Abstract
The Ba and Sr bearing Tl(BaSr)CaCu2O7 (Tl-1212) phase is an interesting superconductor. Optimizing the average copper valence can lead to a high transition temperature, Tc. Single elemental substitution can be used to optimize the Cu valence. This work investigated the effects of multivalent Cr and Mo substitutions at the Tl site of Tl1-xMx(BaSr)CaCu2O7 (M = Cr and Mo). The samples were prepared via the solid-state reaction method. The structure and lattice parameters were determined using the X-ray diffraction method (XRD). A field emission scanning electron microscope (FESEM) was utilized to observe the microstructure. The electrical resistance and AC susceptibility were measured to determine the transition temperature. XRD pattern showed a single Tl(BaSr)CaCu2O7 phase for the non-substituted sample. Other phases, such as Tl-1201 and Ca0.3Sr0.7CuO2 were also observed in the Cr and Mo substituted samples. The non-substituted sample showed onset transition temperature, Tc-onset of 108 K. The highest Tc-onset was observed in the Tl0.9Mo0.1(BaSr)CaCu2O7 (115 K) and Tl0.5Cr0.5(BaSr)CaCu2O7 (111 K) samples. The intergranular critical current density was between 17 and 22 A cm−2. Cr substitutions for x = 0.1 to 0.5 and Mo substitutions for x = 0.1 showed the highest transition temperature. These results can be used as guides to improve the superconducting properties of the Tl-1212 phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The Tl(BaSr)CaCu2O7 (Tl-1212) is obtained by partially replacing Ba with Sr in TlSr2CaCu2O with 1:1 ratio. This 1:1 ratio enhanced the transition temperature of the Tl-1212 phase [1, 2]. The TlSr2CaCu2O7 phase is not easily prepared in pure form and usually non-superconducting due to the high average Cu valence and over-doping [3, 4]. Substitutions of higher valence ions can optimize the average Cu valence, superconducting volume fraction and transition temperature, Tc [5, 6]. The highest onset transition temperature, Tc-onset exhibited by (Tl0.6Pb0.4)(BaSr)CaCu2O7 is 108 K [7]. Ratio other than 1:1 suppressed the superconducting properties of Tl(BaSr)CaCu2O7 [1].
The carriers in the CuO2 planes of the p-type superconductors, such as thallium cuprates are holes. Thus, holes and Cu valence are closely related and can affect the transition temperature [8, 9]. The Tc of the cuprates superconductor depends on the hole concentration and usually follows the parabolic curve of transition temperature against hole concentrations per CuO2, nh. Similar parabolic curves were reported for the bismuth, lanthanum, and yttrium cuprate systems [10]. The optimal Cu valence for the Tl-1212 phase is around 2.30 [11]. The Cu valence can be optimized with elemental substitutions at various sites of the Tl-1212 phase.
Substitution of W and Te for Tl in Tl(BaSr)CaCu2O7 showed suppression of the superconducting properties [12]. However, the substitution of Se and Pb improved the transition temperature and the Tl-1212 volume fraction when heated at 970 °C [7]. Substitutions of Gd at the Ca site suppressed the superconducting properties of the Tl-1212 phase [13].
Substitution of elements with valence states higher than Tl3+ can optimize the Cu valence. The superconducting phase of TlSr2CaCu2O7 can be stabilized with the Cr substitution at the Tl site [14, 15]. Several studies have reported the effects of Cr and Mo substitution on the thallium-based materials [16,17,18,19,20]. Cr substitutions in TlSr2CaCu2O7 were reported to result in the highest transition temperature of 118 K [16]. For Mo substitutions, a double transition was observed at 100 K and 70 K for TlSr2CaCu2O7 [17]. Substitution of multivalent Pb in the Tl-1223 superconductor enhanced Tc and volume fraction of the superconducting phase [21]. Copper fluoride and thallium fluoride enhanced the transition temperature and volume fraction of the Tl-2223 phase [22].
Ga or Ta substitutions at the Ca site suppressed the superconductivity of Tl(Ba,Sr)CaCu2O7. The effects of other elements at the Tl site of Tl1−xXx(Ba,Sr)CaCu2O7 superconductor with X = Cr, Bi, Pb, Se, and Te have been investigated [23]. Se substitution at the Tl site enhanced the transition temperature of the phase. For the Se substituted samples, x = 0.3 showed the highest transition temperature. Elements with a smaller ionic radius helped improve the superconducting properties of the Tl-1212 phase [23]. The ionic radius of the substituting elements must be relatively smaller or within the range of the substitution site to improve the transition temperature.
It is interesting to study the effect of multivalent Cr and Mo on the Ba and Sr bearing Tl(BaSr)CaCu2O7. Cr and Mo exist in a multivalent state of Cr3+/Cr4+ and Mo4+/Mo6+. The main objective of this study was to determine the effects of Cr and Mo substitutions at the Tl site of Tl1–xMx(BaSr)CaCu2O7 for x = 0, 0.1, 0.2, 0.3, 0.5, 0.6 and 0.7 with M = Cr and Mo. X-ray diffraction (XRD) patterns and scanning electron microscope (SEM) of Tl1–xMx(BaSr)CaCu2O7 are reported. This paper also reports the AC susceptibility and electrical properties of the samples.
2 Experimental details
The solid-state reaction method was used to prepare Tl1–xMx(BaSr)CaCu2O7 samples with M = Cr and Mo. Powders of high purity (> 99.9%) BaCO3, SrCO3, CaO, and CuO from Sigma Aldrich and BDH Laboratory were mixed and ground. The mixture was heated in air at 900 °C for 24 h with two intermittent grindings to produce a precursor (BaSrCaCu2Oy). Tl2O3 and Cr2O3 or Mo were added to the precursor powder with formula Tl1-xMx(BaSr)CaCu2O7 for 0 ≤ x ≤ 0.7 and mixed completely. The resultant materials were pressed into pellets with 12.5 mm diameter and 2.5 mm thickness. The pellets were then placed in a preheated tube furnace at 970 °C with oxygen flow for 4 min and furnace cooled. Due to the high volatility, excess Tl2O3 powders were added to take into account the thallium vaporization during the heat treatment.
The crystalline phase was determined by X-ray diffraction (XRD) method using a Bruker model D8 Advanced diffractometer with CuKα radiation from 2θ = 2° to 60°. The patterns were fitted using the Pawley refinement method using the X’Pert HighScore software. The micrographs of the samples were recorded by a field emission scanning microscope (FESEM) using Zeiss Supra55VP. An Oxford Instruments Energy-dispersive X-ray (EDX) analyzer was used to determine the chemical composition.
The electrical property was studied by measuring the dc electrical resistance at low temperatures using the four-point probe technique. The electrical contacts between the probes and the sample were achieved with silver pastes. A CTI Cryogenics Model 22 cryostat and Lakeshore Model 340 temperature controller were used for the low-temperature measurements. The current used throughout the measurement was between 1 and 50 mA. A Cryo Industry AC susceptometer (REF-1808-AS) was used to measure the complex susceptibility (χ = χ′ + iχ′′) at 295 Hz and 5 Oe. The critical current density, Jc at a peak temperature of χ′′, Tp was determined using the Bean’s Model [24, 25] with formula Jc(Tp) = H/(lw)1/2, where H is the applied field, and l and w is the cross-sectional dimension of the sample.
3 Results and discussion
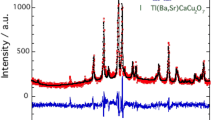
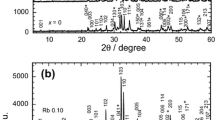
Figure 1 shows the XRD patterns for the Cr-substituted samples, which were refined using the Pawley method using X'Pert HighScore software. The Rp, Rwp, and Rexp parameters and the goodness of fit, χ2 as shown in Table 1, were used as the numerical criteria of refinement. The patterns revealed the presence of the Tl-1212 phase in all samples, which were indexed based on the tetragonal structure with the P4/mmm space group. A single Tl-1212 phase was observed for x = 0 and 0.1 Cr substituted samples. The Tl-1212 phase was suppressed with further substitutions of Cr. Impurity phases, including Tl-1201, Ca0.3Sr0.7CuO2 (CSCO), and Cr2O3 were also observed in the XRD patterns. The Ca0.3Sr0.7CuO2 phase was also found in the Bi-based [26] and other copper oxide-based superconductors containing strontium and calcium [27].
Experimental and calculated XRD patterns of Cr substitution for a x = 0, 0.1, and 0.2, b x = 0.3, 0.4 and 0.5, and c x = 0.6 and 0.7. The plots show the Pawley fit (solid black line) and the measured intensity (red dot). The blue line shows the difference between the measured and calculated intensity. Green, orange, violet, and cyan represent the expected positions of Bragg reflections for Tl(Ba,Sr)CaCu2O7, TlSr2CuO5, Ca0.3Sr0.7CuO2, and Cr2O3, respectively
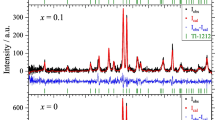
The Tl-1212 superconducting phase was suppressed in the Mo-substituted samples (Fig. 2). This is consistent with previous reports, which showed that transition metals did not enhance the Tl-1212 phase. The a and c lattice parameters for the x = 0 sample are larger (a = 3.832 Å and c = 12.398 Å) than the Cr and Mo substituted samples. This result indicated that Cr and Mo ions may have entered the Tl site since both ions have smaller ionic radii—Cr3+ (0.615 Å), Cr4+ (0.55 Å) and Mo6+ (0.59 Å) compared to Tl3+ (0.885 Å). The reduction in a lattice parameter can be due to the increase in the average Cu valence with Mo and Cr substitutions, which have smaller ionic radii. This led to Cu–O bonding length compression within the Cu–O layers [28]. The decrease in the c lattice parameter can be due to the larger Tl ions being partially replaced by the smaller Cr and Mo ions. In both Cr and Mo substitutions, there were some unknown peaks observed. All the unknown peaks were marked with α and β for Cr and Mo samples, respectively. The impurities caused the formation of these peaks during preparation.
Experimental and calculated XRD patterns of Mo substitution for a x = 0.1, 0.2 and 0.3, b x = 0.4 and 0.5, and c x = 0.6 and 0.7 samples. The plot shows the Pawley fit (solid black line) and the measured intensity (red dot). The blue line shows the difference between the measured and calculated intensity. Green, orange, and violet represent the expected positions of Bragg reflections for Tl(Ba,Sr)CaCu2O7, TlSr2CuO5, and Ca0.3Sr0.7CuO2, respectively
The micrograph for the x = 0 sample is shown in Fig. 3a. A layered microstructure with no clear grain boundaries was observed for the non-substituted and Cr-substituted (x = 0.3) samples. EDX spectra for the non-substituted, Mo, and Cr substituted samples are shown in Fig. 4. The inset tables in Fig. 4 show the weight percent and atomic percent of elements present. Some variations from the Tl-1212 phase in the elemental composition were due to the multiphasic nature of the samples.
The electrical resistance versus temperature curves of Tl1–xCrx(Ba,Sr)CaCu2O7 are shown in Fig. 5. All samples exhibited metallic-normal state behavior above the onset transition temperature, Tc-onset, except for x = 0.7 which showed a semiconductor normal state. The non-substituted sample showed Tc-onset = 108 K and Tc-zero = 91 K. Substitution of Cr with x = 0.2 showed a double transition due to the uncoupling between the superconducting grains [29]. Cr substitution increased Tc-onset from 109 to 111 K up to x = 0.5. Further substitutions (x = 0.6 and 0.7) showed a gradual decrease of Tc-onset. Cr substitutions (0.2 ≤ x ≤ 0.5) optimized the doping level and electrical properties of the Tl-1212 phase. The highest Tc-onset (111 K) and Tc-zero (97 K) were observed for x = 0.5. Cr substitutions broadened the transition width (10 to 22 K). This showed that Cr substitution increased the inhomogeneities of Tl-1212 individual grain. The increase in Tc-onset shown by samples with 0.2 ≤ x ≤ 0.5 can be attributed to increased chemical pressure with the presence of Cr because of the changes in internal lattice strain and the increase of unit cell volume [30].
Figure 6 shows the electrical resistance versus temperature curves for Tl1–xMox(Ba,Sr)CaCu2O7. The sample with x = 0, 0.1, 0.2, 0.3, 0.5 and 0.6 showed a metallic normal state. Sample x = 0.4 and 0.7 showed a semiconducting normal state. The x = 0.1 sample exhibited the highest Tc-onset (115 K). The non-substituted sample exhibited the highest Tc-zero. Further substitution of Mo (0.1 < x ≤ 0.7) suppressed Tc-onset and Tc-zero. This result is consistent with Mo substitution in the La-123 system [31]. This implied that this amount of Mo decreased the Cu valence to the underdoped state and suppressed superconductivity. Mo substitutions showed a higher Tc-onset than Cr substitutions. This result indicated that Mo was a better element than Cr for enhancing the superconductivity of Tl-1212. The concept of average Cu valence and charge neutrality can be used to explain the variation in the transition temperature of the Tl-1212 phase. The optimum average Cu valence to obtain the maximum transition temperature for the Tl-1212 phase is around 2.30. The variation of the transition temperature for the samples is shown in Fig. 7. In Cr and Mo substitutions, the optimized amount was x = 0.5 and x = 0.1, respectively, indicating an average Cu valence of 2.3 in these two samples. Cr ion (Cr3+/Cr4+) and Mo ion (Mo6+) have higher valence states than Tl (Tl3+). If we assume the following valence states for the elements involved: Tl3+, Ba2+, Sr2+, Ca3+, O2− and Cu is in the optimal valence state (i.e., Cu2.3+), for the x = 0.5 sample, the average valence for Cr is Cr3.8+, i.e., in the mixed Cr3+ and Cr4+ states. For the Mo substituted samples, Fig. 7 shows that the highest transition temperature was in the vicinity of x = 0.1 and 0.15. Assuming that the highest transition temperature is at x = 0.15 and using the same concept as above, the average Mo valence in the samples was around Mo6+. Hence, this work indirectly showed that Cr was in the Cr3+ and Cr4+ (mixed) states and Mo was in the Mo6+ state.
The AC susceptibility curves for both Cr and Mo substitutions are shown in Figs. 8 and 9, respectively. The sudden drop in the real part, χ′ indicates diamagnetic shielding. Two peaks in the imaginary susceptibility, χʺ represents AC losses. The first peak (intrinsic peak) at a higher temperature demonstrates intragranular losses. The second peak at a lower temperature indicates an intergranular loss. The applied field is sufficient to penetrate the grains and showed the presence of an intergranular loss peak (Tp). The intrinsic peak was not observed in our measurements due to the low applied magnetic field, H. The flux pinning strength can be determined from the shifting and the width of the intergranular loss peak at a particular applied magnetic field (i.e., 5 Oe in these measurements).
Tcχ′ the for Cr substituted samples was from 74 to 106 K (Fig. 8a and b). At this temperature, the flux started to penetrate the sample. In this series, the intergranular peak loss was the highest in x = 0.3 with Tp = 76 K (Fig. 8a). Tp shifted to lower temperatures and broadened, indicating that Cr substitution suppressed the intergranular coupling and weakened the flux pinning. The substitution of Cr changed the morphology of the samples and affected the intergranular nature of the samples. SEM micrographs showed partial melting, which may have suppressed the coupling between grains. The impurity phase between the superconducting grains can become barriers to the electrical conduction [32, 33]. Cr-substitution suppressed the intergranular coupling and decreased the flux pinning energy of Tl-1212. Jc(Tp) for the Cr substituted samples was between 17 and 22 Acm−2.
In the Mo substituted samples, the highest Tcχ’ and Tp were 109 and 85 K, respectively, was observed in the x = 0.1 sample (Fig. 9a). Unlike Cr substitution, the intergranular loss peak for this sample shifted to a higher temperature. The peak became narrower, indicating the improvement of intergranular coupling and enhancement of flux pinning with Mo substitutions. Further substitutions (0.1 < x ≤ 0.7) weakened the coupling and flux pinning energy as observed from the Tp value which shifted to a lower temperature and broadening of the peak (Fig. 9b). The unknown XRD peaks observed in the Mo-substituted samples suppressed the superconducting properties of Tl-1212. The Jc(Tp) for Mo substitution was between 17 and 20 A cm−2. Tc-onset, Tc-zero, ΔTc, Tcχ′, Tp and Jc(Tp) for Mo and Ru substitutions are shown in Table 1.
4 Conclusions
The effects of Cr and Mo substitutions on Tl1–xMx(BaSr)CaCu2O7 have been studied. Multivalent Cr and Mo significantly affected the transition temperature. Substitutions of Cr with x = 0.5 showed the highest Tc, while the non-substituted and x = 0.1 samples exhibited a single Tl-1212 phase. Mo substitutions showed an optimized level at x = 0.1 with the highest Tc-onset = 115 K. AC susceptibility measurement also showed that Tcχ′ and Tp were the highest in Mo substituted samples for x = 0.1.
This work showed that Cr was in the mixed Cr3+/4+ states and Mo was in the Mo6+ state. Substituting ions with smaller radii, i.e. Cr3+/4+(0.615 Å/0.55 Å) and Mo6+(0.59 Å) improved the transition temperature. Ionic radii and valence states of the substituted elements are important factors in improving the Tc of the Tl-1212 type phase. Substitutions with other multivalent transition metals at the Tl-site of Ba and Sr bearing Tl-1212 are interesting for further investigations in the future.
Data Availability
Data from the work shall be provided upon reasonable request.
References
A. Soeta, T. Suzuki, S. Takeuchi, T. Kamo, K. Usami, S.-P. Matsuda, Relationship between crystal structures and solid solution of Tl-Sr-Ca-Cu-O and Tl-Ba-Ca-Cu-O superconductors. Jpn. J. Appl. Phys. 28(Part 2, No. 7), L1186–L1189 (1989)
M. Kuroda, K. Yokogawa, Preparation and superconducting properties of TlBaSrCaCuO system. Jpn. J. Appl. Phys. 30(11A), L1871–L1872 (1991)
R. Liu, W. Zhou, R. Janes, P. Edwards, An investigation of the new high-Tc Tl-V-Sr-Ca-Cu-O and Tl-V-Sr-Ca-Y-Cu-O superconductors. Supercond. Sci Technol. 4(1S), S310–S312 (1991)
M.A. Subramanian, C.C. Torardi, J. Gopalakrishnan, P.L. Gai, J.C. Calabrese, T.R. Askew, R.B. Flippen, A.W. Sleight, Bulk superconductivity up to 122 K in the Tl-Pb-Sr-Ca-Cu-O system. Science 242(4876), 249–252 (1988)
Z.Z. Sheng, D.X. Gu, Y. Xin, D.O. Pederson, L.W. Finger, C.G. Hadidiacos, R.M. Hazen, A new 1212-type phase: Cr-substituted TlSr2CaCu2O7 with Tc up to about 110 K. Mod. Phys. Lett. B 5(9), 635–642 (1991)
Z.Z. Sheng, Y.F. Li, D.O. Pederson, Derivatives of TlSr2CaCu2O7 from elemental substitutions of Pb for Tl, La for Sr, and Y for Ca. Solid State Commun. 80, 913–915 (1991)
M.G. Ranjbar, M. Ghoranneviss, R. Abd-Shukor, Optimal formation and enhanced superconductivity of Tl-1212 phase (Tl0.6Pb0.4)(Ba, Sr)CaCu2O7. Appl. Phys. A. 124, 456 (2018)
I. Gopalakrishnan, J. Yakhmi, R. Iyer, TlCaBaSrCu2O7−δ, a new 94 K superconductor. Phys. C 172, 450–454 (1991)
M. Paranthaman, A. Manthiram, J. Goodenough, Hole concentration and Tc in Tl2−yBa2Ca1−zYzCu2O8−x. J. Solid State Chem. 98, 343–349 (1992)
R. Wesche, Physical properties of high-temperature superconductors (John Wiley & Sons, New York, 2015), p.93
Z. Sheng, Y. Xin, D. Gu, J. Meason, J. Bennett, D. Ford, Semiconducting TlSr2RCu2O7 (R= rare earth) and its superconducting derivatives. Phys. B: Condens. Mat. 84, 349–352 (1991)
K. Muhammad-Najib, R. Abd-Shukor, AC susceptibility and electrical properties of tungsten substituted (Tl1–xWx)(BaSr)CaCu2O7 superconductor. J. Fiz. Malays. 42, 10011–10017 (2021)
H. Mortada-Hamid, A.P.B. Ilhamsyah, R. Abd-Shukor, Formation of Tl-1212 phase in Ga-substituted Tl(BaSr)Ca1−xGaxCu2O7 superconductor. J Mater Sci: Mater Electron. 31, 5316–5323 (2020)
C. David, R. Christopher, Effect of addition of chromium (VI) oxide on the formation of the Tl–Sr–Ca–Cu–O 1212 phase. J. Mater. Chem. 3, 473–478 (1993)
E. Yusrianto, A.N. Jannah, R. Abd-Shukor, Phase formation and transition temperature of (Tl0.5Pb0.5)Sr2Ca(Cu2–xCrx)O7 (x = 0–0.100) superconductors. Sains Malays. 50, 1775–1786 (2021)
M.G. Ranjbar, R. Abd-Shukor, Formation of Tl-1212 phase in Cr substituted (Tl1–xCrx)Sr2CaCu2O7 superconductor. Phys. Int. 3, 74–78 (2012)
Y. Li, Z. Sheng, Superconductivity at 70 K and 100 K in the MoTlSrCaCuO system. Mod. Phys. Lett B 24&25, 1661–1667 (1991)
K.A. Jasim, S.A. Makki, A.A. Abud-Almohsin, Comparison study of transition temperature between the superconducting compounds Tl0.9Pb0.1Ba2Ca2Cu3O9-δ, Tl0. 9Sb0.1Ba2Ca2Cu3O9-δ and Tl0.9Cr0.1Ba2Ca2Cu3O9-δ. Phys. Procedia 55, 336–341 (2014)
R. Awad, A. Abou-Aly, S. Mahmoud, M.M. Barakat, Thermal expansion measurements using x-ray powder diffraction of Tl-1223 substituted by molybdenum. Supercond. Sci. Technol. 20, 40–405 (2007)
F. Letouze, C. Martin, A. Maignan, C. Michel, M. Hervieu, B. Raveau, Stabilization of new superconducting thallium cuprates and oxycarbonates by molybdenum. Phys. C 254, 33–43 (1995)
R.F. Khattar, K. Habanjar, R. Awad, M. Anas, Comparative study of structural, electrical, and mechanical properties of (Tl, Hg)-1223 high temperature superconducting phase substituted by lead oxide and lead dioxide. J. Low Temp. Phys. (2023)
A. Kamar, A. Srour, M. Roumié, W. Malaeb, R. Awad, A. Khalaf, Comparative study of structural and superconducting properties of (Cu0.5Tl0.5)Ba2Ca2Cu3O10–δ phase substituted by copper fluoride and thallium fluoride. Appl. Phys. A 127, 579 (2021)
J. Nur-Akasyah, R. Abd-Shukor, T.V. Chong, Elemental substitution at Tl-site of Tl1–xXx(Ba, Sr)CaCu2O7 superconductor with X = Cr, Bi, Pb, Se and Te. Materials 16, 4022 (2023)
C.P. Bean, Magnetization of hard superconductors. Phys. Rev. Lett. 8, 250–253 (1962)
C.P. Bean, Magnetization of high-field superconductors. Rev. Mod. Phys. 36, 31–39 (1964)
S. Misture, C. Park, R. Snyder, B. Jobst, B. Seebacher, Powder-diffraction data for several solid solutions with the compositions (CaxSr1−x)CuO2 and (CaxSr1−x)2CuO3. Powder Diffr. 10, 296–299 (1995)
C. Rao, R. Nagarajan, R. Vijayaraghaven, Synthesis of cuprate superconductors. Supercond. Sci. Technol. 6, 1–22 (1993)
N. Hamid, B. Musa, A. Yahya, M. Jumali, R. Abd-Shukor, Effects of elemental substitution involving V and Cr on superconductivity of (Tl, Bi)Sr2(Ca, Y)CU2O7 ceramics. Ceram. Int. 30, 1585–1598 (2004)
E. Hannachi, Y. Slimani, A. Ekicibil, A. Manikandan, F.B. Azzouz, Excess conductivity and AC susceptibility studies of Y-123 superconductor added with TiO2 nano-wires. Mater. Chem. Phys. 235, 121721 (2019)
I. Gopalakrishnan, J. Yakhmi, R. Iyer, TlCaBaSrCu2O7−δ, a new 94 K superconductor. Phys. C: Supercond. Appl. 172, 450–454 (1991)
S. Rayaprol, K. Mavani, C. Thaker, D.S. Rana, R. Kulkarni, D. Kuberkar, D.C. Kundaliya, S.K. Malik, Effect of Mo-substitution on superconductivity, flux pinning and critical currents of La1.5Nd0.5Ca1Ba2Cu5Oz. Phys. C 391, 237–244 (2003)
E. Hannachi, Y. Slimani, F. Ben Azzouz, A. Ekicibil, Higher intra-granular and inter-granular performances of YBCO superconductor with TiO2 nano-sized particles addition. Ceram. Int. 44, 18836–18843 (2018)
J. Nur-Akasyah, M. Ranjbar, R. Abd-Shukor, Influence of Se and Te substitutions at Tl-site of Tl(Ba, Sr)CaCu2O7 superconductor on the AC susceptibility and electrical properties. Ceram. Int. 47, 31920–31926 (2021)
Acknowledgements
The Ministry of Higher Education (MOHE), Malaysia, supported this research under grant no. FRGS/1/2020/STG07/UKM/01/1.
Author information
Authors and Affiliations
Contributions
Conceptualization: RAS; methodology: KMN, KMA and MHJ; formal analysis and investigation: KMN and MHJ; writing—original draft preparation: KMN; writing—review and editing: RAS; funding acquisition: RAS; resources: RAS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Muhammad-Najib, K., Muhammad-Aizat, K., Jumali, M.H. et al. AC susceptibility and electrical properties of Cr and Mo substituted Tl(BaSr)CaCu2O7 Tl-1212 type phase superconductors. Appl. Phys. A 129, 634 (2023). https://doi.org/10.1007/s00339-023-06911-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06911-8