Abstract
Highly dispersed silicon carbide powders were prepared by a solvothermal-assisted sol–gel process with tetraethyl orthosilicate and phenolic resin as starting materials and carbothermal reduction reactions at a relatively low temperature (1400–1600 °C). Investigated by X-ray diffraction, transmission electron microscopy, scanning electron microscope and the nitrogen adsorption–desorption isotherm measurements, the obtained SiC powders had an average crystallite size of 200 nm and high specific surfaces areas of 149 m2/g. Besides, mechanism for preparation of highly dispersed SiC powders was proposed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Silicon carbide (SiC) ceramics have several excellent merits such as high hardness, moderate toughness and excellent wear resistance [1,2,3], high temperature oxidation [4] and corrosion resistance [5, 6], high thermal conductivity and tailorable electrical resistivity [7]. These favorable properties make SiC a promising material applied in aerospace structures, high temperature electric devices, and catalysis in severe environments [8,9,10,11].
At present, the fabrication methods of SiC powders are widely implemented and quite mature, including laser [12], thermal plasma CVD [13], high-energy milling [14], precursor infiltration and pyrolysis (PIP) [15]. However, requirement special equipment or complex technology of these methods limits their application in low cost large scale production of SiC powders. With the advancement in the preparation of powders field, the hydrothermal/solvothermal process have emerged and attracted much attentions in recent years [16,17,18]. The outstanding advantages of the powders by hydrothermal/solvothermal process are excellent homogeneity and narrow particle-size distribution. For sintered materials, the finer the grain, the higher the sintering efficiency [19]. Thus, the highly dispersible powders via solvothermal route are very suitable for sintering. Efforts to prepare uniform SiC powders by a solvothermal-assisted sol–gel process lead to this paper.
In this paper, a simple route to obtain SiC powders via a solvothermal-assisted sol–gel process is proposed. The composition and morphology characteristics of the SiC powders were studied.
2 Experimental
Analytical grade tetraethyl orthosilicate (TEOS; Sigma–Aldrich) was acted as silica precursors. Phenolic resin with high carbon yield of 60 wt% (THC-400; Shanxi Taihang Impedefire Polymer Limited Company, China) was selected as carbon source.
Stoichiometric amount of TEOS (20.8 g, 0.1 mol) were added into a mixture of 100 ml of ethanol. After oil bath concentration at 60 °C for 1–2 h, appropriate amount of phenolic resin was mixed uniformly to obtain the SiC sol. Then, the SiC sol was poured into the Teflon-lined stainless steel autoclave and treated at 200 °C for 12 h. After cooling down the autoclave to room temperature, the obtained precipitation through centrifugation must be cleaned with anhydrous ethanol twice. The preparation of SiC precursor was finished until the precipitates were dried with 60 °C for 48 h. Besides we also tried to get the SiC precursor through a traditional sol–gel route. Without solvothermal treatment, the SiC sol were directly solidified at 80 °C and dried to remove some of the remaining water and ethanol at 200 °C for 5 h. The following pyrolysis process was conducted at a heating rate of 15°C/min to the designed temperature of 1400 and 1600 °C for 1 h.
The composition and crystal structure were characterized by XRD diffractometer (Bruker D8 Advance, Germany; Cu Kα radiation), SEM (S4800, Hitachi, Japan), EDS (S4800, Hitachi, Japan) and TEM (JEM-2100, Japan). The element analysis for carbon content was characterized using a carbon analyzer (CS-600, Leco). The laser particle-size analyzer (Brookhaven Zeta PALS, USA) was used to study the particle-size distribution of the SiC powder. The test is measured at a pH of 7 with a scattering angle of 15°. Micro/mesopores information including the surface area and the pore size distribution were examined by a Quantachrome instrument (USA) through the Barrett-Joyner-Halenda (BJH) method.
3 Results and discussions
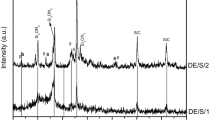
The reaction for SiC formation by carbothermal reaction between carbon (C) and silica (SiO2) was favorable as high as 1793 K in the standard conditions from the thermodynamic perspective estimation [20]. In this paper, the designed temperature (1673 K—1 h and 1873 K—1 h) were employed to prepare SiC powders. XRD patterns of the obtained powders are recorded in Fig. 1.
When the pyrolysis temperature was 1673 K, the weak SiC peaks (JCPDS cards No. 29-1131, 6H-SiC) existed, no other crystalline phases of silica, carbon or other impurities were detected, which indicated the carbothermal reduction between C and SiO2 was almost complete. With temperature further up to 1873 K, the peaks become sharper, suggesting the growth of the SiC crystallites. The peaks with 2θ values of 33.69°, 35.72°, 41.48°, 60.04°, 71.80°, and 75.59° correspond to the crystal planes of 101, 102, 104, 110, 116, and 0012, respectively, for SiC phase. Besides, the weak peak at 33.6° (2θ values) indicates existence of stacking faults in the SiC crystallites [21].
Figure 2 shows the different morphologies of SiC ceramics by a traditional sol–gel route and via a solvothermal-assisted sol–gel process at a same pyrolyzed temperature. The powders obtained by a traditional sol–gel route gathered into some aggregates, and exhibited a loose and porous structure. The holes formed within SiC grains might be caused by the release of CO gas come from the carbothermal reduction reaction [22]. However, the powders obtained by a solvothermal-assisted sol–gel process performed excellent dispersivity, and the particle size was in nanoscale.
The main reason for the different morphology of SiC powders might be attributed to a typical colloid aggregation by polymerization [23]. Phenolic resin molecules first tangle with silica nano-spheres via hydrogen bonding in sol. Then, phenolic resin might be precipitate because of the increase of local concentration. Thus, the resin molecules could be shaped into flocs (nano-spheres linked by polymers) through cross-linking. What’s more, the polymer would become more and more hydrophobic with the dehydration progress. The flocs would become micro-sized spherical particles when the hydrophobic effects could overcome the entropy of epitaxial growth mixing [23]. The SiC precursor by a solvothermal-assisted sol–gel process would be dispersible particles with ultrafine size, and kept the homogeneity form after pyrolysis. The similar effect of colloid aggregation has been found in the ZrO2@C formation by solvothermal process [24].
To investigate the size and morphologies of the SiC powders, SEM analysis was conducted and presented in Fig. 3. The high dispersible SiC powders exhibited a sphere-like morphology (Fig. 3a). The stoichiometric composition judged by EDS analysis was about Si0.5C0.5. Besides, combustion analysis conducting at elevated temperature in air environment (1273 K for 1 h) was implemented to ensure the accurate content of carbon in the SiC powders. The measured value was 51.4 ± 0.6 at.%, which showed good agreement with EDS result. Because of nanoscale effect, the particles were inclined to reunion into small aggregations or actively sintered to be hard aggregates, and finally showed micro–nano grain size of 486 nm. Besides, the particle-size distribution is very narrow as can be seen in the laser granulometry pattern of Fig. 3b.
Observed by means of transmission electron microscope, the SiC powders were well-distributed and the particle size was about 200 nm (Fig. 4a). The HRTEM and Fourier-transformed images indicate a value of 2.17 Å for the SiC (104) planes (Fig. 4b), which was very close to the theoretical lattice spacing of 2.174 Å.
The N2 adsorption–desorption isotherms of the SiC powders is performed in Fig. 5a, and the pore size distribution is showed in the Fig. 5b. According to IUPAC classification, the SiC powders had a type-IV adsorption isotherm. The flatter area in the middle of the curve demonstrates a presence of monolayer by isotherm characteristics. It means that the micro-pores within SiC powders would be filled with nitrogen gas at very low pressures, the monolayer formed at medium pressures in the knee of curve, and capillary condensation occurs at high pressures. Type-IV isotherms are usually related to mesoporous or macroporous characteristics. Figure 5b shows the average pore diameter of SiC powders was about 3.589 nm, confirming the existence of mesoporous. Moreover, the specific surface area is an important indicator for the nanoparticles, the value of SiC powders was about 149.08 m2/g, which was substantially higher than the value of that by solid state reaction process (about 20 m2/g) [25]. The presence of nano-SiC particles and/or the pores in the small aggregation might be accounted for high specific surface area of SiC powders by a solvothermal-assisted sol–gel process.
4 Conclusion
A facile route has been successfully adopted to synthesize SiC powders by joint solvothermal treatment of TEOS and phenolic resin in 200 °C with subsequent carbothermal reaction in 1600 °C. The highly dispersed SiC powders with a small crystallite size of 200 nm had large BET-specific surface area (149.08 m2/g). Besides, the synthesized powders might be promising candidates for the SiC ceramic sintering and this effective technique can also be popularized for other carbide powders.
References
K. Suzuki, M. Sasaki, Microstructure and mechanical properties of liquid-phase-sintered SiC with AlN and Y2O3 additions. Ceram. Int. 31, 749–755 (2005)
J. David, G. Trolliard, A. MaÎtre, Transmission electron microscopy study of the reaction mechanisms involved in the carbothermal reduction of anatase. Acta. Mater. 61, 5414–5428 (2013)
K. Yamada, N. Kamiya, S. Wada, Abrasive wear of Al2O3–SiC composites. J. Ceram. Soc. Jpn. Int. Ed. 99, 797–800 (1991)
Y. Xiang, W. Li, S. Wang, Z.H. Chen, Oxidation behavior of oxidation protective coatings for PIP-C/SiC composites at 1500 °C. Ceram. Int. 38, 9–13 (2012)
W. Li, Y. Xiang, S. Wang, Y. Ma, Z.H. Chen, Ablation behavior of three-dimensional braided C/SiC composites by oxyacetylene torch under different environments. Ceram. Int. 39, 463–468 (2013)
N. Ekinaga, Characteristics of SiC whisker and their applications, in Proceedings of the 1st Japan International SAME Symposium, pp. 883–888, (1989)
S. Ogihara, K. Maeda, Y. Takeda, K. Nakamura, Effect of impurity and carrier concentrations on electrical resistivity and thermal conductivity of SiC ceramics containing BeO. J. Am. Ceram. Soc. 68, 16–18 (1985)
A. Najafi, F. Golestani-Fard, H.R. Rezaie, N. Ehsani, A study on sol–gel synthesis and characterization of SiC nanopowders. J. Sol–Gel Sci. Techn. 59, 205–214 (2011)
H.P. Martin, R. Ecke, E. Müller, Synthesis of nanocrystalline silicon carbide powder by carbothermal reduction. J. Eur. Ceram. Soc. 18, l737-l742 (1998)
A. Najafi, F. Golestani-Fard, H.R. Rezaie, N. Ehsani, Synthesis and characterization of SiC nano powder with low residual carbon processed by sol–gel method. Powder Technol. 219, 202–210 (2012)
B. Li, Y.C. Song, C.R. Zhang, J.S. Yu. Synthesis and characterization of nanostructured silicon carbide crystal whiskers by sol–gel process and carbothermal reduction. Ceram. Int. 40, 12613–12616 (2014)
F. Lusquinos, J. Pou, F. Quintero, M. Perez-Amor, Laser cladding of SiC/Si composite coating om Si–SiC ceramic substrates. Surf. Coat. Techol. 202, 1588–1593 (2008)
X.H. Wang, A. Yamamoto, K. Eguchi et al., Thermoelectric properties of SiC thick films deposited by thermal plasma physical vapor deposition. Sci. Technol. Adv. Mater. 4, 167–172 (2003)
H.R. Orthner, R. Tomasi, W.J. Botta, Reaction sintering of titanium carbide and titanium silicide prepared by high-energy milling. Mater. Sci. Eng. A. 336, 202–208 (2002)
Y. Xiang, Y. Li, F. Cao, The degradation behavior of SiC coated PIP–C/SiC composites in thermal cycling environment. Compos. B 79, 204–208 (2015)
L. Zhao, X.F. Chen, X.C. Wang, Y.J. Zhang, W. Wei, Y.H. Sun et al., One-step solvothermal synthesis of a carbon@TiO2 Dyade structure effectively promoting visible-light photocatalysis. Adv. Mater. 22, 3317–3321 (2010)
S.K. Liu, Z. Chen, K. Xie, Y. Li, J. Xu, C. Zheng, A facile one-step hydrothermal synthesis of a-Fe2O3 nanoplates imbedded in graphene networks with high-rate lithium storage and long cycle life. J. Mater. Chem. A. 2, 13942–13948 (2014)
X.F. Ma, X. Huang, Z. Kang, G.J. Zhang, H.J. Luo. One-pot syntheses and characterization of zirconium carbide microspheres by carbon microencapsulation. Ceram. Int. 41, 6740–6746 (2015)
M.J. Mayo, Processing of nanocrystalline ceramics from ultrafine particles. Int. Mater. Rev. 41, (85–115 (1996)
W.W. Alan, J.N. Kevin, A.C. Gene, P.R. Raymoud, Kinetics of carbothermal reduction synthesis of beta silicon carbide. AICHE. J. 39, 493–503 (1993)
W.S. Seo, K. Koumoto, Stacking faults in β-SiC formed during carbothermal reduction of SiO2. J. Am. Ceram. Soc. 79, (1777–1782 (1996)
J.M. Jiang, S. Wang, W. Li., Z.H. Chen, Low-temperature synthesis of tantalum carbide by facile one-pot reaction. Ceram. Int. 42, 7118–7124 (2016)
L. Sun, M.J. Annen, F. Lorenzano-Porras, P.W. Carr, A.V. Mccormick, Synthesis of porous zirconia spheres for HPLC by polymerization-induced colloid aggregation (PICA). Acta Sedimentologica Sinica. 163, (464–473) (2004)
W.T. Xu, Y.F. Zhou, D.C. Huang, M.X. Yang, C. Yu, K. W, et al. Synthesis and pyrolysis evolution of glucose-derived hydrothermal precursor for nanosized zirconium carbide. Ceram. Int. 42, 10655–10663 (2016)
F.F. Wang, D.P. Xiang, Y.W. Wang, J.B. Li, Rapid synthesis of SiC powders by spark plasma-assisted carbothermal reduction reaction. Ceram. Int. 43, 4970–4975 (2017)
Acknowledgements
The authors sincerely thank the support from the National Natural Science Foundation of China under Grants 61671467 and 21471159.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, H., Jiang, J. & Zhao, H. Synthesis of highly dispersed silicon carbide powders by a solvothermal-assisted sol–gel process. Appl. Phys. A 124, 470 (2018). https://doi.org/10.1007/s00339-018-1885-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-018-1885-x