Abstract
Objectives
Evaluate the influence of an MRI contrast agent application on primary and follow-up staging in pediatric patients with newly diagnosed lymphoma using [18F]FDG PET/MRI to avoid adverse effects and save time and costs during examination.
Methods
A total of 105 [18F]FDG PET/MRI datasets were included for data evaluation. Two different reading protocols were analyzed by two experienced readers in consensus, including for PET/MRI-1 reading protocol unenhanced T2w and/or T1w imaging, diffusion-weighted imaging (DWI), and [18F]FDG PET imaging and for PET/MRI-2 reading protocol an additional T1w post contrast imaging. Patient-based and region-based evaluation according to the revised International Pediatric Non-Hodgkin’s Lymphoma (NHL) Staging System (IPNHLSS) was performed, and a modified standard of reference was applied comprising histopathology and previous and follow-up cross-sectional imaging. Differences in staging accuracy were assessed using the Wilcoxon and McNemar tests.
Results
In patient-based analysis, PET/MRI-1 and PET/MRI-2 both determined a correct IPNHLSS tumor stage in 90/105 (86%) exams. Region-based analysis correctly identified 119/127 (94%) lymphoma-affected regions. Sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy for PET/MRI-1 and PET/MRI-2 were 94%, 97%, 90%, 99%, 97%, respectively. There were no significant differences between PET/MRI-1 and PET/MRI-2.
Conclusions
The use of MRI contrast agents in [18F]FDG PET/MRI examinations has no beneficial effect in primary and follow-up staging of pediatric lymphoma patients. Therefore, switching to a contrast agent–free [18F]FDG PET/MRI protocol should be considered in all pediatric lymphoma patients.
Clinical relevance statement
This study gives a scientific baseline switching to a contrast agent–free [18F]FDG PET/MRI staging in pediatric lymphoma patients. This could avoid side effects of contrast agents and saves time and costs by a faster staging protocol for pediatric patients.
Key Points
• No additional diagnostic benefit of MRI contrast agents at [18F]FDG PET/MRI examinations of pediatric lymphoma primary and follow-up staging
• Highly accurate primary and follow-up staging of pediatric lymphoma patients at MRI contrast–free [18F]FDG PET/MRI
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As the third most common tumor disease, lymphomas represent a significant proportion of pediatric malignancies, accounting for approximately 15% [1]. Generally, lymphoma types are broadly distinguished: Non-Hodgkin’s lymphoma (NHL) is diagnosed more frequently than Hodgkin’s lymphoma (HL) [2]. Highly accurate staging of lymphoma patients plays an important role for therapy, as it is well known that therapeutic strategies and prognosis depend heavily on the tumor stage at initial staging [3,4,5]. Although contrast-enhanced computed tomography (CT) diagnostic is used frequently due to its immediate availability for initial staging, [18F]fluorodeoxyglucose-positron emission tomography ([18F]FDG PET)/CT is considered the current reference imaging method. The additional metabolic information outperforms single CT diagnostics especially for staging of small nodal lymphoma manifestations and for response assessment. Nonetheless, both modalities come with repetitive radiation exposure and an increasing risk of future secondary malignancies [6,7,8,9]. Especially for children, a radiation-saving diagnostic alternative should be the goal. Combining high spatial soft tissue resolution with a functional imaging dataset, [18F]FDG PET/magnet resonance imaging (MRI) is becoming increasingly important as a radiation-saving alternative for initial staging and follow-up imaging of all lymphoma patients, especially in children [10,11,12,13,14]. It enables a reduction of radiation exposure up to 65%, while obtaining high quality at morphologic lymphoma imaging [15,16,17]. Consequently, improvements of the diagnostic work-up therapeutic abilities build the basis of increasingly favorable prognosis for children with lymphoma, measurable with a 5-year survival of 97% for HL and 85% for NHL, respectively [18,19,20]. However, [18F]FDG PET/MRI can easily reach 1 h of examination time for an adequate staging, making it stressful and challenging for pediatric and adult patients [21]. Bearing in mind that lymphoma patients commonly undergo multiple examinations for staging and therapy monitoring, special attention should be paid to the ongoing discussion about the relevance of gadolinium deposition to the brain [15, 22,23,24,25]. Thus, application of an MRI contrast agent should be reduced to a clinically reasonable minimum. Thus, skipping contrast administration and the associated contrast-enhanced sequence could reduce the risk of adverse effects like the discussed gadolinium deposition in the brain and allergic reactions and save time as well as costs.
A pilot study published by Kirchner et al. in 2017 already compared different reading protocols in a small cohort, entailing non-enhanced/contrast-enhanced and diffusion-weighted [18F]FDG PET/MR imaging and whole-body diffusion-weighted MRI for lesion detection and determination of the tumor stage in pediatric lymphoma patients. This study revealed that the application of contrast agents does not lead to a noticeable improvement of the diagnostic accuracy of a PET/MRI staging [13]. Nonetheless, data about the value of MRI contrast agent application in [18F]FDG PET/MRI in the diagnostic work-up of pediatric lymphoma patient is still limited to few small cohort studies [26,27,28,29].
Therefore, the present follow-up study aims to further validate a potentially time- and contrast agent–saving [18F]FDG PET/MRI protocol in the diagnostic work-up of pediatric lymphoma patients.
Material and methods
Patients
The institutional review board (study number 11–4822-BO) approved this study and it was performed in conformance with the Declaration of Helsinki. All patients underwent a clinically indicated contrast-enhanced whole-body [18F]FDG PET/MRI after informed written consent of the parents was obtained. Histopathological verification of lymphoma subtypes was available in all patients. Following a publication of the Council on Child and Adolescent Health (1988) that sees the upper pediatric age limit at 21 and a 2019 published study of Arendt et al which included pediatric lymphoma patients until the age of 25 years, this study included pediatric lymphoma patients < 21 years [26, 30]. Ultimately n = 25 HL patients and n = 7 NHL patients mean aged 14 ± 3 years (range 7–20 years) with a total of 105 examinations, including scans for initial staging (n = 32) and restaging during treatment or at the end of treatment (n = 73), as recommended in the ESMO Guidelines, were included [31, 32].
Whole-body PET/MRI
All [18F]FDG PET/MRI examinations were performed on an integrated 3-T PET/MRI system (Biograph mMR, Siemens Healthcare GmbH) with an average delay of 67 ± 19 min after [18F]FDG injection. To ensure blood glucose levels below 150 mg/dl, blood samples were obtained prior to injection of a bodyweight adapted dosage of [18F]FDG (4 MBq/kg bodyweight). Mean activity was 202 ± 53 MBq. Initial [18F]FDG PET/MRI staging was performed using a whole body protocol (including the head and limbs). All follow-up scan volumes generally covered the skull base to mid-thigh if the head and limbs were unsuspicious in initial staging.
PET data acquisition was performed in up to 5 bed positions by using an acquisition time of 4 min per bed position, depending on children height. PET images were reconstructed using the iterative ordered-subset expectation maximization (OSEM) algorithm (3 iterations, 21 subsets, Gaussian filter 4 mm, matrix size 344 × 344) [33]. Investigating possible differences between (PET/MRI-1) unenhanced PET/MRI (T2 weighted (w) imaging and/or T1w imaging, diffusion-weighted imaging (DWI)) and (PET/MRI-2) contrast-enhanced PET/MRI (T2w imaging and/or T1w pre-contrast imaging and T1w post-contrast imaging, DWI), the readers were asked to exclusively read the corresponding sequences in different combinations out of a longer protocol. Due to MRI protocol adjustments in clinical practice for time saving in some cases, a T2w sequence or a contrast-free T1w sequence was skipped at some pediatric patients. The MRI protocols were set up in accordance with clinical (age-dependent) standards, entailing different kinds of T1w and T2w sequences as well as a transversal DWI echo-planar imaging (EPI) (b values: 0, 500, and 1000 s/mm2). For contrast-enhanced imaging, a transverse volume interpolated breath-hold examination (VIBE) after intravenous administration of a gadolinium-based contrast medium (Dotarem; 0.05 mmol/kg bodyweight, literature accepted standard value) was acquired.
Image analysis
Imaging datasets of PET/MRI-1 and PET/MRI-2 were evaluated using a dedicated OsiriX workstation (Pixmeo SARL). A board-certified radiologist and a board certified nuclear medicine physician with experience in hybrid (more than 5 years) and MR imaging (more than 5 years) performed reading. Imaging datasets of the [18F]FDG PET/MRI examination were analyzed in consensus. In general, reading was subdivided in two different reading sessions entailing the different datasets of PET/MRI-1 and PET/MRI-2. Each dataset was evaluated in a dedicated reading session in a random order with a minimum of 4 weeks apart to avoid recognition bias. Both readers were blinded to patient identity and results of initial or follow-up imaging. Readers were informed about pediatric lymphoma diagnosis and scan indication (initial staging or restaging).
First, readers should evaluate all typical areas for presence or absence of lymphoma manifestation. Lymph nodes were summarized to nodal groups comprising the head/neck (Waldeyer’s ring, bilateral cervical and bilateral supraclavicular), chest (bilateral infraclavicular, prevascular, aortopulmonary, paratracheal, pretracheal, subcarinal, posterior mediastinal, bilateral hilar, and retrocrural), axilla/extremities (bilateral axillary), abdomen (gastrohepatic, periportal, aortocaval, retrocrural, mesenteric, retroperitoneal, and paraaortic), and pelvis (bilateral common iliac, bilateral internal iliac, bilateral external iliac and bilateral inguinal). Lymphoma manifestations at the bilateral pleura, bilateral lung, bilateral breast, myocardium, liver, ovary, and bowel as well as bone lesions were classified as extranodal manifestation.
Afterwards, readers were asked to separately set a tumor stage for each dataset in accordance with the IPNHLSS [34].
No universally applied morphologic criteria for pediatric lymphoma manifestation have been established yet. In accordance with previous publications for lymphoma in adults and according to the Lugano classification, the following morphologic criteria for the manifestation of lymphoma were considered: nodal lesions with a nodal long-axis diameter greater than 1.5 cm (unidimensional measurement), cluster formation or mass-like lesions, and high signal intensity at DWI sequences on high b value (b = 1000 s/mm2) with correlating signal drop in the corresponding ADC map [35,36,37]. In addition, a homogeneously accentuated contrast enhancement of a lesion and the adjacent tissue was considered lymphoma suspicious. Due to the large number of possible lymphoma-affected regions, contrast enhancement was assessed visually and not determined by a cut-off.
The 5-point (Deauville) scale for interpretation of [18F]FDG PET and the revised staging and response criteria of the Lugano classification were entirely focused on adult lymphoma without reference to pediatric lymphoma entities [5, 38]. Nonetheless, for lesion characterization on [18F]PET, visually increased focal FDG uptake in comparison to background and mediastinum and higher than liver activity was considered indicative for involvement with active lymphoma in concordance with the 5-point scale of the Lugano classification.
Standard of reference
All included patients suffered from a histologically proven lymphoma disease. Patients suffering from NHL and unclear bone marrow involvement underwent bone marrow biopsy according to the actual pediatric guidelines [39,40,41]. Due to clinical and ethical standards, a histological confirmation of each lymphoma-suspected lesion was not possible. Therefore, in a final consensus reading a modified standard of reference was established by the two experienced readers on a patient and region basis. Previous and follow-up cross-sectional imaging were provided for the final consensus reading, as it was already performed in previous publications, to enable accurate lesion characterization [13, 42, 43].
Statistical analysis
SPSS Statistics 26 (IBM Inc.) was used for statistical analysis. Data analysis was performed patient-based and region-based. Descriptive analysis was performed and data are presented as mean ± SD. Sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy were calculated for PET/MRI-1 and PET/MRI-2, respectively. Confidence intervals were calculated at 95%. The Wilcoxon test was chosen for evaluation of differences in tumor stage between PET/MRI-1 and PET/MRI-2. The McNemar test was used for paired-group comparison at a region-based analysis. P values < 0.05 were considered to be statistically significant.
Results
In total, n = 32 patients underwent [18F]FDG PET/MRI providing a total of 105 examinations. Mean acquisition time of contrast-enhanced PET/MRI examinations was 51 ± 23 min (range: 22 to 112 min) with a mean acquisition time of 2 ± 1 min (range: 1 to 6 min) of the T1-weighted post-contrast sequence. In all 105 examinations, MRI contrast–enhanced MRI was successfully completed. Eighty of total 105 (76%) examinations suffered from Hodgkin lymphoma, 4/105 (4%) examinations suffered from Burkitt lymphoma, 11/105 (10%) examinations suffered from B cell lymphoma, and 10/105 (10%) examinations suffered from T cell lymphoma (see Table 1).
Region-based analysis
As previously described, PET/MRI-1 and PET/MRI-2 imaging datasets of pediatric lymphoma patients were subdivided into six anatomical regions to differentiate between different anatomical lymphoma manifestations. According to the standard of reference, active lymphoma manifestations were visible at 127 anatomical regions. PET/MRI-1 and PET/MRI-2 correctly detected 119/127 (94%) lymphoma-affected regions. A detailed evaluation of active lymphoma manifestations according to the six anatomical regions is shown in Table 2.
Sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy for PET/MRI-1 and PET/MRI-2 were 94%, 97%, 90%, 99%, and 97%, respectively (see Table 3). No statistically significant difference was seen between both reading protocols (p = 1).
Patient-based analysis
According to the standard of reference, active lymphoma was present in 65/105 (62%) examinations and 40/105 (38%) examinations had no evidence of disease (see Table 4). Sixty-two of 65 active lymphoma manifestations were identified by PET/MRI-1 and PET/MRI-2, and the same three were missed respectively (exemplified in Figs. 1 and 2). PET/MRI-1 and PET/MRI-2 each rated the same three examinations as false positive (3/40; 8%).
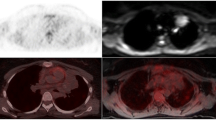
Example of a 13-year-old pediatric patient with missed active lymphoma disease at PET/MRI-1 (A) and PET/MRI-2 (A, B) according to the standard of reference. No visual [18F]FDG uptake (SUVmax: 2.0) was seen. Non-pathological appearance of the lymph node right cervical with borderline size (13 mm). Minimal contrast agent accumulation (white frame) within the lymph node in the T1-weighted (w) contrast-enhanced (CE) sequence. However, in synopsis of the images, this did not change the evaluation
Patient-based lymphoma manifestation was determined in accordance with the revised IPNHLSS. According to the standard of reference, 40/105 (38%) examinations had no evidence of disease, 5/105 (5%) examinations had an IPNHLSS stage 1, 16/105 (15%) examinations had an IPNHLSS stage 2, 34/105 (32%) examinations had an IPNHLSS stage 3, and 10/105 (10%) examinations had an IPNHLSS stage 4 lymphoma manifestation. PET/MRI-1 and PET/MRI-2 determined a correct IPNHLSS tumor stage in 90/105 (86%) examinations and each protocol overrated 7 of 105 (7%) lymphoma-affected examinations. The same 8 examinations of total 105 (8%) were underrated in both reading protocols. There was no significant difference determining IPNHLSS stage between both reading protocols (p = 1). The rated lymphoma stages of the PET/MRI-1 and PET/MRI-2 are given in Table 4.
Discussion
[18F]FDG PET/MRI is increasingly accepted for staging of lymphoma patients, and the present study further confirms the feasibility and high diagnostic accuracy of it in pediatric lymphoma patients [11, 12]. Especially in larger lymphoma treatment centers, [18F]FDG PET/MRI becomes more and more the diagnostic of choice in the work-up of lymphoma patients compared to the current [18F]FDG PET/CT reference imaging method. This is also caused by the fact that radiation exposure when using [18F]FDG PET/MRI is significantly less than the radiation exposure of even a low-dose [18F]FDG PET/CT [44, 45]. Bearing in mind the repetitive scans for therapy planning and follow-up imaging a lymphoma patient needs to undergo the reduction in radiation exposure using [18F]FDG PET/MRI is even more substantial. Consequently, the risk of secondary malignancies due to radiation exposure becomes significantly lower [6, 7]. This is of particular high importance in pediatric patients.
The need for MRI contrast agent application in PET/MRI is clinically not debatable in individual tumor entities for further classification, such as liver or pelvic tumors [46,47,48]. Contrary, first publications have already shown that especially in pediatric lymphoma patients, a MRI contrast agent application might be waived for staging [13, 29]. MRI contrast–free [18F]FDG PET/MRI imaging protocols are able to outperform a radiation-saving [18F]FDG PET/CT low-dose protocol with a more accurate soft tissue contrast and its well-known advantages when imaging parenchymal organs [13, 29]. Furthermore, they profit from simultaneously acquired metabolic PET imaging data that has been shown to be beneficial for staging, therapy monitoring, and differentiation between active and non-active lymphoma diseases [15,16,17, 49].
The omission of contrast administration and the associated contrast-enhanced sequence would avoid side effects of contrast agents like gadolinium deposition to the body, allergic reactions, and incompatibilities and furthermore saves time and costs by a faster [18F]FDG PET/MRI protocol [24]. Thus, the present follow-up study aims to further validate the need of MRI contrast agent application in the diagnostic [18F]FDG PET/MRI work-up of pediatric lymphoma patients by evaluating two different reading protocols.
According to our data evaluation, there was no difference between the diagnostic potential of PET/MRI-1 and PET/MRI-2. Bearing in mind that this cohort consists of initial and follow-up examinations, it can be concluded that pediatric lymphoma patients do not benefit from MRI contrast agent application at initial and follow-up [18F]FDG PET/MRI staging. In detail, information about vascularization gained from contrast MRI sequences does not add information when compared to FDG metabolic identification, MRI diffusion, and morphological localization of lymphoma. Clinical relevant aspects of lymphoma staging for therapy planning are metabolic mapping and morphologic information on size, shape, and infiltration we can achieve from non-contrast [18F]FDG PET/MRI without missing therapeutic relevant information. Apart from tumor detection, the application of contrast agents might be beneficial in cases of large mediastinal tumors, e.g., causing a superior vena cava compression syndrome. A venous vessel compression can lead to venous thrombosis that are better delineated after contrast agent application [50, 51]. However, since these cases are very rare, administration of contrast agents should be strictly discussed and regulated in the future due to the lack of diagnostic benefit shown in this study. For example, administration after clinical examination and suspicion of venous occlusion may be appropriate accordingly.
Figure 2 visualizes one borderline patient with missed lymphoma manifestation cervical (right) at PET/MRI-1 and PET/MRI-2 according to the standard of reference. Although MRI contrast agent uptake might result in a better delineation and potentially give a hint to subsequent lymphoma manifestation, there is no clinical benefit in this case. Given the morph on both reading protocols and without visible [18F]FDG uptake, this lymph node would not be considered suspicious for lymphoma in a clinical setting.
Dependent on the aggressiveness of lymphoma disease, sensitivity and accuracy of whole-body MRI and [18F]FDG PET/CT in detecting bone marrow involvement of adult and pediatric patients is ranging from 45 to 100% [52,53,54,55]. Generally, focal or multifocal [18F]FDG uptake exceeding liver uptake indicates bone marrow involvement of lymphoma in adults [56]. Diffuse [18F]FDG uptake of the bone marrow is more likely associated with lymphoma bone marrow manifestation in NHL than in HL [56, 57]. In our data, it is noticeable that a lower number of lymphomas were detected in examinations who suffered from a stage 4 IPNHLSS lymphoma (bone marrow involvement, 6/105 vs. 10/105) compared to the standard of reference including a bone marrow aspiration for unclear involvement and NHL patients. Missed children with bone marrow involvement of their lymphoma showed a more diffuse [18F]FDG uptake of the bone marrow in our data. In addition to a general misinterpretation by the reader during the data evaluation, another possible explanation for this underestimation of bone marrow involvement could be the increased proportion of the [18F]FDG affine red bone marrow in children compared to adults [58]. During adolescence, the proportion of yellow bone marrow increases, resulting in a decreased metabolic FDG activity of the bone. However, this may also increase again in adults, especially when suffering from hematopoietic diseases. Thus, yellow bone marrow is again converted to red bone marrow for hematopoiesis at patients with anemia, especially common at HL [57]. Furthermore, diffuse inflammatory bone marrow reaction also visualized at lymphoma patients makes it also difficult to identify bone marrow involvement of lymphoma [57]. Although the partially different, anatomical condition supports the fact that staging of adult lymphoma patients cannot be unreservedly transferred to staging of pediatric lymphoma patients, subtle bone marrow involvement of pediatric patients could be more difficult to detect at diffuse [18F]FDG uptake of the bone marrow exceeding liver uptake according to the described findings [13].
In line with the current literature, both reading protocols had a high sensitivity, specificity, positive predictive value, negative predictive value, and accuracy above 89% concordant to previous publications [36, 59]. Our results clearly support the increasing trend of MRI contrast agent–free [18F]FDG PET/MR imaging in pediatric lymphoma patients [26,27,28,29]. This is from particular importance to skip the potential risk of gadolinium deposition to in the brain (dentate nucleus/paleostriatum) and bone due to repetitive application of linear, gadolinium-based contrast agents [23, 24, 60]. Although there is no evidence to date concerning adverse effects of gadolinium-based contrast agents or clinical implications, the potential risk makes it necessary to reduce the MRI contrast agent to a justifiable minimum [24].
In addition, [18F]FDG PET/MRI is a time-consuming examination that can easily reach 1 h of examination time as visualized at our acquisition times (mean: 51 min) and can be hard to challenge, especially for children [29]. There is a need of a compromise between short examination times and adequate image quality. Especially in children the duration of a PET/MRI examination should be as short as possible to increase acceptance and decrease potential anesthesia [61]. On the one hand shortened PET image acquisition times and on the other hand the adaption of the MRI protocol can manage this problem by ending up with a “fast”-PET/MRI protocol [36, 62, 63]. Our data highly support adjusting the MRI part by omitting the post-contrast whole-body T1-weighted MRI sequence for staging. This would save up to 6 min according to the evaluated data. Whole-body DWI might be beneficial for staging, as it seems to be a promising, radiation-free staging alternative of lymphoma patients with nearly same diagnostic abilities compared to PET/CT examinations [27, 64, 65]. Nonetheless, not all study results support the use of whole-body DWI for pediatric lymphoma staging. Thus, a study of Shapira-Zaltsberg and colleagues with focus on pediatric HL highlights the superiority of PET/CT at initial staging and assessment of therapy response [66]. However, at comprehensive [18F]FDG PET/MRI staging of pediatric lymphoma patients, whole-body DWI seems to have no beneficial effect for staging [13]. This might be due to the included PET component that is highly accurate in the detection of lymphoma manifestations. Furthermore, the results by Georgi et al. recommend the high diagnostic potential of T2-weighted transverse fat-saturated sequence for sufficient staging of pediatric lymphoma patients [29]. Additionally, a potential reduction of the PET acquisition times to 2 min is possible as described by Hartung-Knemeyer et al. [67]. Taking all this information into account, a relevant reduction of [18F]FDG PET/MRI examination times could be achieved.
[18F]FDG PET/MRI is predestined for children suffering from lymphoma and should be considered the diagnostic of choice. Since these patients are often treated in larger centers for pediatric medicine, these data will help to establish PET/MRI diagnostics at these. Furthermore, the feasibility of adapting the [18F]FDG PET/MRI examination protocol, which is highlighted by the available publications, may improve patient satisfaction and reduce potential anesthesia, bearing in mind that the patients are children suffering from a severe disease. In this context, higher costs and/or financial aspects should rather be considered secondary and the aim of such examination protocols should be a potential risk reduction to a minimum.
This study is not without limitations. Although histopathological sampling for subtype determination was available in all patients, in accordance with current ethical and clinical guidelines, not every detected lesion could be sampled. Hence, as already published in numerous previous studies on hybrid imaging, a modified standard of reference was applied. Secondly, lymphoma is known to comprise a heterogeneous group of cancers entailing different subtypes. The limited number of pediatric lymphoma patients does not enable a further subgroup comparison because of underpowered statistical analyses. Furthermore, due to the diagnostic focus on HL patients at PET/MRI of the institute, retrospectively HL were predominantly included for data evaluation. Moreover, due to missing QOL data based on the retrospective design, impressions of children undergoing PET/MRI could not be implemented in data evaluation. Nonetheless, this study is one of the larger data collections related to pediatric lymphoma patients at a single institute.
Finally, the use of MRI contrast agents in [18F]FDG PET/MRI examinations does not add relevant diagnostic information in primary and follow-up staging of pediatric lymphoma patients. Therefore, switching to a contrast agent–free [18F]FDG PET/MRI protocol should be considered in all pediatric lymphoma patients.
Abbreviations
- HL:
-
Hodgkin’s lymphoma
- IPNHLSS:
-
International Pediatric Non-Hodgkin’s Lymphoma Staging System
- NHL:
-
Non-Hodgkin’s lymphoma
References
Steliarova-Foucher E, Fidler MM, Colombet M et al (2018) Changing geographical patterns and trends in cancer incidence in children and adolescents in Europe, 1991–2010 (Automated Childhood Cancer Information System): a population-based study. Lancet Oncol. https://doi.org/10.1016/S1470-2045(18)30423-6
Linet MS, Ries LA, Smith MA, Tarone RE, Devesa SS (1999) Cancer surveillance series: recent trends in childhood cancer incidence and mortality in the United States. J Natl Cancer Inst. https://doi.org/10.1093/jnci/91.12.1051
Lin C, Itti E, Haioun C et al (2007) Early 18F-FDG PET for prediction of prognosis in patients with diffuse large B-cell lymphoma: SUV-based assessment versus visual analysis. J Nucl Med. https://doi.org/10.2967/jnumed.107.042093
El-Galaly TC, Hutchings M (2015) Imaging of non-Hodgkin lymphomas: diagnosis and response-adapted strategies. Cancer Treat Res. https://doi.org/10.1007/978-3-319-13150-4_5
Barrington SF, Mikhaeel NG, Kostakoglu L et al (2014) Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. https://doi.org/10.1200/jco.2013.53.5229
Wen JC, Sai V, Straatsma BR, McCannel TA (2013) Radiation-related cancer risk associated with surveillance imaging for metastasis from choroidal melanoma. JAMA Ophthalmol. https://doi.org/10.1001/jamaophthalmol.2013.564
Brenner DJ, Elliston CD (2004) Estimated radiation risks potentially associated with full-body CT screening. Radiology. https://doi.org/10.1148/radiol.2323031095
Brix G, Nosske D, Lechel U (2014) Radiation exposure of patients undergoing whole-body FDG-PET/CT examinations: an update pursuant to the new ICRP recommendations. Nuklearmedizin. https://doi.org/10.3413/Nukmed-0663-14-04
Schäfer JF, Gatidis S, Schmidt H et al (2014) Simultaneous whole-body PET/MR imaging in comparison to PET/CT in pediatric oncology: initial results. Radiology. https://doi.org/10.1148/radiol.14131732
Heacock L, Weissbrot J, Raad R et al (2015) PET/MRI for the evaluation of patients with lymphoma: initial observations. AJR Am J Roentgenol. https://doi.org/10.2214/AJR.14.13181
Afaq A, Fraioli F, Sidhu H et al (2017) Comparison of PET/MRI with PET/CT in the evaluation of disease status in lymphoma. Clin Nucl Med. https://doi.org/10.1097/rlu.0000000000001344
Sher AC, Seghers V, Paldino MJ et al (2016) Assessment of sequential PET/MRI in comparison with PET/CT of pediatric lymphoma: a prospective study. AJR Am J Roentgenol. https://doi.org/10.2214/AJR.15.15083
Kirchner J, Deuschl C, Schweiger B et al (2017) Imaging children suffering from lymphoma: an evaluation of different 18F-FDG PET/MRI protocols compared to whole-body DW-MRI. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-017-3726-0
Picardi M, Cavaliere C, Della Pepa R et al (2021) PET/MRI for staging patients with Hodgkin lymphoma: equivalent results with PET/CT in a prospective trial. Ann Hematol. https://doi.org/10.1007/s00277-021-04537-5
Platzek I, Beuthien-Baumann B, Langner J et al (2013) PET/MR for therapy response evaluation in malignant lymphoma: initial experience. MAGMA. https://doi.org/10.1007/s10334-012-0342-7
Drzezga A, Souvatzoglou M, Eiber M et al (2012) First clinical experience with integrated whole-body PET/MR: comparison to PET/CT in patients with oncologic diagnoses. J Nucl Med. https://doi.org/10.2967/jnumed.111.098608
Heusch P, Nensa F, Schaarschmidt B et al (2015) Diagnostic accuracy of whole-body PET/MRI and whole-body PET/CT for TNM staging in oncology. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-014-2885-5
Sherief LM, Elsafy UR, Abdelkhalek ER et al (2015) Hodgkin lymphoma in childhood: clinicopathological features and therapy outcome at 2 centers from a developing country. Medicine (Baltimore). https://doi.org/10.1097/md.0000000000000670
Burkhardt B, Zimmermann M, Oschlies I et al (2005) The impact of age and gender on biology, clinical features and treatment outcome of non-Hodgkin lymphoma in childhood and adolescence. Br J Haematol. https://doi.org/10.1111/j.1365-2141.2005.05735.x
Sorge I, Georgi TW, Hirsch FW (2021) Lymphomerkrankungen im Kindes- und Jugendalter. Radiologe. https://doi.org/10.1007/s00117-021-00873-9
Gatidis S, Schmidt H, Gücke B et al (2016) Comprehensive oncologic imaging in infants and preschool children with substantially reduced radiation exposure using combined simultaneous 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging: a direct comparison to 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Invest Radiol. https://doi.org/10.1097/rli.0000000000000200
Doniselli FM, Albano D, Chianca V, Cimmino MA, Sconfienza LM (2017) Gadolinium accumulation after contrast-enhanced magnetic resonance imaging: what rheumatologists should know. Clin Rheumatol. https://doi.org/10.1007/s10067-017-3604-y
Stanescu AL, Shaw DW, Murata N et al (2020) Brain tissue gadolinium retention in pediatric patients after contrast-enhanced magnetic resonance exams: pathological confirmation. Pediatr Radiol. https://doi.org/10.1007/s00247-019-04535-w
Raczeck P, Fries P, Bücker A, Schneider G (2019) Gadolinium deposition-“gadolinium deposition disease.” Radiologe. https://doi.org/10.1007/s00117-019-0522-9
Schneider GK, Stroeder J, Roditi G et al (2017) T1 Signal measurements in pediatric brain: findings after multiple exposures to gadobenate dimeglumine for imaging of nonneurologic disease. AJNR Am J Neuroradiol. https://doi.org/10.3174/ajnr.A5270
Arendt CT, Beeres M, Leithner D et al (2019) Gadolinium-enhanced imaging of pediatric thoracic lymphoma: is intravenous contrast really necessary? Eur Radiol. https://doi.org/10.1007/s00330-018-5859-3
Littooij AS, Kwee TC, Barber I et al (2016) Accuracy of whole-body MRI in the assessment of splenic involvement in lymphoma. Acta Radiol. https://doi.org/10.1177/0284185115571657
Albano D, Micci G, Patti C et al (2021) Whole-body magnetic resonance imaging: current role in patients with lymphoma. Diagnostics (Basel). https://doi.org/10.3390/diagnostics11061007
Georgi TW, Stoevesandt D, Kurch L et al (2022) Optimized whole-body positron emission tomography magnetic resonance imaging sequence workflow in pediatric Hodgkin lymphoma patients. J Nucl Med. https://doi.org/10.2967/jnumed.122.264112
Co C, Health A (1988) Age limits of pediatrics. Pediatrics. https://doi.org/10.1542/peds.81.5.736
Ghielmini M, Vitolo U, Kimby E et al (2013) ESMO Guidelines consensus conference on malignant lymphoma 2011 part 1: diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) and chronic lymphocytic leukemia (CLL). Ann Oncol. https://doi.org/10.1093/annonc/mds517
Eichenauer DA, Engert A, Andre M et al (2014) Hodgkin’s lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. https://doi.org/10.1093/annonc/mdu181
Quick HH (2014) Integrated PET/MR. J Magn Reson Imaging. https://doi.org/10.1002/jmri.24523
Rosolen A, Perkins SL, Pinkerton CR et al (2015) Revised International Pediatric Non-Hodgkin Lymphoma Staging System. J Clin Oncol. https://doi.org/10.1200/jco.2014.59.7203
Herrmann K, Queiroz M, Huellner MW et al (2015) Diagnostic performance of FDG-PET/MRI and WB-DW-MRI in the evaluation of lymphoma: a prospective comparison to standard FDG-PET/CT. BMC Cancer. https://doi.org/10.1186/s12885-015-2009-z
Grueneisen J, Sawicki L, Schaarschmidt B et al (2016) Evaluation of a fast protocol for staging lymphoma patients with integrated PET/MRI. PLoS One. https://doi.org/10.1371/journal.pone.0157880
Johnson SA, Kumar A, Matasar MJ, Schöder H, Rademaker J (2015) Imaging for staging and response assessment in lymphoma. Radiology. https://doi.org/10.1148/radiol.2015142088
Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C (2009) Report on the First International Workshop on Interim-PET-Scan in Lymphoma. Leuk Lymphoma. https://doi.org/10.1080/10428190903040048
Flerlage JE, Hiniker SM, Armenian S et al (2021) Pediatric Hodgkin lymphoma, version 3.2021. J Natl Compr Cancer Netw. https://doi.org/10.6004/jnccn.2021.0027
(2017) Non-Hodgkin-Lymphome im Kindesalter. AWMF-Leitlinie. AWMF-Reg.-Nr. 025/013
(2018) LL Hodgkin-Lymphom. AWMF-Leitlinie 025/012
Sawicki LM, Grueneisen J, Schaarschmidt BM et al (2016) Evaluation of 18F-FDG PET/MRI, 18F-FDG PET/CT, MRI, and CT in whole-body staging of recurrent breast cancer. Eur J Radiol. https://doi.org/10.1016/j.ejrad.2015.12.010
Buchbender C, Hartung-Knemeyer V, Beiderwellen K et al (2013) Diffusion-weighted imaging as part of hybrid PET/MRI protocols for whole-body cancer staging: does it benefit lesion detection? Eur J Radiol. https://doi.org/10.1016/j.ejrad.2013.01.019
Ferdová E, Ferda J, Baxa J (2017) (18)F-FDG-PET/MRI in lymphoma patients. Eur J Radiol. https://doi.org/10.1016/j.ejrad.2017.01.023
Martin O, Schaarschmidt BM, Kirchner J et al (2019) PET/MRI versus PET/CT in whole-body staging: results from a unicenter observational study in 1003 subsequent examinations. J Nucl Med. https://doi.org/10.2967/jnumed.119.233940
Nougaret S, Nikolovski I, Paroder V et al (2019) MRI of tumors and tumor mimics in the female pelvis: anatomic pelvic space–based approach. Radiographics. https://doi.org/10.1148/rg.2019180173
Corines MJ, Nougaret S, Weiser MR, Khan M, Gollub MJ (2018) Gadolinium-based contrast agent during pelvic MRI: contribution to patient management in rectal cancer. Dis Colon Rectum. https://doi.org/10.1097/dcr.0000000000000925
Welle CL, Guglielmo FF, Venkatesh SK (2020) MRI of the liver: choosing the right contrast agent. Abdom Radiol (NY). https://doi.org/10.1007/s00261-019-02162-5
Uslu L, Donig J, Link M, Rosenberg J, Quon A, Daldrup-Link HE (2015) Value of 18F-FDG PET and PET/CT for evaluation of pediatric malignancies. J Nucl Med. https://doi.org/10.2967/jnumed.114.146290
Rizvi I, Zaman S, Zaidi N et al (2012) Superior vena cava syndrome caused by Hodgkin’s lymphoma in an adolescent girl. BMJ Case Rep. https://doi.org/10.1136/bcr.01.2012.5487
Schönning A, Karlén J, Frisk T et al (2017) Venous thrombosis in children and adolescents with Hodgkin lymphoma in Sweden. Thromb Res. https://doi.org/10.1016/j.thromres.2017.02.011
Albano D, Patti C, Lagalla R, Midiri M, Galia M (2017) Whole-body MRI, FDG-PET/CT, and bone marrow biopsy, for the assessment of bone marrow involvement in patients with newly diagnosed lymphoma. J Magn Reson Imaging. https://doi.org/10.1002/jmri.25439
Adams HJ, Kwee TC, Vermoolen MA et al (2013) Whole-body MRI for the detection of bone marrow involvement in lymphoma: prospective study in 116 patients and comparison with FDG-PET. Eur Radiol. https://doi.org/10.1007/s00330-013-2835-9
Agrawal K, Mittal BR, Bansal D et al (2013) Role of F-18 FDG PET/CT in assessing bone marrow involvement in pediatric Hodgkin’s lymphoma. Ann Nucl Med. https://doi.org/10.1007/s12149-012-0665-5
Cheng G, Chen W, Chamroonrat W, Torigian DA, Zhuang H, Alavi A (2011) Biopsy versus FDG PET/CT in the initial evaluation of bone marrow involvement in pediatric lymphoma patients. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-011-1815-z
Adams HJA, Kwee TC, de Keizer B et al (2014) Systematic review and meta-analysis on the diagnostic performance of FDG-PET/CT in detecting bone marrow involvement in newly diagnosed Hodgkin lymphoma: is bone marrow biopsy still necessary? Ann Oncol. https://doi.org/10.1093/annonc/mdt533
Adams HJA, de Klerk JMH, Fijnheer R et al (2016) Variety in bone marrow 18F-FDG uptake in Hodgkin lymphoma patients without lymphomatous bone marrow involvement: does it have an explanation? Nucl Med Commun. https://doi.org/10.1097/mnm.0000000000000400
Fan C, Hernandez-Pampaloni M, Houseni M et al (2007) Age-related changes in the metabolic activity and distribution of the red marrow as demonstrated by 2-deoxy-2-[F-18]fluoro-d-glucose-positron emission tomography. Mol Imag Biol. https://doi.org/10.1007/s11307-007-0100-9
Giraudo C, Raderer M, Karanikas G et al (2016) 18F-Fluorodeoxyglucose positron emission tomography/magnetic resonance in lymphoma: comparison with 18F-fluorodeoxyglucose positron emission tomography/computed tomography and with the addition of magnetic resonance diffusion-weighted imaging. Invest Radiol. https://doi.org/10.1097/rli.0000000000000218
Radbruch A, Weberling LD, Kieslich PJ et al (2015) Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology. https://doi.org/10.1148/radiol.2015150337
Schaefer JF, Berthold LD, Hahn G et al (2019) Whole-body MRI in children and adolescents - S1 Guideline. Rofo. https://doi.org/10.1055/a-0832-2498
Grueneisen J, Schaarschmidt B, Heubner M et al (2015) Implementation of FAST-PET/MRI for whole-body staging of female patients with recurrent pelvic malignancies: a comparison to PET/CT. Eur J Radiol. https://doi.org/10.1016/j.ejrad.2015.08.010
Lindemann ME, Stebner V, Tschischka A, Kirchner J, Umutlu L, Quick HH (2018) Towards fast whole-body PET/MR: investigation of PET image quality versus reduced PET acquisition times. PLoS One. https://doi.org/10.1371/journal.pone.0206573
Kharuzhyk S, Zhavrid E, Dziuban A, Sukolinskaja E, Kalenik O (2020) Comparison of whole-body MRI with diffusion-weighted imaging and PET/CT in lymphoma staging. Eur Radiol. https://doi.org/10.1007/s00330-020-06732-w
Spijkers S, Littooij AS, Kwee TC et al (2021) Whole-body MRI versus an FDG-PET/CT-based reference standard for staging of paediatric Hodgkin lymphoma: a prospective multicentre study. Eur Radiol. https://doi.org/10.1007/s00330-020-07182-0
Shapira-Zaltsberg G, Wilson N, Trejo Perez E et al (2020) Whole-body diffusion-weighted MRI compared to (18 F)FDG PET/CT in initial staging and therapy response assessment of Hodgkin lymphoma in pediatric patients. Can Assoc Radiol J. https://doi.org/10.1177/0846537119888380
Hartung-Knemeyer V, Beiderwellen KJ, Buchbender C et al (2013) Optimizing positron emission tomography image acquisition protocols in integrated positron emission tomography/magnetic resonance imaging. Invest Radiol. https://doi.org/10.1097/RLI.0b013e3182823695
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Julian Kirchner.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all patients in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
No study subjects or cohorts have been previously reported.
Methodology
• Retrospective data evaluation
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jannusch, K., Morawitz, J., Schweiger, B. et al. [18F]FDG PET/MRI in children suffering from lymphoma: does MRI contrast media make a difference?. Eur Radiol 33, 8366–8375 (2023). https://doi.org/10.1007/s00330-023-09840-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09840-5