Abstract
The effects of rare-earth elements M = Er, Gd and La, and alkali metals M = Na, K and Rb substitution on (Tl0.7M0.3)Sr2CaCu2O7 (Tl-1212) are reported. The rare-earth elements were chosen by considering the ionic radius, i.e., from the smallest (Er+3) to the largest (La+3) ion. The samples were prepared via the solid-state reaction method. X-ray diffraction patterns showed the presence of major Tl-1212 and minor Tl-1201 and Ca0.3Sr0.7CuO2 phase in almost all samples. The resistance versus temperature curves for the rare-earth-substituted samples showed onset temperature between 87 and 90 K and zero resistance temperature between 69 and 81 K. Alkali metal substitutions exhibited Tl-1212 phase but showed no superconducting transition although the electrical resistivity was much lower than the rare-earth-substituted samples. AC susceptibility measurement showed superconducting transition Tcχ, between 59 and 80 K for the rare-earth element-substituted samples. The average grain size, the transition temperature, Tcχ and the peak temperature Tp of the imaginary part of the susceptibility χ″ decreased as the ionic radius of the rare earths was reduced.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The thallium-based cuprate superconductor has been of interest due to the high transition temperatures. It can form several phases with different number of CuO or TlO layers [1,2,3]. The TlSr2CaCu2O7 and TlBa2CaCu2O7 also known as Tl-1212 phase is superconducting between 70 and 80 K [4]. The Tl-1212 phase structure is analogue to the widely studied YBa2Cu3O7 [5]. Synthesis of Tl-1212 phase in single form is not easy due to the high average Cu (+ 2.5) valence and over-doped hole state. Reducing the hole carriers concentration to the optimal value (+ 2.3) can stabilize the Tl-1212 superconducting phase [6]. Partial substitution of elements with higher valences at different sites in TlSr2CaCu2O7 enhances the superconducting properties and stabilizes the Tl-1212 phase [7]. The appropriate ionic radius of the substituted elements is crucial in the formation of the Tl-1212 phase and to enhance the transition temperature, Tc [8, 9].
There are several reports on the rare-earth elemental substitution with trivalent ions, which enhances Tc of TlSr2CaCu2O7. Substitution of Gd, Er, La at Ca site of Tl0.5Pb0.5Sr2CaCu2Ox facilitated the formation of Tl-1212 phase with Tc between 105 and 109 K [10,11,12]. La substitution at Sr site of Tl0.5Pb0.5Sr2CaCu2Ox improved Tc up to 105 K [12]. Gd and La substitutions in TlBa2CaCu2O7 increased Tc from 80 to 110 K [5, 13]. Rare-earth (R) substitution in TlSr2(Sr,R)Cu2O7 − δ exhibited superconducting transition at 90 K with purity of Tl-1212 phase over 90% [14, 15]. Substitution of Er in TlBa2Ca2Cu3O9 − δ suppresses Tc with increasing Er content [16]. Rare-earth elemental substituted (Tl,Pb)Sr2Ca2Cu3O9 enhances the formation of the Tl-1223 phase with Tc between 75 and 115 K [17]. In Tl2Ba2CaCu2O8 added with diamagnetic CdTe, improvement in the superconducting properties was also observed [18].
There are also reports on the substitution and addition of alkali metals in the Tl-1212 phase. Substitution of A = Li, Na and Rb into (Tl0.85Cr0.15)Sr2 − xAxCaCu2O7 enhances the inter-grain critical current density and increased the grain size [19]. It was also suggested that Rb+1 was substituted at the Tl+1 site, and Li and Na were not substituted at any site in the Tl-1212 phase of (Tl0.85Cr0.15)Sr2 − xAxCaCu2O7 [19]. Na, K, and Rb added Tl2Ba2Ca2Cu3O11 (Tl-2223) phase improved Tc to over 113 K with purity of Tl-2223 phase above 60% [20]. There is no significant change in Tc for Rb-substituted TlBa2SrCu2O7 [21]. The formation of Tl-1223 phase increased significantly with increasing K content [22]. Appropriate amounts of Li addition facilitated the formation of Tl-2223 phase and improved Tc [23].
In this paper, we report the effects of rare-earth elements (M = Er, Gd and La) and alkali metals (M = Na, K and Rb) substitution on (Tl0.7M0.3)Sr2CaCu2O7. The ratio 0.3 was chosen because in general the Tl-1212 phase shows optimal superconducting transition between 0.15 (for Cr substitution) and 0.5 (for rare-earth substitution). Hence, in this work 0.3 was chosen because it lies between these optimal ratios for the different elements. The motivation of this study was to investigate the effect of different ionic radius of rare-earth elements and alkali metals on the superconductivity of the Tl-1212 phase. Hence, the objectives of this study were to determine if the substitution of trivalent rare-earth elements and monovalent alkali metals with different ionic radius could stabilize the Tl-1212 phase and produces a higher transition temperature. The effective ionic radius with six-fold coordination number (CN) of La+3 (1.032 Å) is the largest, followed by Gd+3 (0.93 Å) and Er+3 (0.89 Å). Ionic radius of alkali metal Rb+1 (1.52 Å) is the largest followed by K+1(1.38 Å) and Na+1(1.02 Å).
The phases and microstructure were determined by X-ray diffraction method and field emission scanning electron microscope (FESEM), respectively. The chemical composition was determined by energy-dispersive X-ray analysis (EDX). The DC electrical resistance was measured by four-probe method. The transition temperature (Tcχ′) and inter-grain current density were determined by AC susceptibility measurement.
2 Experimental details
The samples were prepared by the solid-state reaction method with nominal starting composition (Tl0.7M0.3)Sr2CaCu2O7 with M = Er, Gd, La, Na, K and Rb. A precursor was prepared by mixing stoichiometric amounts of high purity (> 99%) powders of SrO, CaO, CuO, Rb2CO3, K2CO3, Na2CO3, Gd2O3, Er2O3 and La2O3 in an agate mortar. The powders were heated in air at 900 °C for 24 h followed by furnace cooling to room temperature with several intermittent grindings. The powders were then sintered at 900 °C for 24 h to obtain a homogenous black powder. Tl2O3 with an appropriate amount was added to the precursor and mixed completely. Excess 10% of Tl2O3 was added to compensate for thallium lost during the heating process. The mixed powders were pressed into pellets with 13 mm diameter and 2 mm thickness and heated in a tube furnace at 1000 °C in flowing O2 for 5 min followed by furnace cooling to room temperature.
The phase was identified by X-ray powder diffraction method using Bruker D8 Advance Diffractometer with CuKα source. At least ten diffraction peaks were employed to calculate the lattice parameters. Field emission scanning electron microscope (FESEM) micrographs were obtained using a Merlin Gemini scanning electron microscope. The chemical composition of the samples was identified by energy-dispersive X-ray analysis (EDX) using an Oxford Instrument analyser.
The DC electrical resistance versus temperature measurements were carried out using four-probe technique with silver paste contacts. A closed-cycle refrigerator from CTI Cryogenics Model 22 and a temperature controller from Lake Shore Model 340 were used for electrical resistance–temperature measurements.
AC susceptibility was measured by a susceptometer from Cryo Industry model number REF-1808-ACS. The samples were cut into bar shape for the measurement. The frequency of the AC signal was 295 Hz and the magnetic field was Ha = 5 Oe. The critical current density Jc at the peak temperature Tp of χ″ was calculated using the Bean model [24] with the formula Jc(Tp) = Ha/(lw)1/2 where Ha is the magnetic field, l and w are the dimensions of the cross section of the bar-shaped sample.
3 Results and discussion
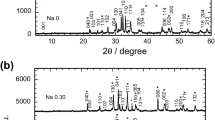
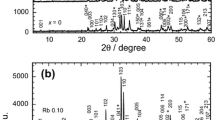
The X-ray powder diffraction patterns of the rare-earth elemental and alkali metal-substituted samples are shown in Fig. 1a and Fig. 1b, respectively. The pattern for non-substituted TlSr2CaCu2O7 is shown in Fig. 1b. All samples showed Tl-1212, Tl-1201 and Ca0.3Sr0.7CuO2 (CSCO) phase. The reflection peaks due to the Tl-1201 and CSCO phases are indicated with (*) and (#), respectively. The Tl-1212 phase with tetragonal unit cell (space group, P4/mmm) is dominant for all samples except for Rb-substituted sample. The Rb-substituted sample showed CSCO as the major phase with minor phases of Tl-1212 and Tl-1201.
The lattice parameter for Rb-substituted sample is a = 3.781 Å and b = 12.076 Å. The c lattice parameter decreased as K and Na were substituted and this may be due to K+1(1.38 Å) and Na+1(1.02 Å) were substituted for larger Tl+1(1.50 Å). A variation of a lattice parameter was observed in K- and Na-substituted samples, which indicated that the K+1(1.38 Å) and Na+1(1.02 Å) might be substituted into other ion sites such as Sr+2(1.18 Å), Ca+2(1.00 Å) and Cu+2(0.73 Å) due to the proximity of the ionic radii. However, these assertions need to be supported by a more direct method.
Figures 2 and 3 show SEM micrographs and statistic histograms of the grain size of (Tl0.7M0.3)Sr2CaCu2O7 for M = (a) Er, (b) Gd, (c) La, (d) Na, (e) K, and (f) Rb-substituted samples. The average grain size of all substituted samples is shown in Table 1. The average grain size was calculated based on 100 grains using the ImageJ software. The average grain size for K-substituted sample is the highest which is 1.47 µm. Rb-substituted sample showed the smallest average grain size (0.14 µm) indicating that Rb hindered the grain growth. The average grain size for rare-earth-substituted samples was about 1.21–0.49 µm. It was observed that the average grain size for rare-earth-substituted samples decreased as the ionic radius of the rare-earth was reduced. The Er- and La-substituted samples showed a higher porosity compared with other substituted samples. All substituted samples showed a closed packed microstructure. Generally, the grain size differed drastically between the rare-earth-substituted samples and alkali metal-substituted samples. The differences in texture and grain connectivity were observed that affected the transport current density, which are discussed below.
The EDX spectra and weight percent of one superconducting sample (a) La and two non-superconducting samples, (b) K, and (c) Rb substitution are shown in Fig. 4. The peaks of La, K and Rb were observed in the spectra besides Tl, Sr, Ca, Cu, and O peaks. Insert shows the weight percent of the corresponding elements. The EDX analysis showed the Tl-1212 chemical composition with some variations due to the limitation of EDX in determining light elements such as oxygen and also the presence of other phases such as Tl-1201 and Ca0.3Sr0.7CuO2.
The electrical resistance versus temperature curves for rare-earth-substituted samples are shown in Fig. 5a. The curves for alkali metals and non-substituted samples TlSr2CaCu2O7 are shown in Fig. 5b. Metallic normal state behavior above the onset transition temperature, Tc-onset was exhibited by all rare-earth elemental substituted samples. The Rb-, K- and Na-substituted samples also showed metal-like normal state properties, but no superconducting transition was observed. The non-substituted TlSr2CaCu2O7 showed anomalous behavior at 75 K. However, it did not show any superconducting transition down to 40 K. All rare-earth elemental substituted samples showed superconducting transition between 87 K and 91 K. The highest transition temperature was for Er substitution with Tc-onset = 91 K and zero transition temperature, Tc-zero = 75 K. However, no superconducting transition was observed for alkali metal substitutions. This can be explained in term of valence states of elements. Alkali metal is monovalent element (+ 1) while Tl is assumed to be in trivalent state (+ 3). Our results show that substitution of lower valence states elements into higher valence states elements suppressed the superconductivity. This result agreed with previous study that showed a higher valence substitution can stabilized Tl-1212 phase in contrast with lower valence substitution [7]. Thus, substitution of rare-earth elements enhanced Tc of TlSr2CaCu2O7 in contrast with alkali metals substitution, which did not show superconducting transition down to 50 K. Rare-earth elemental substituted samples showed lower Tc compared to previous studies [11,12,13,14,15,16,17] (Tc > 100 K) due to the fact that the carrier concentration is not optimized in this study. The purpose of this study was to compare the effect of trivalent rare-elements and monovalent alkali metals with the same stoichiometric ratio on superconductivity of Tl-1212 phase. Thus, the optimal carrier concentration is not essential.
The electrical resistivity at room temperature for Gd substitution was the highest (35.583 mΩ-cm) and the lowest was K substitution (0.004 mΩ-cm). In general, rare-earth substitutions exhibited higher electrical resistivity at room temperature compared to alkali metal substituted samples. Usually low normal state electrical resistivity is required to induce superconductivity in the cuprates. However, in this work we found that although the alkali metal-substituted samples exhibited a much lower room temperature resistivity (ρ297K) compared with the rare-earth-substituted samples (Table 1), the former did not show superconducting properties down to 40 K. Hence, optimal carrier concentration does not necessarily result in superconducting phase in the cuprates.
Figure 6 shows the AC susceptibility of rare-earth elemental substituted samples. The susceptibility transition temperature, Tcχ′ of bulk superconductivity due to the diamagnetic shielding is noted by a sudden decrease in the real part χ′ of the complex susceptibility (χ = χ′ + iχ″). AC losses are represented by the peaks of the imaginary part of the complex susceptibility χ″. A narrower peak at higher temperature caused by intrinsic losses and a broad peak at low temperature due to coupling losses should be observed in large AC fields. Our measurements in H = 5 Oe did not show peak signifying the intrinsic loss indicating grain coupling took place immediately after the occurrence of superconductivity within the grains.
T cχ′ was the highest for La-substituted sample (80 K), followed by Gd substitution (68 K) and the lowest was for Er substitution (59 K). Tp also shifted to lower temperatures and broadened as the ionic size was reduced in La- (Tp = 62 K), Gd- (Tp = 58 K) and Er (Tp = 39 K)-substituted samples (inset of Fig. 6). The inter-grain critical current density at the peak temperature Jc(Tp) = 22 A cm−2 for La-, 24 A cm−2 for Gd- and 18 A cm−2 for Er-substituted samples. This indicated that rare-earth substitution with smaller ionic radius suppressed flux pining and weakened the inter-granular coupling more than rare earth with larger ionic radius (La). Tc-onset, Tc-zero, ΔTc, Tcχ′, Tp, Jc(Tp), ρ297K, lattice parameter for Tl-1212 phase, and major and minor, and average grain size for all samples are shown in Table 1. The La-substituted sample showed a higher Tp and Jc(Tp) compared with the Er-substituted sample. This can be partly explained by the better microstructure, i.e., lower porosity, larger grain size and better connection between the grains in the La-substituted (Fig. 2c) compared with the Er-substituted sample (Fig. 2a). The higher Tp and Jc(Tp) can also be explained in terms of the optimization of superconductivity in the grains where the La sample showed a much higher transition Tc, and Tcχ′, compared with Er-substituted sample. Hence, optimization of the superconducting grains contributed to increase in Tp and Jc(Tp).
In conclusion, the effects of rare-earth elements Er, Gd and La and alkali metals Na, K and Rb substitutions on (Tl0.7M0.3)Sr2CaCu2O7 have been investigated. The substitution of rare-earth elements resulted in the Tl-1212 phase and superconducting transition. On the other hand, alkali metal substitution resulted in the formation of Tl-1212 phase, but no superconducting transition was observed even though the normal state resistivity was much lower that the rare-earth-substituted samples. The average grain size, Tcχ′ and Tp decreased as the ionic radius of the rare earth was reduced. Rare earth with smaller ionic radius showed weakened inter-granular coupling and suppressed flux pining. The ionic radius, valence state and appropriate substitution site should be considered in the formation of new Tl-1212 superconducting materials.
References
R.M. Hazen, L.W. Finger, R.J. Angel, C.T. Prewitt, N.L. Ross, C.G. Hadidiacos, P.J. Heaney, D.R. Veblen, Z.Z. Sheng, A. El Ali, A.M. Hermann, Phys. Rev. Lett. 60, 1657 (1988)
S.S.P. Parkin, V.Y. Lee, A.I. Nazzal, R. Savoy, R. Beyers, S.J. La Placa, Phys. Rev. Lett. 61, 750 (1988)
Z.Z. Sheng, A.M. Hermann, Ten Years of Superconductivity: 1980–1990 Springer, Dordrecht, 1993, pp. 305–308
Z.Z. Sheng, A.M. Hermann, D.C. Vier, S. Schultz, S.B. Oseroff, D.J. George, R.M. Hazen, Phys. Rev. B. 38, 7074 (1988)
S. Nakajima, M. Kikuchi, Y. Syono, N. Kobayashi, Y. Muto, Phys. C. 168, 57 (1990)
N.A. Hamid, B. Musa, A.K. Yahya, M.H. Jumali, R. Abd-Shukor, Ceram. Int. 30, 1585 (2004)
Y.F. Li, O. Chmaissem, Z.Z. Sheng, Phys. C. 248, 42 (1995)
R. Abd-Shukor, K.S. Tee, J. Mater. Sci. Lett. 17(2), 103 (1997)
Z.Y. Chen, Z.Z. Sheng, Y.Q. Tang, Y.F. Li, D.O. Pederson, Solid. State. Commun. 83, 895 (1992)
D.A. Zając, W.M. Woch, J. Stępień, C. Kapusta, A. Kołodziejczyk, H. Sudra, G. Gritzner, Radiat. Phys. Chem. 93, 33 (2013)
J.M. Liang, R.S. Liu, Y.T. Huang, S.F. Wu, P.T. Wu, L.J. Chen, Phys. C. 165, 347 (1990)
M.R. Presland, J.L. Tallon, Phys. C. 177, 1 (1991)
V. Badri, U.V. Varadaraju, Solid. State. Commun. 93, 1003 (1995)
Y. Xin, Z.Z. Sheng, D.X. Gu, D.O. Pederson, Phys. C. 177, 183 (1991)
Y. Xin, Y.F. Li, D. Ford, D.O. Pederson, Z.Z. Sheng, Jpn. J. Appl. Phys. 30(9A), L1549 (1991)
A.I. Abou-Aly, N.H. Mohammed, R. Awad, I.H. Ibrahim, Supercond. Sci. Technol. 13, 1107 (2000)
M.H. Eder, G. Gritzner, Supercond. Sci. Technol. 18, 87 (2005)
K. Muhammad-Aizat, R. Abd-Shukor, Sains. Malays. 47(7), 1579 (2018)
J. Nur-Akasyah, R. Abd-Shukor, J. Mat. Sci. Mater. Electron. 29, 3690 (2018)
Y. Xin, Y.F. Li, D.X. Gu, D.O. Pederson, Z.Z. Sheng, Phys. C. 184, 185 (1991)
B. Morosin, E.L. Venturini, R.G. Dunn, P. Newcomer Provencio, N. Missert, R.R. Padilla, Phys. C. 302, 119 (1998)
T.-M. Chen, J.S. Ho, Phys. C. 282–287, 915 (1997)
T.S. Kayed, H. Özkan, N.M. Gasanly, Supercond. Sci. Technol. 14, 738 (2001)
C.P. Bean, Rev. Mod. Phys. 36, 31 (1964)
Acknowledgements
This research was supported by the Ministry of Higher Education of Malaysia under grant no. FRGS/1/2017/STG02/UKM/01/1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest regarding this paper.
Rights and permissions
About this article
Cite this article
Bakar, I.P.A., Muhammad-Aizat, K., Nur-Akasyah, J. et al. AC susceptibility and electrical properties of rare-earth- and alkali metal-substituted (Tl0.7M0.3)Sr2CaCu2O7 with M = Er, Gd, La, Na, K and Rb. Appl. Phys. A 125, 21 (2019). https://doi.org/10.1007/s00339-018-2316-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-018-2316-8