Abstract
In this paper, the effects of the addition of silicon nitride seeds on the phase composition, particle size, and shape of silicon nitride powders obtained by carbothermal reduction-nitridation were studied. Environmentally friendly natural raw material, diatomaceous earth, was used as a Si precursor. Three different carbon sources were used: activated carbon, carbonized sucrose, and carbon cryogel as reducing agents in the molar ratio C/SiO2 = 5. To obtain better-quality Si3N4 powder, the commercial α-Si3N4 powder was added into starting mixtures as seeds in four different quantities. The X-ray diffraction, specific surface area, infrared spectroscopy with Fourier transform, and scanning electron microscopy were employed to characterize the obtained powders. Sucrose as a carbon source enables a major reduction of SiO2 and the onset of β-Si3N4 crystallization at a lower temperature (1350 °C) as well as the complete absence of diatomaceous earth relics. It indicates that the carbothermal reduction-nitridation takes place faster in contrast to the other two carbon sources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to unique combination of properties, such as high temperature stability, thermal shock resistance, high hardness and toughness, and wear and corrosion resistance, silicon nitride (Si3N4) powder is important for the production of specific structural materials that pave the way for widespread use in the field of engineering [1]. Silicon nitride (Si3N4) has found application in the automotive, rocket, electronic, petrochemical, metallurgical, and chemical industries in the production of ball bearings, cutting tool, sealing rings, nozzles, and gas turbines.
The main issue in the commercial production of this material is the high cost of the raw materials and additives used in the synthesis of Si3N4 powder, which are most often rare earth oxides (RE2O3; RE: Y, Ce, Yb, Lu) as well as very high synthesis temperature [2-4]. Direct nitridation of silicon as one of the methods for silicon-nitride synthesis is performed in an atmosphere of N2, N2/H2 or NH3 at temperatures higher than 1100 °C, but lower than the melting point of Si (1410 °C). Alpha modification of silicon nitride (α-Si3N4) is the main reaction product (Eq. 1), as β modification of Si3N4 occurs only in the presence of the liquid phase. The liquid phase is formed in the presence of impurity elements that form low-temperature eutectics [5].
One of the most promising methods for the production of non-oxide ceramic powders with important technical uses is the carbothermal reduction reaction (CRR). The process of carbothermal reduction and nitridation is a reaction between a mixture of carbon and SiO2 precursors in a flowing atmosphere of N2 in the temperature range of 1400–1500 °C. The carbothermal process starts also from very cheap and harmless raw materials (SiO2, C, N2). Additionally, the reaction is endothermic and no special infrastructure is necessary [6-8].
The overall reaction takes place in several steps. The first step is to obtain SiO (g) by reducing SiO2(s) with C(s) (Eq. 2):
The resulting carbon monoxide can further reduce SiO2 to SiO (Eq. 3):
C can also react with carbon dioxide and form CO (Eq. 4):
The gaseous product (SiO) from reactions denoted by Eqs. 2 and 3 reacts with N2 and form Si3N4 according to Eqs. 5 and 6:
The final product obtained by this method depends on many factors: C/SiO2 ratio, nitrogen flow rate, reaction temperature, SiO2, and C particle sizes, as well as their specific surfaces, impurities, etc. The carbothermal reduction and nitridation method have significant advantages over the direct nitridation method. This procedure offers the possibility of an economically attractive production route from naturally occurring materials and the obtained powders are fine (nano range) and well homogenized [9-15].
The advantages of diatomaceous earth as a natural mineral raw material are reflected in its availability and quantity in landfills after the exploitation of coal in surface mines. Diatomite is a siliceous, sedimentary rock consisting principally of the fossilized skeletal remains (frustules) of diatom, a unicellular aquatic plant related to the algae. The terms diatomite and kieselguhr are used synonymously for diatomaceous earth. The highly developed surface area with numerous finely developed pores (micro to nanometer scale) over the surface of diatom frustules and siliceous composition provides an opportunity to use diatomite as a Si precursor for the synthesis of various non-oxide powders based on Si3N4 by carbothermal reduction and nitridation (CRN). The silica of the fossilized diatom skeleton closely resembles opal or hydrous silica in composition (SiO2·nH2O) [13]. In addition to bound water, varying between 3.5 and 8%, the siliceous skeleton may also contain, in solid solution, or as part of the SiO2 complex, alumina, small amounts of organic components, and lesser amounts of iron, alkaline earth, and alkali metals. These oxides promote the conversion process by the formation of a liquid phase with a low melting point, thus significantly decreasing the temperature of carbothermic reduction and nitridation reactions. Also, these oxides can favorably affect the sintering process of the obtained powders by acting as additives, which can be further beneficial for the mechanical and high-temperature properties during the sintering of ceramic materials based on Si3N4 [16]. In the work of others and our previous work, it was shown that diatomaceous earth, due to its chemical composition, genesis, and submicrometer particle size and porosity, can be successfully used to obtain non-oxide powders (Si3N4, SiC, Si3N4,/SiC) [9, 12-15, 17-20]. Kang et al. showed that the added silicon nitride seeds can further enhance the properties of the obtained powders by controlling particle size and morphology during synthesis [21]. The mentioned group of authors also used commercial alpha silicon nitride H. Starck LC 12-SX as seeds in an amount of 0–2.0 mass%. Depending on abundant sources of silica and carbon source, the CRN method is expected to produce high-purity, fine silicon nitride powder [22, 23].
In this paper, the effect of three different carbon sources and the amount of silicon nitride seeds on the phase composition, particle size, and morphology of synthesized silicon nitride powders was preliminarily examined. Diatomaceous earth was used as a naturally abundant source of silica whereas activated carbon, carbonized sucrose, and carbon cryogel were used as carbon sources. Commercial powder, α-Si3N4 as seeds was added in starting mixtures as nucleation centers to accelerate reactions that occur during the process of carbothermal reduction and nitridation as well as to obtain fine silicon nitride powder. Phase evolution during carbothermal reaction and nitridation in three starting reacting mixtures without seeds was also followed. One of the goals of this work was to find the effect of each carbon sources on the phase composition and morphology of the obtained Si3N4 powders.
Experimental
Chemical treatment of diatomaceous earth
As-recieved diatomaceous earth was chemically treated with a 1 M HCl solution (p.a. 37%, BDH Prolabo) to reduce the content of iron oxide Fe2O3. The chemical composition of as-received and chemically treated diatomaceous earth (hereafter DE) is given in Table 1.
Preparation of starting mixtures
During the preparation of the starting mixtures, diatomaceous earth and the corresponding carbon source were homogenized in a FRITSCH-Pulverisette 9 vibratory mill for 2 h in a tungsten carbide (WC) vessel with an engine speed of 900 min−1. The used silica source was a diatomaceous earth from the Kolubara basin (Republic of Serbia). As mentioned above, three different sources of carbon were used for carbothermal reduction, industrially obtained activated carbon (Miloje Zakić – Kruševac, hereafter AC), sucrose (C11H22O11, Alfa Aesar, p.a., SBET = 4 m2/g, hereafter S) and chemically synthesized carbon cryogel (hereafter CC). Carbon cryogel was obtained by pyrolyzing RF cryogels in an inert atmosphere as described in the literature [24]. Constant C/SiO2 ratio = 5 was used throughout all experiments, calculated according to remained carbon in carbonized sucrose and carbon cryogel. The stoichiometric ratio C/SiO2 was chosen to be 5, because this ratio gave the best results in the CRN reaction, although the other stoichiometric ratios had been examined [17]. It has been experimentally proven that the C/SiO2 (activated carbon used as a source of carbon) molar ratio has a significant role in the synthesis of silicon carbide [13]. If this ratio is below 4 [1-3], the reaction of formation SiC is incomplete, while if the molar ratio is more than 5, in addition to SiC, there is an excess of carbon in the system. It is experimentally determined by X-ray diffraction analysis that in samples with a molar ratio of C/SiO2 = 1, 2, 3 thermally treated at temperatures from 1250 to 1500 °C, in addition to newly formed Si3N4/SiC, unreacted SiO2 also remains [18]. Silicon dioxide remains unreacted, due to the insufficient carbon content required for the complete reduction of silicon dioxide. In samples with a molar ratio of C/SiO2 = 5, an excess of carbon remains in the system in addition to Si3N4/SiC. In this case, it is necessary to introduce an additional thermal treatment (annealing at 700 °C in an air atmosphere) to remove the excess carbon and obtain pure Si3N4/SiC powder. Excess carbon ensures better contact between the powder particles of the starting reactants, to obtain Si3N4/SiC as the final product.
As reference mixtures, seed-free mixtures with the same carbon sources were prepared at first. These mixtures were subjected to thermal treatments at 1350 °C and 1450 °C for 2 h, in the controlled nitrogen flow atmosphere. The temperature conditions were selected according previous experimental practice.
Three series of samples of diatomite and corresponding carbon-containing seeds (commercial powder α-Si3N4) were homogenized for 2 h in a vibratory mill. Commercial powder Si3N4 was subsequently added and further homogenization was continued for 1 h in a vibratory mill. The first series of samples represent mixtures of diatomaceous earth and activated carbon containing 5–20 wt.% α-Si3N4. The second series of samples represent mixtures of diatomaceous earth containing surose and 5–20 wt.% α-Si3N4 was performed differently. Mixture DE/S was obtained by immersing diatomaceous earth in a solution (saturated solution of sucrose/distilled water) and homogenizing on a magnetic stirrer at room temperature, after which it was dried at 110 °C. The obtained mixture was carbonized for 4 h at 1000 °C, under a flowing atmosphere of nitrogen. The third series of samples represent mixtures of diatomaceous earth and carbon cryogel containing 5–20 wt.% α-Si3N4. Also, these samples were subjected to thermal treatment at 1350 °C and 1450 °C for 2 h, in the controlled nitrogen flow atmosphere. The gas flow was kept at 0.05 l/min until 200 °C. The heating of the alumina reactor was carried out in a furnace with a SiC heating element. Abbreviations of synthesized samples are presented in Table 2.
Specific surface area, SBET was calculated using the gravimetric McBain method. The reaction products were analyzed by X-ray diffraction (XRD) analysis, infrared spectroscopy, and scanning electron microscopy. Average particle size measurements were done by laser diffraction (LD), method with Mastersizer 2000 device. Sample powders were recorded on a diffractometer which is part of the SIEMENS D500 automated system. Patterns were recorded with CuKα radiation (λ = 1.54184 Å), at a current in an X-ray tube of 20 mA and a voltage of 35 kV, passed through a Ni-filter. The Debye–Scherrer formula D = kλ/β cos θ [25] was used to determine the average crystallite size. With β representing full width at half maximum (FWHM) in radian, λ is the wavelength (nm), θ is Bragg’s diffraction angle, and shape constant k is 0.9.
Infrared (IR) spectra were recorded on a spectrophotometer model Perkin-Elmer 597 in the range of 4000–200 cm-1, using the KBr pastille method.
The morphology of the powders was examined with a scanning electron microscope JEOL JSM-3500, an electron beam with a diameter below 1 μm at a voltage of 30 keV. Prior to analysis, the powder samples were steamed with gold.
Determination of carbon content
Residual carbon content from the reaction products was removed by oxidation in air at a temperature of 700 °C. The mass of each individual sample was measured immediately after the end of the carbothermal reduction process and after oxidation in the air. The difference between these two masses is the residual carbon content. In this way, the reactivity of each individual mixture with the change of temperature and time of thermal treatment was also examined.
Results and discussion
The reaction of carbothermal reduction and nitridation takes place at the contact points of SiO2-solid carbon. It is known that the carbon structure significantly determines the specific surface area and porosity parameters affecting the rate of the carbothermal reduction reaction [13]. Since the contact surface between the carbon source and the SiO2 source is very important for the rate and mechanism of the reaction, a specific surface was the determined area of the pulverized starting reducing agents and starting mixtures. Activated carbon and carbon cryogel materials are known to be highly specific surfaces and developed porosity. Also, the dimensions of the pores are very important because they directly influence both, the availability of the surface to the carbon source and the intermolecular forces acting on the surface. Specific surface area, SBET, mean particle sizes, porous characteristics of pulverized starting materials reducing agents, and starting mixtures are shown in Tables 3, 4, and 5. In order to determine the contribution of each carbon precursors during the grinding and homogenization process, all three starting carbon sources were pulverized separately under the same preparing conditions (Table 4).
After grinding of the starting reducing agents, there is an increase in the specific surface area in the case of carbon cryogel and activated carbon without significant change in terms of porosity parameters (Table 4). In the case of carbonized sucrose, there is a significant increase in specific surface area. Namely, during the grinding process, micropores that developed during carbonization process of sucrose open, which is probably the cause of a significant increase in the specific surface area (Tables 3 and 4). Therefore, it can be expected that mixture containing sucrose as a carbon source (DE/S) will be the most reactive in the carbothermal reduction process.
XRD patterns of the examined samples without seeds (DE/AC/1, DE/AC/2, DE/S/1, DE/S/2 DE/CC/1, and DE/CC/2) sintered at 1350 °C and 1450 °C (labeled in Table 2) are shown in Fig. 1. At a lower temperature of 1350 °C (DE/AC/1), the cristobalite (SiO2) phase is still present, in samples for all three carbon sources (Fig. 1). However, silicon oxi nitride, sinoite (Si2ON2), β-Si3N4, and β′-sialon (Si3Al3O3N5) are also present indicating that reduction of SiO2 started at 1350 °C. So, the obtained composite powders are comprised of silicon carbide (SiC, moissanite), as the major crystalline phase at 1450 °C in all three starting mixtures is present with β-Si3N4 and β′-sialon (Si3Al3O3N5). As the β modification of Si3N4 appears only with the presence of a liquid phase, the formation of β-Si3N4 is encouraged by the presence of impurities (alkaline and alkaline earth metal oxides from diatomaceous earth) that form low-temperature eutectics or by nitriding above the melting temperature of silicon.
The formation of this final product (SiC) can be explained by equations given elsewhere in detail [19].
The instability of the Si3N4 phase at 1450 °C in the presence of CO (Fig. 1) can be explained by the following reaction (Eq. 7):
Thus, CO (g) could be one of the reasons for decreasing of the Si3N4 phase.
Therefore, at a lower temperature, the carbothermal reduction reaction takes place much faster in the initial stage. If the solid sample has a large specific surface and contains a large number of micropores, the attractive forces from the opposite walls of the micropores overlap, and the force acting on the adsorbent molecule (reaction gas molecule: SiO, CO) will be much higher compared to the attractive forces acting in open pores of larger dimensions. Based on the presented results, we can conclude that in the initial stage of carbothermal reduction when dominant reactions involve the release of various gaseous products (SiO and CO) carbothermal reduction reactions occur faster in samples with large specific surface area and a high proportion of micropores (developed microporosity). Hadjar et al. also confirmed the fact that the surface properties of diatomaceous earth during the process of carbothermal reduction are greatly influenced by the surface properties of the reducing agent [13].
X-ray diffraction patterns of powders obtained by thermal treatment of the seeded samples at 1350 °C and 1450 °C (Table 2), designated DE/AC/1/5–20, are shown in Fig. 2a and b. Weak and diffuse peaks with d values: 4.32; 3.88; 3.37; 2.89; 2.60, 2.55, 2.32 2.16, and 2.08 Ǻ for sample DE/AC/1/5 originate from α-Si3N4 (Fig. 2a). A wide background in the area of 2θ angles from 20 to 25° indicates low crystallinity of the powders obtained at 1350 °C. Reflections characteristic for SiO2 modification, cristobalite (d values: 4.07 and 2.51 Ǻ) are also observed. The presence of cristobalite represents unreacted SiO2 from diatomaceous earth and indicates that under these conditions carbothermal reduction and nitridation are not complete. The intensity of characteristic α-Si3N4 peaks, increases with increasing content of α-Si3N4 seeds, which is expected. Kang et al. showed that the added silicon nitride seeds are strikingly influenced by the α-Si3N4 and the reduction rate of silica is relatively slow when seeds are absent [21]. Also, the α-Si3N4 is producible at a relatively high partial pressure of SiO (g) (reactions (2) and (3), introduction part) while β-Si3N4 is considered to be obtained because of the lower partial pressure of SiO (g) [21]. These observations were confirmed by the results shown in Figs. 1 and 2.
The same series of samples, designated as DE/AC/2/5–20 were subjected to another heat treatment at 1450 °C (Fig. 2b) to determine the effect of temperature on the reaction products. The transformation products (DE/AC/2/5) showed very strong α-Si3N4 peaks, followed by the reflections of β-Si3N4 (d values: 3.29; 2.65; and 2.49 Ǻ) and β′-sialon (Si3Al3O3N5) (d values: 3.32; 2.72; and 2.51 Ǻ). Si2ON2 phase (sinoite) and silicon carbide (SiC) are also detected [12]. This behavior can be explained by the partial melting of diatomite at this temperature. Thus, the micro-porous structure is harmed and the surface area is dramatically reduced with the presence of a liquid phase which will decrease the transport of reaction gasses. Under normal conditions, α-Si3N4 was produced by the CRN process, but liquid phases of oxides such as Na2O, K2O, MgO, CaO, and Fe2O3 contained in the diatomite occurred due to eutectic reaction, led to the formation of β-Si3N4 instead of α-Si3N4 [12, 19]. The intensity of peaks of α-Si3N4 on the XRD pattern of the sample obtained at 1450 °C DE/AC/2/20 was gradually decreased with an increase in the content of seeds while peaks of β -Si3N4 and β′-sialon (Si3Al3O3N5) almost completely disappear.
Aluminum-rich species in diatomaceous earth (the presence of aluminum silicate, clay component in the diatomaceous earth) improved the nitridation process thereby enabling the formation of SiAlONs [19, 25]. The formation of β′-sialon (Si3Al3O3N5) according to the following reaction:
This phase (Si3Al3O3N5) has also very good mechanical and physical properties as composite powder with Si3N4 and SiC, can be interesting for the production of specific structural materials [26-29].
XRD patterns of powders obtained by thermal treatment of samples designated as DE/S/1/5–20 and DE/S/2/5–20 at 1350 °C and 1450 °C are shown in Fig. 2c and d. XRD pattern of sample DE/S/1/5 is characterized by low crystallinity, which is indicated by a wide background in the area of angles 2θ = 20–25°. Weak reflections (d values: 3.29; 2.65; and 2.49 Ǻ) indicate the beginning of β-Si3N4 crystallization (α to β-Si3N4 phase transformation). This data also indicates the beginning of carbothermal reduction. Also, cristobalite is present (d values: 4.08Ǻ). For sample DE/S/1/10, reflections with d values: 3.32; 2.72, and 2.51 Ǻ indicate the beginning of β′-sialon (Si3Al3O3N5) crystallization at 1350 °C. The presence of silicon cabide (SiC) is also indicative.
At 1450 °C, samples DE/S/2/5–20, silicon oxi nitride, sinoite (Si2ON2), of α-Si3N4, β-Si3N4, β′-sialon (Si3Al3O3N5), and silicon cabide (SiC) reflections are observed (Fig. 2c). The presence of β′-SiAlON (Si3Al3O3N5) is indicated by reflections with d values: 3.32; 2.72; and 2.51 Ǻ.
XRD patterns of samples DE/CC/1/5–20 and DE/CC/2/5–20 (thermaly treated at 1350 °C and 1450 °C) are shown in Fig. 2d and f, respectively. At 1350 °C, characteristic peak intensities of α-Si3N4 increase as expected (d values: 4.32; 3.37; 2.88; 2.60; 2.55Ǻ) while SiO2 content decreases with increasing content of commercial α-Si3N4 powder (d values: 4.08 and 2.5 Ǻ, cristobalite) and low crystallinity is indicated by a wide background in the area of angles 2θ = 20–25° (Fig. 2e).
After thermal treatment at 1450 °C, at the lowest content of commercial α-Si3N4 powder (sample DE/CC/2/5) reflections with d values: 3.29; 2.65; and 2.49 Ǻ and are indicative for phase transformation α in β-Si3N4 (Fig. 2f). Based on a comparison of the intensity of reflections characteristic for β-Si3N4, this phase reaches a maximum in sample DE/CC/2/10. Diffuse and weak reflections (d values: 3.32; 2.72; and 2.51 Ǻ) indicate the beginning of crystallization of β-Si3N4 reflections are observed, β′-SiAlON (Si3Al3O3N5) and silicon carbide (SiC). With a further increase in the commercial α-Si3N4 powder, content over 10%, the intensity of β-Si3N4 reflections decreases, and SiO2 appear in the form of cristobalite. Based on the comparison of the reflection intensities of the sample DE/CC/2/5 and DE/CC/2/10 obtained at 1450 °C (Fig. 1f) are significantly higher crystallinity compared to the same samples obtained at 1350 °C (Fig. 2e). This data indicates that an increase in temperature of 100 °C has a favorable effect on the course of carbothermal reduction and nitridation process for samples with a smaller content of commercial α-Si3N4 powder (DE/CC/2/5 and DE/CC/2/10). However, increasing content of commercial α-Si3N4 powder (over 10 wt.%) and CTRN temperature to 1450 °C did not produce any further increase in reduction and nitridation for a series of samples containing seeds.
Using sucrose, at 1350 °C crystallization of β′-sialon (Si3Al3O3N5) and β-Si3N4 begins at 10 wt.% of α-Si3N4 (sample DE/S/1/10, Fig. 2c). This indicates that sucrose is a more efficient carbon source at a lower temperature, for the reaction of carbothermal reduction and nitridation than activated carbon and carbon cryogel. An explanation can also be found in the method of preparation of the starting mixtures. A mixture of diatomaceous earth and activated carbon as well as a mixture of diatomaceous earth and carbon cryogel are physical mixtures of two powdered materials. In contrast to that, in a mixture of diatomaceous earth and sucrose, carbon is introduced in the form of a solution which allowed its penetration in all free space in diatomaceous earth particles and remains in the channels of diatomaceous earth during carbonization. During the carbonization process sucrose turns into carbon that envelops the exterior and interior of diatomite particles. This data results in higher reactivity of diatomaceous earth and sucrose because the surface which is in contact with carbon is much larger than in the case of the other two powder mixtures (i.e., carbon sources).
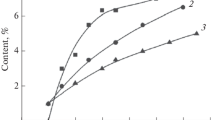
Figure 3 presents a graphical representation of changes in residual carbon content with changing of seed content and thermal treatment temperature for easier following and understanding of phase composition confirmed by X-ray diffraction. Oxidation of the obtained powder after the completion of the carbothermal reduction process is in practice a common way to determine the residual carbon content as an important indicator of the mixture reactivity [12]. The reactivity trend of each mixture depends on the type of carbon precursor. As can be seen from the graphs, the carbon content of all three carbon precursors decreases as temperature increases. However, with the increase of the temperature to 1450 °C, a larger amount of carbon participates in the reduction reaction of SiO2 from diatomaceous earth, and therefore the carbothermal reduction takes place faster for samples containing sucrose as a carbon precursor (Fig. 3). These results also indicate that the carbon source is extremely important for the speed of reactions that take place during carbothermal reduction, which is confirmed by XRD patterns in Figs. 1 and 2 [10-20].
In all three cases of carbon sources, the average crystalline size of the α-Si3N4 and β-Si3N4 slightly decreases with the increasing amount of seeds (Table 6). Crystalline size for all processing temperatures is in the nanometer range (about 20–30 nm) for α-Si3N4 and for β-Si3N4 (about 10–25 nm). Although values of the average particle size of the commercial α-Si3N4 powder are in the micrometer range (about 44 µm, Table 7) the obtained α and β-Si3N4 powders are below this value.
The IR spectra of samples obtained at 1350 °C and 1450 °C, designated as DE/AC/1/5–20 and DE/AC/2/5–20 are shown in Fig. 4a and b. The wide stretched bands of relatively low intensity at about 1100 cm−1 and 470 cm−1 are assigned to the characteristic absorption of the Si–O bond [26], which are characteristic of cristobalite. The low crystallinity of these samples is indicated by wide and stretched bands. The absorption band in the region of about 490 cm−1 is assigned to the characteristic absorption of the Si–N bond and belongs to α-Si3N4, consistent with the XRD results.
The spectra of samples DE/AC/2/5 and DE/AC/2/10 obtained at 1450 −C show the absorption bands in the area of about 490 cm−1 and at 570 cm−1 slightly lower intensity are generally assigned to the characteristic absorption of the Si–N bond and belong to α and β-Si3N4 (Fig. 4b). As the content of α-Si3N4 increases to 15 wt.%, the intensity of the absorption band at 570 cm−1 decreases. The band at 1040 cm−1 is common for SiAlONs and silicon nitride which certifies the existence of SiN4 tetrahedrons in the lattice. In the range of 930–1050 cm−1, the spectrum of SiAlON most closely resembles (overlaps) that of β-Si3N4. Absorption bands in the area of 800–600 cm−1 appear due to the replacement of Si–N bond with Al–N and Al–O bonds in the β-Si3N4 lattice at dissolving of Al2O3 in it, and is common for SiAlONs [27]. However, in this range, an intense absorption band in the region of 800–760 cm−1, is the result of valence vibrations of Si–C (absorption band in the area around 843 cm−1) is common for SiC.
IR spectra of samples, designated as DE/S/1/5–20 and DE/S/2/5–20 obtained at 1350 °C and 1450 °C, respectively are shown in Fig. 4c and d. The spectra of samples DE/S/1/5 and DE/S/1/10, (1350 °C) show broad bands of low intensity in the range of about 1100 cm−1 and 469 cm−1 are characteristic of SiO2 (cristobalite) and indicate low crystallinity of the obtained powder. The disappearance of the peak at about 900 cm−1 in the spectrum of sample DE/S/1/10 may be related to forming of the SiAlON phase during carbothermal reaction and nitridation [26]. Also in the spectrum of sample DE/S/1/10 bands characteristic of α and β-Si3N4 (490 and 570 cm−1) can be observed. A rather weak absorption band in the area around 850 cm−1 is common for SiC (sample DE/S/2/20, obtained at 1450 °C).
IR spectra of samples, designated as DE/CC/1/5–20 and DE/CC/2/5–20 obtained at 1350 °C and 1450 °C are shown in Fig. 4e and f. Wide, stretched bands of low intensity in the region of about 1100 and 469 cm−1 for samples DE/CC/1/5 and DE/CC/1/10 are characteristic absorption of the Si–O bond and belong to SiO2 modification, cristobalite. At 1450 °C, in spectra of samples DE/CC/2/5 and DE/CC/2/10 absorption bands of higher intensity in the region at about 490 cm−1 and 570 cm−1 belong to α and β-Si3N4. Also in the spectrum of samples DE/CC/2/15 and DE/CC/2/20 bands at about 1100 cm−1 and 470 cm−1 are assigned to the characteristic absorption of the Si–O bond [26]. Absorption band in the area around 850 cm−1 is common for SiC (samples DE/CC/1/5–20 and DE/CC/2/5–20). Results obtained by infrared spectroscopy are in good agreement with results obtained with XRD analysis.
The morphological features of obtained powders were studied by scanning electron microscopy (SEM). Figure 5 shows the SEM of obtained samples DE/AC/1/5 (a), DE/AC/2/5 (b), DE/AC/1/20 (c), and DE/AC/2/20 (d).
Morphologically, the powders obtained at both 1450 °C and 1350 °C were largely agglomerated. In the sample DE/AC/1/5 obtained at 1350 °C, there is a complete absence of diatomaceous earth relics (Fig. 5a), in contrast to sample DE/AC/1/20 obtained at the same temperature (Fig. 4c). The morphological characteristics of the starting diatomaceous earth were retained (sample DE/AC/1/20, Fig. 5c). For the sample DE/AC/2/5 obtained at 1450 °C larger irregular particles were observed (Fig. 5b). The powder DE/AC/2/20 consists of very fine nanometer polygonal particles, which are characteristic of silicon nitride crystals (Fig. 5d).
SEM of samples DE/S/1/10, DE/S/2/10 (Fig. 6a and b) and DE/S/1/20, DE/S/2/20 (Fig. 6c and d). At 1350 °C both samples DE/S/1/10 and DE/S/1/20, an almost complete absence of diatomaceous earth relics is observed (Fig. 6a and c). This data indicates that the reaction of carbothermal reduction and transformation of α → β-Si3N4 in this mixture takes place faster and at 1350 °C (XRD, Fig. 1). At 1450 °C the obtained powders with regular polygonal shape and a higher crystallinity are characteristic for α-modification of silicon nitride, while the elongated particles are typical for β-Si3N4 (Fig. 6b and d).
SEM of samples DE/CC/1/10 (a), DE /CC/2/10 (b), DE /CC/1/20 (c), and DE/CC/2/20 (d). Sample DE /CC/1/10, obtained at 1350 °C and 1450 °C individual powder particles of irregular shape are observed (Fig. 7a). Unlike sample DE /CC1/10, sample DE /CC/1/20 (Fig. 7c) has relics of diatomaceous earth, which indicates lower powder crystallinity. Also, the powders obtained at 1350 °C, as in the case of a mixture of diatomaceous earth and activated carbon (Fig. 5c), consist mainly of agglomerated relics of diatomaceous earth. These relics indicate that this temperature is not sufficient for complete carbothermal reduction, also confirmed by X-ray diffraction and infrared spectroscopy (Figs. 1 and 4). However, the powder obtained at 1450 °C consists of agglomerated grains of polygonal habitus (Fig. 7b and d).
Conclusion
The process of carbothermal reduction and nitridation was used in the synthesis of silicon nitride powders as an inexpensive method using cheap and harmless raw materials.
Diatomaceous earth and various sources of carbon were thermally treated for 2 h at 1350 and 1450 °C. Three different carbon sources were used: activated carbon, carbonized sucrose, and carbon cryogel. The obtained starting powders were homogenized with commercial α-Si3N4 powder (H. Starck LC 12-SX) in four different quantities 5, 10, 15, and 20 wt. %. The effect of added silicon nitride seeds on the phase composition and particle morphology was investigated.
From the results, the following conclusions are summarized:
The results indicate that the onset of β-Si3N4 crystallization at 1350 °C is observed at a content of 10 wt.% commercial α-Si3N4 powder when sucrose was used carbon source. This indicates that sucrose is a far more efficient carbon source at a lower temperature, for the reaction of carbothermal reduction and nitridation relative to activated carbon and carbon cryogel. At 1450 °C, by using the same mixture with the addition of 10% of α-Si3N4 seeds, a composite powder composed of α-Si3N4, β-Si3N4/Si3Al3O3N5 was obtained. An explanation can be found in the method of preparation of the starting mixtures, where carbon source was introduced into SiO2 precursor via solution. Major reduction of SiO2 diatomaceous earth took place at 1450 °C and phase transformation of α → β-Si3N4 when active carbon and carbon cryogel were used as carbon sources. Thus, reaction products depend on the reaction temperature and the carbon sources rather than the seed content.
Powders obtained at higher temperatures were composite powder composed of α-Si3N4, β-Si3N4/Si3Al3O3N5, the crystalline size is in the nanometer range (about 20–30 nm) for α-Si3N4 and smaller for β-Si3N4 (about 10–25 nm). The nanometer crystallite size of obtained composite powders allows designing of the advanced materials with improved physical and chemical properties.
Sucrose also revealed morphological advantages over two other carbon sources, activated carbon and carbon cryogel. The complete absence of diatomaceous earth relics in those samples indicated the onset of the carbothermal reduction and nitridation at a lower temperature (1350 °C) leading to the polygonal particles at a higher temperature (1450 °C) which is characteristic for silicon nitride crystals.
In this regard, sucrose can be considered a more efficient carbon source relative to activated carbon and carbon cryogel [30].
References
Schwartz M. M.: Handbook of structural ceramics. McGraw-Hill, New York (1992)
Gao, J., Xiao, H., Du, H.: Effect of Y2O3 addition ammono sol-gel synthesis and sintering of Si3N4-SiC nanocomposite powder. Ceram. Int. 29, 655–661 (2003)
Raju, C.B., Verma, S., Sahu, M.N., Jain, P.K., Choundary, S.: Silicon nitride/SiAlON ceramics-a review. Indian J. Eng. Mater. Sci. 8, 36–45 (2001)
Karakus, N., Kurt, A.O., Toplan, H.O.: Synthesizing high α-phase Si3N4 powders containing sintering additives. Ceram. Int. 35, 2381–2385 (2009)
Matović, B.: Synthesis of silicon nitride powder. Proc. XLVIII ETRAN Conference, Čačak, June 6–10, IV, (2004)
Hofmann, H., Vogt, U., Kerber, A., van Dijen, F.: Silicon nitride powder from carbothermal reaction. MRS Proc. 287, 105–120 (1993)
Ličko, T., Figusch, V., Púchyová, J.: Synthesis of silicon nitride by carbothermal reduction and nitriding of silica: control of kinetics and morphology. J. Eur. Ceram. Soc. 9, 219–230 (1992)
Li, J., Tian, J., Dong, L.: Kinetics of the rection between silicon nitride and carbon. J. Mat. Sci. Lett. 19, 1767–1768 (2000)
Kljajević, Lj., Šaponjić, A., Ilić, S., Nenadović, S., Kokunešoski, M., Egelja, A., Devečerski, A.: Fabrication of non-oxide ceramic powders by carbothermal-reduction from industrial minerals. Ceram. Int. 42, 8128–8135 (2016)
Ashkin, D.: Nitridation behaviour of silicon with clay and oxide additions: rate and phase development. J. Eur. Ceram. Soc. 17, 1613–1624 (1997)
Kurt, A.O., Davies, T.J.: Synthesis of Si3N4 using sepiolite and various sources of carbon. J. Mat. Sci. 36, 5895–5901 (2001)
Arik, H.: Synthesis of Si3N4 by the carbo-thermal reduction and nitridation of diatomite. J. Eur. Ceram. Soc. 23, 2005–2014 (2003)
Hadjar, H., Hamdi, B., Jaber, M., Brendlé, J., Kessaïssia, Z., Balard, H., Donnet, J.B.: Elaboration and characterization of new mesporous materials from diatomite and charcoal. Microporous. Mesoporous. Mater. 107, 219–226 (2008)
Mazzoni, A.D., Aglietti, E.F.: Study of carbonitriding from diatomaceous earth. Mater. Chem. Phys. 37, 344–348 (1994)
Mizuhara, Y., Noguchi, M., Ishihala, T., Satoh, A.: Preparation of fiberlike silicon nitride from diatomaceous earth. J. Am. Ceram. Soc. 74, 846–848 (1991)
Petzow, G., Herrmann, M.: High performance non-oxide ceramics II, in: M. Janson (Ed.), Structure and Bonding, 102, Springer-Verlag, Berlin, (2002)
Matović, B., Šaponjić, A., Bošković, S.: Carbonitriding reactions of diatomaceous earth: phase evolution and reaction mechanisms. J. Serb. Chem. Soc. 71(6), 677–683 (2006)
Matovic, B., Saponjic, A., Devecerski, A., Miljkovic, M.: Fabrication of SiC by carbothermal-reduction reactions of diatomaceous earth. J. Mater. Sci. 42, 5448–5451 (2007)
Šaponjić, A., Babić, B., Devečerski, A., Matović, B.: Preparation of nanosized non-oxide powders using diatomaceous earth. Sci. Sinter. 41, 151–159 (2009)
Šaponjić, A., Matović, B., Babić, B., Zagorac, J., Poharc-Logar, V., Logar, M.: Cost-effective synthesis of Si3N4-SiC nanocomposite powder. Optoelectron. Adv. Mater. Rapid Commun. 4, 1681–1684 (2010)
Kang, I.H., Komeya, K., Meguro, T., Naito, M., Hayakawa, O.: Effect of silicon nitride seeds addition on the particle size and the crystal form of resulting powder in carbothermal reduction-nitridation of silica. J. Ceram. Soc. Jpn. 104, 471–475 (1996)
Cochran, G.A., Conner, C.L., Eisman, G.A., Weimer, A.W., Carroll, D.F., Dunmead, S.D., Hwang, C.J.: The synthesis of a high quality, low cost silicon nitride power by the carbothermal reduction of silica. Key. Eng. Mater. 89–91, 3–8 (1994)
Komeya, K., Inoue, H.: Synthesis of the alpha form of silicon nitride from silica. J. Mater. Sci. 10, 1243–1246 (1975)
Pekala, R.W.: Organic aerogels from the polycondensation of resorcinol with formaldehyde. J. Mater. Sci. 24, 3221–3227 (1989)
Ziegler, G.: Structural and morphological investigations of ceramic powders and compacts. Powder. Metall. Int. 10, 70–73 (1978)
Dou, K., Jiang, Y., Xue, B., Wei, C., Li, F.: Carbothermal reduction nitridation of fly ash, diatomite and raw illite: formation of nitride powders with different morphology and photoluminescence properties. Crystals 10, 409–421 (2020)
Antsiferov, V.N., Gilev, V.G., Karmanov, V.I.: Infrared spectra and structure of Si3N4, Si2ON2, and SIALONS. Refract. Ind. Ceram. 44, 108–114 (2003)
Ziegler, G., Heinrich, J., Wotting, G.: Review Relationships between processing, microstructure and properties of dense and reaction-bonded silicon nitride. J. Mater. Sci. 22, 3041–3086 (1987)
Jack, K.H.: Review Sialons and related nitrogen ceramics. J. Mater. Sci. 11, 1135–1158 (1976)
Sučik, G., Kuffa, T., Hršak, D.: The use of ditomaceous earth in preparation of sialon powder. METABK 43(2), 93–95 (2004)
Acknowledgements
This research was granted by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia. Grant no. 451-03-47/2023-01/200017
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by [Aleksandra Šaponjić], [Svetlana Ilić], and [Tanja Barudzija]. Conceptualization: [Aleksandra Šaponjić], methodology: [Branko Matović], formal analysis and investigation: [Aleksandra Šaponjić], writing—review and editing: [Aleksandra Šaponjić], [Svetlana Ilić] [Ana Radosavljević Mihajlović], [Maja Kokunešoski], and [Branko Matović]. The first draft of the manuscript was written by [Aleksandra Šaponjić] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Šaponjić, A., Ilić, S., Barudzija, T. et al. Seeded silicon nitride powders obtained by carbothermal reduction—nitridation of diatomite and various sources of carbon. J Aust Ceram Soc 59, 823–835 (2023). https://doi.org/10.1007/s41779-023-00876-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-023-00876-w