susceptometer from Cryo Industry model number REF-1808-ACS. The samples were cut into bar shape for this measurement. The frequency of the AC signal was 295 Hz and the applied magnetic field was H = 5 Oe. The critical current density Jc at the peak temperature Tp of χ″ was calculated using the Bean model with formula Jc(Tp) = H/(lw)1/2, where H is the applied field and l and w are the dimensions of the cross section of the bar-shaped sample.
3 Results and discussion
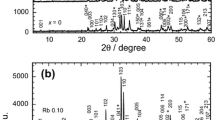
The X-ray powder diffraction patterns of (Tl0.85Cr0.15)Sr2−xNaxCaCu2O7 superconductor are presented in Fig. 1a for x = 0, 0.02, 0.05, and 0.07 and Fig. 1b for x = 0.10, 0.15, 0.20, and 0.30 composition. The x = 0, 0.02, 0.07, and 0.15 samples exhibited the Tl-1212 phase with tetragonal structure (space group P4/mmm) as the major phase and x = 0.05, 0.10, 0.20, and 0.30 samples exhibited Ca0.3Sr0.7CuO2 with orthorhombic structure [space group Cmcm (63)] as the major phase. Earlier studies had reported (Ca,Sr)CuO as a minor phase in Bi-2223 [17] and Bi-2212 [22] superconductors. It is interesting to note that the CSCO phase also exists in the Tl-1212 superconductor. Previous works showed that Na entered the Tl-1223 [13, 23], Tl-1212 [14], and Bi-2212 [16,17,18, 21,22,23] crystal structure. No unreacted Na2CO3 was observed in our XRD patterns, which indicated that Na might have entered the Tl-1212 crystal structure. The Tl-1212-type phase can be improved by the appropriate amount of Na substitution, which is x = 0.02 and 0.07. An extra peak from (Ca0.9Sr0.1CuO2) at 2θ = 36.4° is observed in Fig. 1b, as Na was increased to x = 0.20 and 0.30.
The volume fraction of CSCO (VCSCO) was found to be higher than the Tl-1212 phase (VTl-1212) in some samples. Sample with x = 0.05 and 0.10 showed the highest amount of CSCO. The CSCO phase was also observed in sample synthesized using the single-sample synthesis concept based on multi-element ceramic. In samples prepared with seven components: BaO2, CaCO3, SrCO3, La2O3, PbCO3, ZrO2, and CuO oxides and carbonates, the highest Tc was observed at 118 K due to the Pb-1223 phase [26]. CSCO was also observed in these samples.
Figure 2a, b shows the FESEM micrographs for x = 0 and 0.10, respectively. The FESEM micrographs showed that the grain size increased with the increase of Na. This result is in agreement with recent studies, which showed that the grain size increased with increasing Na substitution/addition [14,15,16,17,18,19,20,21,22,23,24]. As shown in Fig. 2a, partial melting was observed in the Na-free sample. A possible explanation for this might be due to the final sintering temperature (1000 °C), which is higher than the volatization point of Tl2O3 (717 °C).
Figure 3a, b shows the elemental analysis using EDX spectra for x = 0 and 0.10 samples. Insert shows the weight percent of the elements in each sample. EDX spectrum of the x = 0.10 sample in Fig. 3b confirmed the presence of Na.
The onset transition temperature, Tc-onset, is defined as the temperature, where there is a sudden drop in resistance and can be obtained from the crossing point of the linear fit of the highest slope and the metallic high-temperature part of the ρ(T) curve for each sample. The zero-resistance temperature, Tc-zero, is the temperature, where the resistance drops to zero and can be estimated from the extrapolation of the linear part of the resistivity to the temperature axis. The normalized resistance versus temperature curves show that the onset transition temperature (Tc-onset) was between 97 and 106 K for x = 0 to 0.30 (Fig. 4). The zero-resistance temperature (Tc-zero) was between 88 and 99 K. The normal state resistance versus temperature curves showed a metallic behavior for all samples. The electrical resistivity at room temperature for x = 0 was around 1.94 mΩ-cm. When Na was substituted, the resistivity increased to around 7.84 mΩ-cm for the x = 0.10 sample and then decreased to 3.3 mΩ-cm for the x = 0.3 sample. The room temperature resistivities ρ297K, Tc-onset, and Tc-zero for all samples are shown in Table 1. The x = 0.02 sample showed the highest transition temperature and critical current density.
The transition temperature as measured by AC susceptibility (\({T_{{\text{c}}{\chi ^\prime }}}\)) was determined from the sudden decrease in the real part χ′ of the complex susceptibility (χ = χ′ + iχ″). The sudden decrease in χ′ below the transition temperature was due to diamagnetic shielding and can be identified as the onset of bulk superconductivity. The peak temperature (Tp) in the imaginary part of the susceptibility χ″ represents the AC losses. Tp shifts depending on the pinning force strength. The weaker the pinning, the larger Tp shift to lower temperature. Below Tp, the amplitude of χ″ falls due to the decrease in flux penetration. This can be seen from the graph of AC susceptibility versus temperature of (Tl0.85Cr0.15)Sr2−xNaxCaCu2O7 (Fig. 5).
The transition temperature (\({T_{{\text{c}}{\chi ^\prime }}}\)) measurement for all samples was around 89–98 K (Fig. 5). \({T_{{\text{c}}{\chi ^\prime }}}\) changed slightly with Na substitution. The peak temperature, Tp, of the imaginary part of the susceptibility χ″ increased with Na substitution indicating the enhanced inter-grain coupling between the superconducting grains.
At Tp, the AC field amplitude is equal to the full flux penetration field. Two losses are expected observed in large AC fields that include a broad peak at low temperature due to coupling losses and a narrower peak due to intrinsic losses. However, our measurements in H = 5 Oe did not show peak signifying the intrinsic loss due to small inter-granular potential and no flux penetration into the grains due to the low field.
Table 1 shows Tc-onset, Tc-zero, ρ297K, \({T_{{\text{c}}{\chi ^\prime }}}\), Tp, and the inter-grain critical current density Jc (Tp) of the samples. The inter-grain critical current density Jc at the peak temperature Tp of χ″ was calculated using the Bean model [27]. Jc (Tp = 68 K) for x = 0 is 14 A cm−2. All the substituted samples showed increase in Jc (Tp). This may due to the enlargement of the grain size (Fig. 2), as Na was substituted.
In conclusion, the effect of Na substitution on the formation and superconductivity of Tl-1212-type phase (Tl0.85Cr0.15)Sr2−xNaxCaCu2O7 (x = 0–0.30) was investigated. Substitution of Na at Sr-site in (Tl0.85Cr0.15)Sr2−xNaxCaCu2O7 was found crucial in the formation of Tl-1212 type phase. The x = 0, 0.02, 0.07, and 0.15 samples showed the Tl-1212 as the dominant phase with the x = 0.02 showing the highest transition temperature and critical current density. All Na-substituted samples showed higher transition temperature and enhanced critical current density and improved inter-grain coupling compared with the non-substituted sample (x = 0). The grain size also increased with Na substitution resulting in an increase in Tp and Jc (Tp). This work showed that instead of using the trivalent rare-earth elements, monovalent alkali metal Na can also be used to improve the superconducting properties of the Tl-1212 phase.
References
Z.Z. Sheng, A.M. Hermann, Nature 332, 138 (1988)
S. Nakajima, M. Kikuchi, Y. Syono, N. Kobayashi, Y. Muto, Physica C 168, 57 (1990)
Z.Z. Sheng, A.M. Hermann, D.C. Vier, S. Schultz, S.B. Oseroff, D.J. George, R.M. Hazen, Phys. Rev. B 38, 7074 (1988)
Z.Z. Sheng, Y. Xin, D.X. Gu, J.M. Meason, J. Bennett, D. Ford, D.O. Pederson, Y. Xin, Z. Phys. B 84, 349 (1991)
Z.Z. Sheng, Y. Gu, Y. Xin, Y. Xin, L.W. Finger, C.G. Hadidiacos, R.M. Hazen, Mod. Phys. Lett. B 5, 635 (1991)
R. Abd-Shukor, S.V. Lo, J. Mater. Sci. Lett. 20, 159 (2001)
R. Abd-Shukor, N.A.N. Jaafar, J. Mater. Sci. Mater. Electron. 10(9), 677 (1999)
I. Hamadneh, Y.W. Kuan, L.T. Hui, R. Abd-Shukor, Mater. Lett. 60, 734 (2006)
I.P. Abu Bakar, K. Muhammad-Aizat, J. Nur-Akasyah, M.S. Mohd-Syahmi, N.R. Mohd Suib, W.S. Chiu, R. Abd-Shukor, Appl. Phys. A 125, 21 (2019)
K. Muhammad-Aizat, R. Abd-Shukor, Sains Malays. 47, 1579 (2018)
N.A. Khan, S. Hussain, Physica C 470, 51 (2010)
M. Mumtaz, N.A. Khan, A. Ullah, J. Low Temp. Phys. 163, 203 (2011)
N. Hassan, M. Irfan, N.A. Khan, S. Khan, A. Shakoor, A. Majid, A.M. Jadoon, S.S. Hayyat, J. Appl. Phys. 111, 053914 (2012)
J. Nur-Akasyah, R. Abd-Shukor, J. Mater. Sci. Mater. Electron. 29(5), 3690 (2018)
B.G. Krishna, R. Gundakaram, S.V. Suryanarayana, R.D.K. Misra, Physica C 200, 425 (1992)
B. Özkurt, J. Mater. Sci. Mater. Electron. 24(7), 2426 (2013)
B. Özkurt, J Mater Sci. Mater. Electron. 25(8), 3295 (2014)
B. Özkurt, J. Supercond. Nov. Magn. 28(5), 1501 (2015)
M. Gürsul, A. Ekicibil, B. Özçelik., M.A. Madre, A. Sotelo, J. Supercond. Nov. Magn. 28(7), 1913 (2015)
B. Özçelik, M. Gürsul, A. Sotelo., M.A. Madre, J. Mater. Sci. Mater. Electron. 26(5), 2830 (2015)
M. Çalɪş, B. Özkurt, M.E. Aytekin, E. Gün, M.E. Kɪr, U. Öztornacɪ, J. Mater. Sci. Mater. Electron. 27(3), 2670 (2016)
B. Özçelik, O. Nane, A. Sotelo, H. Amaveda, M.A. Madre, J. Mater. Sci. Mater. Electron. 28(8), 6278 (2017)
B. Özkurt, J. Mater. Sci. Mater. Electron. 28(12), 8857 (2017)
N.A. Khan, S. Hussain, J. Alloy Compd. 475(1–2), 652 (2009)
R.D. Shannon, Acta Cryst. A32, 751 (1976)
M. Iranmanesh, N.D. Zhigadlo, T. Tohsophon, J.R. Kirtley, W. Assenmacher, W. Mader, J. Hulliger, Solid State Sci. 88, 6 (2019)
C.P. Bean, Rev. Mod. Phys. 31, 36 (1964)
Acknowledgements
This research was supported by Universiti Kebangsaan Malaysia under grant number AP-2015-006 and the Ministry of Higher Education of Malaysia under Grant no. FRGS/1/2017/STG02/UKM/01/1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nur-Akasyah, J., Muhammad-Aizat, K., Mohd-Suib, N.R. et al. Effect of alkali metal Na on the formation and superconductivity of Tl-1212-type phase (Tl0.85Cr0.15)Sr2−xNaxCaCu2O7. Appl. Phys. A 125, 202 (2019). https://doi.org/10.1007/s00339-019-2502-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-019-2502-3