Abstract
We studied how host-associations and geography shape the genetic structure of sister species of marine snails Coralliophila radula (A. Adams, 1853) and C. violacea (Kiener, 1836). These obligate ectoparasites prey upon corals and are sympatric throughout much of their ranges in coral reefs of the tropical and subtropical Indo-Pacific. We tested for population genetic structure of snails in relation to geography and their host corals using mtDNA (COI) sequences in minimum spanning trees and AMOVAs. We also examined the evolutionary relationships of their Porites host coral species using maximum likelihood trees of RAD-seq (restriction site-associated DNA sequencing) loci mapped to a reference transcriptome. A maximum likelihood tree of host corals revealed three distinct clades. Coralliophila radula showed a pronounced genetic break across the Sunda Shelf (ΦCT = 0.735) but exhibited no genetic structure with respect to host. C. violacea exhibited significant geographic structure (ΦCT = 0.427), with divergence among Hawaiian populations, the Coral Triangle and the Indian Ocean. Notably, C. violacea showed evidence of ecological divergence; two lineages were associated with different groups of host coral species, one widespread found at all sites, and the other restricted to the Coral Triangle. Sympatric populations of C. violacea found on different suites of coral species were highly divergent (ΦCT = 0.561, d = 5.13%), suggesting that symbiotic relationships may contribute to lineage diversification in the Coral Triangle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Our understanding of evolution in marine ecosystems is framed by theories developed in terrestrial environments (Miglietta et al. 2011). Historically, researchers have invoked geographic-based models of speciation without gene flow (i.e. allopatry) to explain the majority of diversity in terrestrial systems (Barraclough and Vogler 2000). However, such models are not a natural fit for the marine realm (Palumbi 1994; Puebla 2009). Most marine organisms have planktonic larvae that increase the potential for gene flow between geographically separated regions (Riginos and Liggins 2013). Even species with relatively modest mean dispersal distances can have dispersal kernels with long tails (Kinlan and Gaines 2003), providing sufficient genetic connectivity to limit population divergence (Slatkin 1987), even across broad geographic scales.
While uncommon, geographic barriers to gene flow in the ocean do exist, albeit with varying degrees of permeability. Landmasses are the most obvious, isolating biota in different ocean basins (Briggs and Bowen 2013), both currently (e.g. Isthmus of Panama, see Lessios 2008 for review) and in the past (e.g. Sunda Shelf, see Ludt and Rocha 2015 for review). However, vast expanses of open-ocean can isolate remote archipelagos like Hawai'i (e.g. Polato et al. 2010; Iacchei et al. 2016; Waldrop et al. 2016) or populations spanning the Eastern Pacific Barrier (e.g. Baums et al. 2012). Additionally, large freshwater outflows like the Amazon can form barriers to gene flow for shallow-water marine species (Rocha 2003).
Dispersal barriers are critical to the evolution and distribution of marine biodiversity, including in the world’s most diverse marine ecosystem, the Coral Triangle (Barber et al. 2011; Carpenter et al. 2011; Gaither et al. 2011; Gaither and Rocha 2013). Low sea levels during the Pliocene and Pleistocene (Williams and Benzie 1998), and more recent phenomena such as the Halmahera Eddy (Kool et al. 2011), create potent dispersal barriers for various reef organisms (see Barber et al. 2011 and Carpenter et al. 2011 for reviews). Still, allopatric divergence alone may be insufficient to explain the Coral Triangle’s exceptional species diversity. Processes such as ecological divergence and assortative mating could promote divergence with gene flow, but remain relatively unexplored in marine systems (Krug 2011; Miglietta et al. 2011).
Ecological divergence is the evolution of reproductive isolation among populations driven by opposing selection in ecological niches or environments (Schluter and Conte 2009). While widely documented in terrestrial ecosystems, ecological barriers to gene flow in the ocean have only recently been reported (Krug 2011; Bird et al. 2012). In terrestrial and freshwater systems, ecological divergence often takes place in sympatry via assortative mating in different microhabitats or on different hosts in species with strong symbioses (Hatfield and Schluter 1999; Matsubayashi et al. 2010). Evidence suggests that ecological factors (Johannesson et al. 2010; Bird et al. 2011; Prada and Hellberg 2013; Moura et al. 2015) including symbiotic relationships (Munday et al. 2004; Sotka 2005; Faucci et al. 2007; Prada et al. 2014b; Fritts-Penniman 2016) may similarly drive ecological divergence in the marine environment.
Marine snails in the genus Coralliophila are symbionts of anthozoans (Oliverio et al. 2009). The sister species C. radula and C. violacea (Oliverio et al. 2009) are ectoparasites, exhibiting obligate relationships with corals in the family Poritidae (Fujioka and Yamazato 1983). These snails are sedentary and feed suctorially on photosynthetic products sent by corals to regenerate injured sites (Oren et al. 1998). As adults they live in groups and rarely move (Soong and Chen 1991; Oren et al. 1998). Dispersal is achieved via planktonic larvae brooded by protandrous hermaphroditic females (Soong and Chen 1991). Both species have extensive geographic ranges, occurring sympatrically in coral reefs throughout the tropical and subtropical Indo-Pacific.
The purpose of this study was to enhance our understanding of the evolutionary processes generating marine biodiversity in the Coral Triangle. Specifically, we tested the hypothesis that co-distributed populations of C. radula and C. violacea would exhibit concordant patterns of phylogeographic structure, patterns that resulted from physical processes shaping the phylogeography of other marine organisms in the Coral Triangle. However, because parasitic relationships with poritid host corals create the possibility of ecological divergence, we first tested for genetic structure that could result from ecological segregation among sympatric populations of snails utilising different host corals.
Materials and methods
Field sampling
During 2011–2013, we collected Coralliophila radula and C. violacea from Indo-Pacific locations (N = 14 and N = 17 respectively, Table 1, Fig. 1). These sites span the Sunda Shelf Barrier, an area where phylogeographic structure is commonly observed (Barber et al. 2011), and also include known areas of isolation (i.e. Hawai'i). At each site, we collected 1–15 C. radula and 1–16 C. violacea from 1–6 colonies of each host coral species (N = 1–4) (Table 2). In total, we collected 235 C. radula and 328 C. violacea from 114 coral colonies (Table 2), representing 12 named Porites species (Fig. 2). Approximately 50–100% of each snail’s foot tissue was preserved in 95% ethanol and stored at room temperature for DNA analysis.
Population sampling locations across the Indo-West Pacific for ectoparasitic snails (Coralliophila radula, C. violacea) and on a suite of host corals (Porites spp.). Location names and coordinates are shown in Table 1
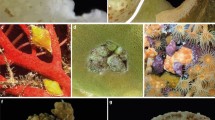
Photo vouchers of the host coral species (a–l) of m C. violacea (Kiener, 1836) and n C. radula (A. Adams, 1855). Photos and tissue samples of each coral colony were taken. a Porites lobata (Dana, 1846), Bali, Indonesia. b Porites solida (Forskål, 1775), Hawai’i, USA. c P. annae (Crossland, 1952), Aceh, Indonesia. d P. evermanni (Vaughan, 1907), Hawai’i, USA. e P. attenuata (Nemenzo, 1955), Bali, Indonesia. f P. compressa (Dana, 1846), Hawai’i, USA. g P. rus (Forskål, 1775), North Sulawesi, Indonesia. h P. lutea (Milne Edwards and Haime, 1851) Sumatra, Indonesia. i P. cylindrica (Dana, 1846), East Nusa Tenggara, Indonesia. j P. nigrescens (Dana, 1848), Sumatra, Indonesia. k P. negrosensis (Veron, 1990) Bali, Indonesia. l P. tuberculosis (Veron, 2000) North Sulawesi, Indonesia. Coral species were identified using Veron (2000) and for P. evermanni Forsman et al. (2015)
Porites corals are notoriously difficult to identify in situ because of their morphological plasticity and small corallites (Forsman et al. 2015), while genetically similar colonies can have vastly different morphologies and vice versa (Forsman et al. 2009, 2015; Prada et al. 2014a). Therefore, to define coral species both morphologically and genetically, we collected detailed information about each snail’s host; tagged photos of coral colonies in situ; took macrophotos with a transparent ruler to measure corallites; and sampled tissues for genetic analysis.
DNA extraction and sequencing
We sequenced 1–16 snails from each coral colony. We extracted DNA using 10% Chelex™ (BioRad, Hercules, CA, USA) (Walsh et al. 2013) and DNeasy® Blood and Tissue Kit (Qiagen, Valencia, CA, USA) and then amplified a 668-bp-length fragment of COI mtDNA using primers HCO-2198, LCO-1490 (Folmer et al. 1994). Following an initial denaturation at 94 °C for 1.5 min, the thermocycling parameters were as follows: 94 °C for 30 secs, 50 °C for 30 secs and 72 °C for 45 secs for 35 cycles with a final 10-min extension at 72 °C. All PCR product clean up and DNA sequencing was done by the University of California, Berkeley DNA Sequencing Facility.
We extracted coral DNA using DNeasy® Blood and Tissue Kit (Qiagen, Valencia, CA, USA). Only extractions with high-quality DNA at high enough concentrations were chosen (N = 51) for library preparation. We chose to sequence coral using a restriction site-associated DNA sequencing (RAD-seq) approach because recent work has shown that these data have a greater potential of resolving relationships within Porites than more commonly used genes such as ITS or COI (Forsman et al. 2017). Coral Illumina libraries were prepared according to methods detailed in the BestRAD protocol (Ali et al. 2016) using the SbfI restriction enzyme, and sequenced using a NextSeq 500 (Illumina, Inc.) sequencer on a mid-output 300 cycle with paired-end reads. All library preparation and sequencing was conducted at the Eagle Fish Genetics Laboratory.
RAD-seq data processing
Coral reads were processed using the iPyrad v0.7.17 pipeline (http://ipyrad.readthedocs.io/) (see Appendix S2 for parameter input file). Sequence data were demultiplexed, low-quality base calls were filtered out, and adapter sequences removed and dereplicated. To focus on coral genes and exclude any DNA from symbiotic micro-organisms, we then mapped reads to a reference transcriptome (Porites lobata available at reefgenomics.org, Bhattacharya et al. 2016) using the programme BWA v0.7.16 (Li and Durbin 2009). From there, highly similar reads were clustered together and aligned. Then, the joint estimate of heterozygosity and sequencing error rates were calculated and used in consensus base calling. Any samples that did not sequence well were removed from further analyses (N = 11). Only loci with less than 20% missing data across taxa were kept, and reads were thinned to one single-nucleotide polymorphism (SNP) per locus to removed linked loci.
Data analysis
Determining host coral identities and evolutionary relationships
To identify coral species, test for cryptic diversity and determine phylogenetic relationships among corals, we aligned our sequences (see Table S1 in Appendix S1) using the iPyrad pipeline and exported alignments in PHYLIP format to Geneious v11 (Kearse et al. 2012). Maximum likelihood analyses were performed using RAxML v8.2.11 with 1000 rapid bootstraps.
Ecological and geographic analyses of genetic structure of Coralliophila
For the snails, we aligned and edited complementary sequences, and confirmed translations in Geneious (Kearse et al. 2012). Sequences were aligned using the MAFFT plugin in Geneious. We trimmed final sequence alignments to 576 bp for C. radula and 617 bp for C. violacea and then reduced all sequences to unique haplotypes using FaBox v1.41 (Villesen 2007). We calculated standard diversity statistics (haplotype and nucleotide diversity) in Arlequin v3.5 (Excoffier and Lischer 2011).
Since genetic structure in the two Coralliophila could be partitioned by host coral or geography, we tested for divergence associated with corals in sympatry. As a first pass to visually examine for genetic structure, we built phylogenetic trees for each snail species using the RAxML plugin in Geneious and labelled each tip with the associated host coral and location. After inspecting these trees, C. violacea showed clear partitioning based on groups of host coral species. These two groupings corresponded to the major clades (clade 1, clade 2/3) present in the Porites RAD-seq tree (Fig. 3). Given the well-documented taxonomic challenges in Porites (Forsman et al. 2009, 2015; Prada et al. 2014a) and possible cryptic species, we opted to group host coral supported clades (Fig. 3) that were seen in both the Porites tree and the C. violacea tree, using these groupings (Gold = clade 1, Green = clade 2/3) for all tests of genetic structure in relation to host coral. We created minimum spanning trees (MSTs) using Gephi v 0.8.2 (Bastian et al. 2009) based on pairwise differences calculated in Arlequin, only including haplotypes from populations where C. radula and C. violacea were found on different host coral clades (Gold = clade 1, Green = clade 2/3) at the same location. To formally, test for structure based on host we plotted coral groups onto MSTs and analysed molecular variance (AMOVA) in Arlequin, partitioning the genetic data by coral group. To estimate the relative contributions of geographic divergence versus divergence in sympatry, we calculated pairwise ΦST values among locations within host-associated lineages, and then calculated pairwise ΦST between host-associated populations within individual sampling locations.
RAxML phylogenetic tree of 40 RAD sequences (10,882 bps) mapped to the transcriptome of P. lobata, from 11 named Porites species. Tip labels are the sample code followed by the species name. * = nodes with > 80% bootstrap support. Green and Gold groups are those observed to be used by divergent lineages of C. violacea
To test for phylogeographic partitions, we constructed MSTs and then plotted the resulting haplogroups onto a map of the study area. We then ran AMOVAs with and without a priori geographic partitions to test for genetic structure related to divergence related to isolation across the Sunda Shelf with significance determined by 100,000 random replicates.
Results
Of the snail species, C. radula was less abundant (235 vs. 328), found at fewer locations (14 vs. 17; Table 2) and found on fewer coral species (8 vs. 12; Table 2). The sister species of Coralliophila exhibited ecological niche overlap in the coral species they inhabited, sharing at least seven named host coral species (Table 2). In addition, looking at the hosts that they shared, Coralliophila spp. were found in syntopy on about half of all the sampled coral colonies.
Sequences and genetic diversity
We successfully sequenced the DNA of 11 named species of Porites from 40 colonies using RAD-seq and the dataset was deposited in the Dryad Data Repository (https://doi.org/10.5061/dryad.jv853v1) (Appendix S1). We obtained 235 COI sequences from C. radula (567 bp) and 328 from C. violacea (617 bp), yielding totals of 192 and 296 unique haplotypes, respectively. All sequences were deposited in GenBank (Accession numbers: MG917096–MG917657).
Both snail species had high haplotype diversity (C. radula: h = 0.966–1.00 and C. violacea: h = 0.900–1.00, Table 3) in all populations except one: C. violacea (Pulau Keluang; h = 0.667, Table 3). Nucleotide diversity was low in both species (C. radula: π = 0.011–0.024 and C. violacea: π = 0.011–0.040), although across all locations C. violacea had a higher average number of polymorphic sites (N = 67/617 bp) than C. radula (N = 41/567 bp).
Phylogenetics of host coral species
The RAxML tree of 40 Porites coral RAD sequences (Table S1 in Appendix S1, Fig. 3) resolved some of the named species as reciprocally monophyletic (i.e. P. rus, P. annae, P. evermanni). At a deeper level, three well-supported clades were apparent. Clade 1 included three species with branching morphologies P. cylindrica, P. negrosensis and P. nigrescens. These colonies were not reciprocally monophyletic to the named species level, but this could be due to cryptic diversity. Clade 2 was composed of three species with nodular growth forms of varying sizes: P. annae, P. evermanni and P. rus. All three species were well supported. Clade 3 contained named species of various morphologies including massive types P. lobata, P. lutea and P. solida, as well as two named branching species P. attenuata and P. compressa. While colonies from the same species clustered together, they did not have high support values.
Analysis of ecological divergence in Coralliophila
To investigate the genetics of snails for structure in relation to host coral, we first built MSTs of haplotypes from locations where snails from Porites groups (Gold = clade 1, Green = clade 2/3) were sampled. There was no evidence of genetic structure in relation to host in C. radula (Fig. 4a), which we confirmed with AMOVA analyses (ΦCT = –0.018, p = 1.000, Table 4).
Sympatric host coral groups (colours same as in Fig. 3) plotted onto minimum spanning trees of a 57 COI haplotypes from 65 Coralliophila radula. Haplogroups separated by more than 13 steps are indicated with numbers. b 188 COI haplotypes from 200 C. violacea. Circles sizes are proportional to the frequency of haplotypes. Haplogroups separated by more than 20 steps are indicated with numbers. Line thickness scales with the number of mutational steps between haplogroups
In contrast, the MST of C. violacea on sympatric hosts showed two lineages (A and B) largely concordant with host groups (Gold = clade 1, Green = clade 2/3; Figs. 2, 3). Lineage A of C. violacea (Fig. 4b) was found predominately on nine named species of Porites (P. annae, P. attenuata, P. compressa, P. evermanni, P. lobata, P. lutea, P. rus, P. solida) belonging to the Green group of Porites (clade 2/3, Fig. 3). Lineage B of C. violacea (Fig. 4b) was found on three different named Porites species (P. cylindrica, P. negrosensis and P. nigrescens), from the Gold group of Porites (clade 1, Fig. 3) as well as P. tuberculosus. AMOVA also showed marked genetic differentiation between hosts in sympatry (ΦCT = 0.561, p = 0.003, d = 5.13%, Table 4), but no geographic structure among populations within host (ΦSC = 0.003, p = 0.328, Table 4). Despite these distinctions, we found occasional mismatches between C. violacea mtDNA background and their host (Fig. 4b). Some C. violacea collected from Porites clade 1 species fell in MST lineage A (mean = 11.7%, Fig. 4b). However, we identified only one mismatch the other way, when host corals were sympatric (Fig. 4b).
Phylogeographic analyses of Coralliophila
Because there was no ecological divergence observed in C. radula, we tested for phylogeographic structure using all haplotypes. The MST revealed three deeply divergent haplogroups separated by 18 or more steps that were concordant with geography (Fig. 5). The red group was restricted to the Indian Ocean. The blue group was the most common, present at all sites in the Pacific Ocean. The yellow group was rarest and found only within the Coral Triangle.
Coralliophila radula. a Minimum spanning tree of COI haplotypes. Circle size corresponds to haplotype frequency. Haplogroups with 18 or more mutational steps between them are coloured. Line thickness scales with the number of mutational steps between haplogroups. b Map showing the geographic distribution of haplogroups. Circle size corresponds to the number of individuals sampled at each location
Due to the strong genetic associations by host coral group in C. violacea, we tested for phylogeographic structure separately within samples collected from each coral group. The MST of C. violacea collected from Porites clade 2/3 distinguished six haplogroups (Fig. 6). The blue group was most common, present at all sites, and dominated Coral Triangle sites. The red group dominated sites in the Indian Ocean (> 75%). The yellow group was found almost exclusively within the Coral Triangle with the exception of Pulau Weh and was concordant with snails found on hosts of the mismatched genetic group. The purple group was restricted to Hawai'i. There were also two rare, but divergent, haplogroups (turquoise, pink) only seen at sites within the Coral Triangle (Hòn Mun, Ticao, Díli and Bunaken).
Coralliophila violacea collected from Porites species in the Green group (clades 2/3) only. a Minimum spanning tree of 204 COI haplotypes from 234 snails. Circle size corresponds to the number of individuals with that haplotype. Haplotypes are coloured by groups with 21 or more mutational steps between them, or groups of haplotypes dominating a geographic area. b Map showing the geographic distribution of haplogroups. Circle size corresponds to the number of individuals sampled at each location
Non-hierarchical AMOVAs of all haplotypes showed significant genetic structure in C. radula (ΦST = 0.531, P = 0.000; Table 4) and C. violacea (ΦST = 0.213, P = 0.000; Table 4). The per cent variation in C. radula was almost equal among (53%) and within (47%) populations. However, in C. violacea more variation was present within (79%) than among (21%) populations (Table 4).
Hierarchical AMOVA analyses comparing C. radula populations from the Indian Ocean, and the Coral Triangle + Hawai'i, spanning the Sunda Shelf, revealed a prominent genetic break (ΦCT = 0.735, P = 0.011, 5% sequence divergence), with the most variation (74%) between ocean basins (Table 4). However, only 0.5% of the variation was among populations within oceans (ΦSC = 0.018, p = 0.039, Table 4). More isolated populations like Hawai'i were significantly different from a few populations in the Coral Triangle (Dumaguete, Pemuteran, Pulau Mengyatan) but only marginally so (pairwise ΦST = 0.120–0.140, Table 5).
Where sample sizes were sufficient, a non-hierarchical AMOVA of C. violacea from Porites clade 1 in the Coral Triangle showed no significant genetic structure among populations (2% var.; ΦST = 0.020, P = 0.091). However, a couple of pairwise ΦST distances were significant: populations in Lembeh were different from Komodo (ΦST = 0.048), and South Bali (ΦST = 0.109).
Because Hawaiian populations of C. violacea from Porites species in the Green group (clade 2/3) were distinct in the MST (Fig. 6), we defined three partitions: (1) Indian Ocean, (2) Coral Triangle and (3) Hawai'i for AMOVAs. Genetic structure was strong (ΦCT = 0.427, P = 0.002, Table 6) with 43% of the variation among regions (Table 6). Snails from Hawai'i were the most genetically distinct, resulting in the highest pairwise ΦST values (ΦST = 0.475–0.689, Table 6). Populations from Hòn Mun in Vietnam were also genetically distinct from all other populations except Díli in Timor-Leste (ΦST = 0.081–0.447, Table 6).
Discussion
Although phylogeographic studies in the Coral Triangle typically focus on allopatric divergence, results from the corallivorous snail C. violacea showed evidence for ecological divergence via host-shifting. Two lineages of C. violacea were strongly concordant with the groups of Porites species from which these snails were collected. Even within locations, there was genetic divergence among snails collected from different groups of host coral species. Given the high prevalence of symbioses in coral reef ecosystems, the recovery of ecological divergence in C. violacea suggests that ecology could be an important driver of lineage diversification in the epicentre of marine biodiversity, the Coral Triangle (e.g. Hoeksema 2007; Gaither and Rocha 2013).
Both C. radula and C. violacea showed evidence of phylogeographic structure across the Sunda Shelf, as predicted for ecologically similar, sympatrically distributed sister taxa. This classic phylogeographic pattern is observed in a wide diversity of Indo-Pacific marine taxa (Carpenter et al. 2011; Barber et al. 2011; Bowen et al. 2013) and is typically attributed to eustatic sea level fluctuations. In addition to divergence across the Sunda Shelf, C. violacea populations in Hawai'i were also highly divergent from all other locations, indicating divergence at both the centre and in more isolated areas of this species’ geographic range (see Bowen et al. 2013).
Ecological barriers
Within the Coral Triangle, two sympatric haplogroups of C. violacea were concordant with host coral groups. Ecological divergence among populations inhabiting sympatric host taxa is commonly reported for terrestrial species, particularly phytophagous insects such as fruit flies (Bush 1969), pea aphids (Peccoud et al. 2009), butterflies (Fordyce 2010) and stick insects (Nosil et al. 2012). However, marine studies have not typically found evidence for genetic structure among populations on different, sympatrically distributed hosts (e.g. Sotka et al. 2003; Johnston et al. 2012; Li and O’Foighil 2012), with the exception of sponge-dwelling snapping shrimp (Duffy 1996), and Phestilla nudibranchs that are also parasites of Porites (Fritts-Penniman 2016). However, ecological barriers have been reported at the species level in Symbiodinium and their anthozoan hosts (LaJeunesse 2005; Bongaerts et al. 2011).
While sympatric populations of parasites from different hosts can be genetically distinct, frequently they can still exchange genes (Dres and Mallet 2002). Indeed, the small number of mismatched mtDNA haplotypes on the C. violacea host MST could be the result of either incomplete lineage sorting, or current/historical gene flow. However, even reduced gene flow resulting from segregation by the host can, over time, result in speciation (Matsubayashi et al. 2010). Phylogenetic studies of symbiotic marine taxa have discovered host-specific cryptic species in anemone dwelling snapping shrimp (Hurt et al. 2013) and anthozoan-associated barnacles (Tsang et al. 2009), snails (Gittenberger and Gittenberger 2011), nudibranchs (Faucci et al. 2007; Fritts-Penniman 2016) and fishes (Munday et al. 2004; Litsios et al. 2012; Tornabene et al. 2013).
The significant genetic patterns we report in C. violacea could be the result of different host-associated haplogroups having distinct host preferences and experiencing differential selection. Previous studies have hinted at host preferences in C. violacea (Fujioka and Yamazato 1983), as well as differential selection on different host morphologies (Chen et al. 2004). However, those studies did not characterise the genetics of the host corals, making their results difficult to interpret in the context of this work. It also emphasises the importance of collecting both host and symbiont data for DNA testing, especially given the challenges of coral taxonomy and possibility of cryptic species.
It is not clear what mechanisms are driving the strong association between the divergent C. violacea haplogroups and their assemblages of host corals. However, possibilities may include: (1) larval settlement cues as found with corallivorous Phestilla nudibranchs (Ritson-Williams et al. 2009), (2) differences in nutritional quality (Yamashiro et al. 1999; Baums et al. 2003), or (3) potential secondary metabolites/pigments development (Wang et al. 2008) due to reduced physical defences (Connell 1973). Whether the mechanism is secondary metabolites, settlement cues, or nutrients, interactions between parasites and hosts are most likely chemically mediated, representing a fruitful avenue of research for understanding the ecological and evolutionary dynamics of host-parasite associations.
Geographic barriers
Co-distributed species with equivalent ecologies and life histories should be impacted in similar ways by broadly acting physical processes (Avise 2000; Marko and Hart 2011). Both C. radula and C. violacea exhibited strong genetic divergence between Indian and Pacific Ocean populations spanning the Sunda Shelf. During the Pleistocene, sea levels repeatedly dropped by 100–140 m, cyclically exposing the Sunda Shelf, and creating a partial barrier between the two oceans that lasted for ~ 15,000–30,000 years (Voris 2000). Genetic structure among marine organism populations spanning the Sunda Shelf is typically attributed to these sea level changes (Gaither and Rocha 2013), and numerous marine molluscs show phylogeographic structure across this region (Crandall et al. 2008; DeBoer et al. 2008; Kochzius et al. 2008; Nuryanto and Kochzius 2009).
The 5% COI sequence divergence we observed in C. radula suggests that separation across the Sunda Shelf began at the latest in the Pliocene/Pleistocene (~ 2.5 Ma) assuming a heuristic molecular clock with a conservative divergence rate of 1%/myr for molluscan COI (Marko 2002; 0.7–1.2%/myr). Therefore, time dependency of substitution rates in other marine invertebrates from this region yield estimates of 2.3–6.7%/myr (Crandall et al. 2011), suggesting that divergence could have occurred less than 1 Ma. Either way, these values place divergence within periods of modern glacial cycles and resulting sea level fluctuations.
The genetic isolation of Hawaiian populations of C. violacea is seen in many other Indo-Pacific species (summarised in Gaither et al. 2011), and the levels of population structure we saw were also similar to other findings (COI, ΦST = 0.08–0.89; Skillings et al. 2011). Yet surprisingly, there was only weak genetic structure between populations of the sister species C. radula in Hawai'i and a few Coral Triangle populations. It remains unclear why species with nearly identical ecological niches and life history strategies, and which inhabit the same hosts and overlap in the majority of their geographic ranges, would have concordant patterns in one part of their range, but discordant patterns in the other. It is unlikely that they differ drastically in planktonic larval duration, so possible explanations include different population demographics, and/or the timing of colonisation or expansion into different parts of their ranges. For example, as evidenced by star polytomies in MSTs and relative abundances, C. radula may have experienced recent population expansions in the Coral Triangle, whereas C. violacea may have expanded in the Indian Ocean. Similarly, subtle ecological differences might structure populations in ways we cannot untangle without information about individuals (e.g. microhabitat) for each specimen collected. For instance, while both species were found on the host coral colonies, they may specialise on different microhabitats (Fernández-González et al. 2015) or nutrients within a host.
A tale of two species
As with the discordant phylogeographic structure between C. radula and C. violacea, it is puzzling why C. violacea has diverged on different host corals, while C. radula has not. There are two possible explanations. First, the two snail species may be responding differently to the same selective pressures because of different evolutionary histories affecting the genetic background upon which selection acts (Prunier et al. 2012). Second, C. radula may in fact be diverging on different hosts and selection is occurring in the face of gene flow at certain loci, but this process began too recently or is too weak to be evident in neutral loci. Previous studies reported adaptations to different hosts by herbivorous marine invertebrates, and genetically mediated differences in fitness on hosts (e.g. Sotka et al. 2003), yet could not find any genetic structure in mtDNA. Finally, differences in fitness and selection between host-associated populations could be maintained under on-going gene flow, a process demonstrated in numerous other systems (e.g. Mullen and Hoekstra 2008). To help settle this question, whole-genome or reduced representation sequencing of C. violacea is needed to look for candidate loci under selection and estimate gene flow among populations.
Allopatric speciation was such a dominant model of speciation, that early terrestrial studies reporting sympatric speciation mediated by ecological differences (ecological speciation) were met with scepticism (Bird et al. 2011). Today, however, a growing body of the literature indicates ecological speciation is more common than previously thought. While studies of ecological divergence in the ocean are still in their infancy, the pervasiveness of obligate host relationships in the marine environment suggests that ecologically mediated divergence and speciation could be important in the evolution of marine biodiversity, particularly in hyper-diverse regions like the Coral Triangle.
References
Ali OA, O’Rourke SM, Amish SJ, Meek MH, Luikart G, Jeffres C, Miller MR (2016) RAD Capture (Rapture): flexible and efficient sequence-based genotyping. Genetics 202:389–400
Avise JC (2000) Phylogeography: the history and formation of species. Harvard University Press, Cambridge
Barber PH, Cheng SH, Erdmann MV, Tenggardjaja K, Ambariyanto (2011) Evolution and conservation of marine biodiversity in the Coral Triangle: Insights from stomatopod Crustacea. In: Held C, Koenemann S, Schubart CD (eds) Phylogeography and population genetics in Crustacea. Crustean Issues. CRC Press, Boca Raton, pp 129–156
Barraclough TG, Vogler AP (2000) Detecting the geographical pattern of speciation from species-level phylogenies. Am Nat 155:419–434
Bastian M, Heymann S, Jacomy M (2009) Gephi: an open source software for exploring and manipulating networks. International AAAI Conference on Weblogs and Social Media
Baums IB, Miller MW, Szmant AM (2003) Ecology of a corallivorous gastropod, Coralliophila abbreviata, on two scleractinian hosts. I: Population structure of snails and corals. Mar Biol 142:1083–1091
Baums IB, Boulay JN, Polato NR, Hellberg ME (2012) No gene flow across the Eastern Pacific Barrier in the reef-building coral Porites lobata. Mol Ecol 21:5418–5433
Bhattacharya D, Agrawal S, Aranda M, Baumgarten S, Belcaid M, Drake JL, Erwin D, Foret S, Gates RD, Gruber DF, Kamel B, Lesser MP, Levy O, Liew YJ, MacManes M, Mass T, Medina M, Mehr S, Meyer E, Price DC, Putnam HM, Qiu H, Shinzato C, Shoguchi E, Stokes AJ, Tambutté S, Tchernov D, Voolstra CR, Wagner N, Walker CW, Weber APM, Weis V, Zelzion E, Zoccola D, Falkowski PG (2016) Comparative genomics explains the evolutionary success of reef-forming corals. Elife 5:e13288. https://doi.org/10.7554/eLife.13288
Bird CE, Holland BS, Bowen BW, Toonen RJ (2011) Diversification of sympatric broadcast-spawning limpets (Cellana spp.) within the Hawaiian archipelago. Mol Ecol 20:2128–2141
Bird CE, Fernandez-Silva I, Skillings DJ, Toonen RJ (2012) Sympatric speciation in the post “Modern Synthesis” era of evolutionary biology. Evol Biol 39:158–180
Bongaerts P, Riginos C, Hay KB, van Oppen MJ, Hoegh-Guldberg O, Dove S (2011) Adaptive divergence in a scleractinian coral: physiological adaptation of Seriatopora hystrix to shallow and deep reef habitats. BMC Evol Biol 11:303
Bowen BW, Rocha LA, Toonen RJ, Karl SA (2013) The origins of tropical marine biodiversity. Trends Ecol Evol 28:359–366
Briggs JC, Bowen BW (2013) Marine shelf habitat: Biogeography and evolution. J Biogeogr 40:1023–1035
Bush GL (1969) Sympatric host race formation and speciation in frugivorous flies of the genus Rhagoletis (Diptera, Tephritidae). Evolution 23:237–251
Carpenter KE, Barber PH, Crandall ED, Ablan-Lagman MCA, Ambariyanto Mahardika GN, Manjaji-Matsumoto BM, Juinio-Meñez MA, Santos MD, Starger CJ, Toha AHA (2011) Comparative phylogeography of the Coral Triangle and implications for marine management. J Mar Biol 2011:1–14
Chen M-H, Soong K, Tsai M-L (2004) Host effect on size structure and timing of sex change in the coral-inhabiting snail Coralliophila violacea. Mar Biol 144:287–293
Connell J (1973) Population Ecology of Reef-Building Corals. In: Jones O, Endean R (eds) Biology and Geology of Coral Reefs V2: Biology 1. Academic Press, INC, pp 205–244
Crandall ED, Frey MA, Grosberg RK, Barber PH (2008) Contrasting demographic history and phylogeographical patterns in two Indo-Pacific gastropods. Mol Ecol 17:611–626
Crandall ED, Sbrocco EJ, DeBoer TS, Barber PH, Carpenter KE (2011) Expansion dating: Calibrating molecular clocks in marine species from expansions onto the Sunda Shelf following the Last Glacial Maximum. Mol Biol Evol 29:707–719
DeBoer TS, Subia MD, Ambariyanto Erdmann M V, Kovitvongsa K, Barber PH (2008) Phylogeography and limited genetic connectivity in the endangered boring giant clam across the Coral Triangle. Conserv Biol 22:1255–1266
Dres M, Mallet J (2002) Host races in plant-feeding insects and their importance in sympatric speciation. Philos Trans R Soc B Biol Sci 357:471–492
Duffy JE (1996) Resource-associated population subdivision in a symbiotic coral-reef shrimp. Evolution 50:360–373
Excoffier E, Lischer H (2011) ARLEQUIN: An integrated software package for population genetics data analysis. Whats New 1–174
Faucci A, Toonen RJ, Hadfield MG (2007) Host shift and speciation in a coral-feeding nudibranch. Proc R Soc B Biol Sci 274:111–119
Fernández-González S, Pérez-Rodríguez A, de la Hera I, Proctor HC, Pérez-Tris J (2015) Different space preferences and within-host competition promote niche partitioning between symbiotic feather mite species. Int J Parasitol 45:655–662
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Fordyce JA (2010) Host shifts and evolutionary radiations of butterflies. Proc R Soc B Biol Sci 277:3735–3743
Forsman ZH, Barshis DJ, Hunter CL, Toonen RJ (2009) Shape-shifting corals: molecular markers show morphology is evolutionarily plastic in Porites. Evol Biol 9:45–54
Forsman Z, Wellington GM, Fox GE, Toonen RJ (2015) Clues to unraveling the coral species problem: distinguishing species from geographic variation in Porites across the Pacific with molecular markers and microskeletal traits. PeerJ 3:e751
Forsman ZH, Knapp ISS, Tisthammer K, Eaton DAR, Belcaid M, Toonen RJ (2017) Coral hybridization or phenotypic variation? Genomic data reveal gene flow between Porites lobata and P. compressa. Mol Phylogenet Evol 111:132–148
Fritts-Penniman A (2016) Ecological speciation and cryptic diversity of coral-associated nudibranchs. University of California, Los Angeles, PhD dissertation
Fujioka Y, Yamazato K (1983) Host selection of some Okinawan coral associated gastropods belonging to the genera Drupella, Coralliophila and Quoyula. Galaxea 2:59–73
Gaither MR, Jones SA, Kelley C, Newman SJ, Sorenson L, Bowen BW (2011) High connectivity in the deepwater snapper Pristipomoides filamentosus (Lutjanidae) across the Indo-Pacific with isolation of the Hawaiian Archipelago. PLoS One 6:e28913
Gaither MR, Rocha LA (2013) Origins of species richness in the Indo-Malay-Philippine biodiversity hotspot: Evidence for the centre of overlap hypothesis. J Biogeogr 40:1638–1648
Gittenberger A, Gittenberger E (2011) Cryptic, adaptive radiation of endoparasitic snails: Sibling species of Leptoconchus (Gastropoda: Coralliophilidae) in corals. Org Divers Evol 11:21–41
Hatfield T, Schluter D (1999) Ecological speciation in sticklebacks: environment-dependent hybrid fitness. Evolution 53:866–873
Hoeksema B (2007) Delineation of the Indo-Malayan centre of maximum marine biodiversity: the Coral Triangle. In: Renema W. (eds) Biogeography, Time, and Place: Distributions, Barriers, and lslands. Topics in Geobiology, vol 29. Springer, Dordrecht, pp 117–178
Hurt C, Silliman K, Anker A, Knowlton N (2013) Ecological speciation in anemone-associated snapping shrimps (Alpheus armatus species complex). Mol Ecol 22:4532–4548
Iacchei M, Gaither MR, Bowen BW, Toonen RJ (2016) Testing dispersal limits in the sea: Range-wide phylogeography of the pronghorn spiny lobster Panulirus penicillatus. J Biogeogr 43:1032–1044
Johannesson K, Panova M, Kemppainen P, Andre C, Rolan-Alvarez E, Butlin RK (2010) Repeated evolution of reproductive isolation in a marine snail: unveiling mechanisms of speciation. Philos Trans R Soc B Biol Sci 365:1735–1747
Johnston L, Miller MW, Baums IB (2012) Assessment of host-associated genetic differentiation among phenotypically divergent populations of a coral-eating gastropod across the Caribbean. PLoS One 7:e47630
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649
Kinlan B, Gaines S (2003) Propagule dispersal in marine and terrestrial environments: a community perspective. Ecology 84:2007–2020
Kochzius M, Nuryanto A, Genetics M (2008) Strong genetic population structure in the boring giant clam, Tridacna crocea, across the Indo-Malay Archipelago: implications related to evolutionary processes and connectivity. Mol Ecol 17:3775–3787
Kool JT, Paris CB, Barber PH, Cowen RK (2011) Connectivity and the development of population genetic structure in Indo-West Pacific coral reef communities. Glob Ecol Biogeogr 20:695–706
Krug PJ (2011) Patterns of speciation in marine gastropods: A review of the phylogenetic evidence for localized radiations in the sea. Am Malacol Bull 29:169–186
LaJeunesse TC (2005) “Species” radiations of symbiotic dinoflagellates in the Atlantic and Indo-Pacific since the Miocene-Pliocene transition. Mol Biol Evol 22:570–581
Lessios HA (2008) The great American schism: divergence of marine organisms after the rise of the Central American Isthmus. Annu Rev Ecol Evol Syst 39:63–91
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760
Li J, O’Foighil D (2012) Host-specific morphologies but no host races in the commensal bivalve Neaeromya rugifera. Invertebr Biol 131:197–203
Litsios G, Sims CA, Wüest RO, Pearman PB, Zimmermann NE, Salamin N (2012) Mutualism with sea anemones triggered the adaptive radiation of clownfishes. BMC Evol Biol 12:212
Ludt WB, Rocha LA (2015) Shifting seas: The impacts of Pleistocene sea-level fluctuations on the evolution of tropical marine taxa. J Biogeogr 42:25–38
Marko PB (2002) Fossil calibration of molecular clocks and the divergence times of geminate species pairs separated by the Isthmus of Panama. Mol Biol Evol 19:2005–2021
Marko PB, Hart MW (2011) The complex analytical landscape of gene flow inference. Trends Ecol Evol 26:448–456
Matsubayashi KW, Ohshima I, Nosil P (2010) Ecological speciation in phytophagous insects. Entomol Exp Appl 134:1–27
Miglietta MP, Faucci A, Santini F (2011) Speciation in the sea: Overview of the symposium and discussion of future directions. Integr Comp Biol 51:449–455
Moura AE, Kenny JG, Chaudhuri RR, Hughes MA, Reisinger RR, de Bruyn PJN, Dahlheim ME, Hall N, Hoelzel AR (2015) Phylogenomics of the killer whale indicates ecotype divergence in sympatry. Heredity 114:48–55
Mullen LM, Hoekstra HE (2008) Natural selection along an environmental gradient: A classic cline in mouse pigmentation. Evolution 62:1555–1570
Munday PL, Herwerden LV, Dudgeon CL (2004) Evidence for sympatric speciation by host shift in the sea. Curr Biol 14:1498–1504
Nosil P, Gompert Z, Farkas TE, Comeault AA, Feder JL, Buerkle CA, Parchman TL (2012) Genomic consequences of multiple speciation processes in a stick insect. Proc R Soc B Biol Sci 279:5058–5065
Nuryanto A, Kochzius M (2009) Highly restricted gene flow and deep evolutionary lineages in the giant clam Tridacna maxima. Coral Reefs 28:607–619
Oliverio M, Barco A, Richter A, Modica MV (2009) The coralliophiline (Gastropoda: Muricidae) radiation: Repeated colonizations of the deep sea? Nautilus 123:113–120
Oren U, Brickner I, Loya Y (1998) Prudent sessile feeding by the corallivore snail Coralliophila violacea on coral energy sinks. Proc R Soc London Ser B 265:2043–2050
Palumbi SR (1994) Genetic divergence, reproductive isolation, and marine speciation. Annu Rev Ecol Syst 25:547–572
Peccoud J, Ollivier A, Plantegenest M, Simon J-C (2009) A continuum of genetic divergence from sympatric host races to species in the pea aphid complex. Proc Natl Acad Sci 106:7495–7500
Polato NR, Concepcion GT, Toonen RJ, Baums IB (2010) Isolation by distance across the Hawaiian Archipelago in the reef-building coral Porites lobata. Mol Ecol 19:4661–4677
Prada C, Hellberg ME (2013) Long prereproductive selection and divergence by depth in a Caribbean candelabrum coral. Proc Natl Acad Sci 110:3961–3966
Prada C, De Biasse MB, Neigel JE, Yednock B, Stake JL, Forsman ZH, Baums IB, Hellberg ME (2014a) Genetic species delineation among branching Caribbean Porites corals. Coral Reefs 33:1019–1030
Prada C, McIlroy SE, Beltrán DM, Valint DJ, Ford SA, Hellberg ME, Coffroth MA (2014b) Cryptic diversity hides host and habitat specialization in a gorgonian-algal symbiosis. Mol Ecol 23:3330–3340
Prunier R, Holsinger KE, Carlson JE (2012) The effect of historical legacy on adaptation: Do closely related species respond to the environment in the same way? J Evol Biol 25:1636–1649
Puebla O (2009) Ecological speciation in marine v. freshwater fishes. J Fish Biol 75:960–996
Riginos C, Liggins L (2013) Seascape genetics: populations, individuals, and genes marooned and adrift. Geogr Compass 7:197–216
Ritson-Williams R, Shjegstad SM, Paul VJ (2009) Larval metamorphosis of Phestilla spp. in response to waterborne cues from corals. J Exp Mar Bio Ecol 375:84–88
Rocha LA (2003) Patterns of distribution and processes of speciation in Brazilian reef fishes. J Biogeogr 30:1161–1171
Schluter D, Conte GL (2009) Genetics and ecological speciation. Proc Natl Acad Sci 106:9955–9962
Skillings DJ, Bird CE, Toonen RJ (2011) Gateways to Hawai'i: genetic population structure of the tropical sea cucumber Holothuria atra. J Mar Biol 2011:1–16
Slatkin M (1987) Gene flow and the geographic structure of natural populations. Science 236:787–792
Soong K, Chen JL (1991) Population structure and sex-change in the coral-inhabiting snail Coralliophila violacea at Hsiao-Liuchiu, Taiwan. Mar Biol 111:81–86
Sotka EE (2005) Local adaptation in host use among marine invertebrates. Ecol Lett 8:448–459
Sotka EE, Wares JP, Hay ME (2003) Geographic and genetic variation in feeding preference for chemically defended seaweeds. Evolution 57:2262–2276
Tornabene L, Ahmadia GN, Berumen ML, Smith DJ, Jompa J, Pezold F (2013) Evolution of microhabitat association and morphology in a diverse group of cryptobenthic coral reef fishes (Teleostei: Gobiidae: Eviota). Mol Phylogenet Evol 66:391–400
Tsang LM, Chan BKK, Shih F-L, Chu KH, Allen Chen C (2009) Host-associated speciation in the coral barnacle Wanella milleporae (Cirripedia: Pyrgomatidae) inhabiting the Millepora coral. Mol Ecol 18:1463–1475
Villesen P (2007) FaBox: An online toolbox for FASTA sequences. Mol Ecol Notes 7:965–968
Voris H (2000) Maps of Pleistocene sea levels in Southeast Asia: shorelines, river systems and time durations. J Biogeogr 27:1153–1167
Waldrop E, Hobbs JPA, Randall JE, Dibattista JD, Rocha LA, Kosaki RK, Berumen ML, Bowen BW (2016) Phylogeography, population structure and evolution of coral-eating butterflyfishes (Family Chaetodontidae, genus Chaetodon, subgenus Corallochaetodon). J Biogeogr 43:1116–1129
Walsh PS, Metzger DA, Higuchi R (2013) Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 54:506–513
Wang CY, Liu HY, Shao CL, Wang YN, Li L, Guan HS (2008) Research progress on chemical defensive substances from soft corals and gorgonians. Acta Ecol Sin 28:2320–2328
Williams ST, Benzie JAH (1998) Evidence of a phylogeographic break between populations of a high-dispersal starfish: congruent regions within the Indo-West Pacific defined by color morphs, mtDNA and allozyme data. Evolution 52:87–99
Yamashiro H, Oku H, Higa H, Chinen I, Sakai K (1999) Composition of lipids, fatty acids and sterols in Okinawan corals. Comp Biochem Physiol - B Biochem Mol Biol 122:397–407
Acknowledgements
This work was supported by three National Science Foundation programs (OISE-0730256, OISE-1243541 and OCE-0349177), and a US Agency for International Development Grant (497-A-00-10-00008-00). The Lemelson Foundation Fellowship, Conchologists of America, Sigma Xi and the UCLA Department of Ecology and Evolutionary Biology provided additional funding to S. Simmonds. We acknowledge support from the Indonesia government including the Indonesian Ministry of Research and Technology (RISTEK), Indonesian Institute of Sciences (LIPI), Nature Conservation Agency (BKSDA) and the National Marine Park offices of Bunaken and Wakatobi. Sampling was covered under research permits obtained in Indonesia (RISTEK 2011, 198/SIP/FRP/SMNl/2012, 187/SIP/FRP/SM/VI/2013), Timor-Leste (Direccao nacional de Pescase Aquicultura 0042/DNPA/IOP/VII/11), Vietnam, the Philippines (Department of Agriculture-Bureau of Fisheries and Aquatic Resources), the Maldives (Ministry of Fisheries and Aquaculture Permit No. (OTHR)30-D/INDIV/2013/116) and Hawai'i (Department of Land and Natural Resources SAP 2013-11). Thanks to C. Meyer for comments that stimulated this study. Thank you to A. Fritts-Penniman, B. Stockwell, M. Weber, H. Nuetzel and D. Willette for collecting specimens. Thanks also to Z. Forsman and C. Reboton for help identifying Porites. Thank you to M. Campbell, N. Vu and D. Eardley at the Eagle Fish Genetics Laboratory (Eagle, Idaho) for sequencing the coral DNA. Thanks to those that provided advice about or assistance with fieldwork and permits: A. Fritts-Penniman, D. Cahyani, A. Wahyu, Z.A. Muchlisin, E. Rudi, A. Muhardy, F. Hadinata, P. Usef, A. Ackiss, G. Arlotti, D. Smith, M. Sweet and R. Pooley. Diving support provided by Bali Diving Academy, Critters@Lembeh, Lorenso’s Cottages, Cubadak Paradiso Village, Komodo National Park, Freddies Santai Sumurtiga, Lumba-lumba Diving Centre, Cendrawasih Bay National Park, Papua State University, Papua Diving, Kri Eco Resorts and the Korallion Lab. We also thank the Indonesian Biodiversity Research Center at Udayana University, Institute for Environmental and Marine Sciences at Silliman University, Nha Trang University and the Korallion Lab in the Maldives for institutional support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Topic Editor Dr. Line K. Bay
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Simmonds, S.E., Chou, V., Cheng, S.H. et al. Evidence of host-associated divergence from coral-eating snails (genus Coralliophila) in the Coral Triangle. Coral Reefs 37, 355–371 (2018). https://doi.org/10.1007/s00338-018-1661-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-018-1661-6