Abstract
Objectives
The aim of the current study was to assess the utility of F-18-fluoro-2-deoxy-d-glucose (F-18 FDG) positron emission tomography/computed tomography (PET/CT) in assessing bone marrow involvement (BMI) compared to bone marrow biopsy (BMB) in initial staging of Hodgkin’s lymphoma (HL) in pediatric patients.
Methods
Data of 38 pediatric patients (mean age 9.8 years, range 3–18 years) with HL were analyzed for the involvement of bone marrow. All patients underwent non-contrast F-18 FDG PET/CT study. BMB was done in 31 patients from the bilateral iliac crests. Scans were interpreted by two nuclear medicine physicians blinded to the details of BMB.
Results
Of the 31 patients who underwent BMB, 5 patients had lymphomatous involvement on BMB. PET/CT was positive in four of these five patients. In 26 patients negative on BMB, PET was negative in 23 patients and positive in 3 patients for BMI. The sensitivity and negative predictive value of F-18 FDG PET/CT was 87.5 and 96 %, respectively, for BMI.
Conclusions
F-18 FDG PET/CT can predict BMB results with high accuracy. F-18 FDG PET/CT may be used at initial staging of pediatric Hodgkin’s lymphoma as it uncovers unsuspected BMI and BMB may be omitted in patients with PET-positive BMI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lymphomas account for 10–15 % of pediatric cancers. In children, 40 % of lymphomas are Hodgkin’s lymphoma (HL) [1, 2]. During preadolescent period mixed cellularity and nodular sclerosis are the two main histopathological subtypes of HL, while adolescents have a predominant incidence of the nodular sclerosis type HL [3]. Therapeutic options for HL are various combinations of chemotherapy and radiotherapy, which depend on the stage of the disease at the time of diagnosis, separating early/localized stages and advanced/disseminated stages [4–6]. This emphasizes the need of adequate staging before initiating a risk-adapted therapy, taking into account the balance between efficacy and risks of toxicity and long-term adverse effects [7]. Staging based on the Ann Arbor classification usually includes computed tomography (CT) and bone marrow biopsy (BMB) [8, 9]. Bone marrow involvement (BMI) upstages the disease to stage IV, which changes both treatment and prognosis. BMB is considered as the reference standard for the depiction of BMI [9]. However, this procedure is invasive, painful and explores a limited part of the bone marrow. CT scan may depict cortical bone lesions or late bone changes but has low sensitivity for early BMI [9].
F-18-fluoro-2-deoxy-d-glucose (F-18 FDG) is a glucose analogue that provides unique information about glucose metabolism of normal and abnormal tissues, in particular, in malignant diseases. Recently, F-18 FDG positron emission tomography/computed tomography (PET/CT) has gained widespread use in the management of HL. Abundant literature is available on value of F-18 FDG PET/CT in the evaluation of BMI in adult lymphoma patients [10–16]. The role of F-18 FDG PET/CT in the initial evaluation of BMI in pediatric lymphoma patients has also been reported with high sensitivity and accuracy [17]. However, the study included patients with both HL and NHL and also some patients with indolent lymphomas. The aim of the current study is to assess the utility of F-18 FDG PET/CT in identifying BMI compared to BMB in initial staging of Hodgkin’s lymphoma in pediatric patients.
Materials and methods
Retrospective analysis of data from 38 pediatric patients (30 males, 8 females; mean age 9.8 years; range 3–18 years) with HL was performed. The characteristics of patients included in this study are shown in Table 1. All patients underwent conventional staging, including physical examination, complete blood count, serum biochemical test, chest X-ray and USG abdomen. BMB was done in 31 patients from the bilateral iliac crest. BMB was not performed in 7 patients with disease stage IIA or lower, as the probability of a positive biopsy was very low in this group (<1 %) [9, 10, 17, 18]. All patients also underwent non-contrast F-18 FDG PET/CT study within week before the BMB but for four patients in whom it was performed within 1 week after BMB. Scans were interpreted without the details of BMB. BMB and PET/CT were part of the routine protocol for initial staging.
Studies were performed on a dedicated PET/CT scanner (Discovery, STE-16, GE Healthcare, Milwaukee, USA). All the patients were non-diabetic and fasted for at least 6 h prior to the F-18 FDG injection. Acquisition was started 45–60 min after intravenous injection of 3.7 MBq/kg of F-18 FDG. Whole-body scans were acquired in overlapped bed positions from base of skull to mid thigh with the arms extended above the head and 2-min acquisition for each bed position. All patients were imaged without sedation. CT was performed using tube current of 80–150 mA, without injection of contrast media. After transmission scan, 3-D PET acquisition was performed at 2 min per bed position. Data obtained from CT acquisition were used for low noise attenuation correction of PET emission data and for fusion of attenuation corrected PET images with corresponding CT images. Image reconstruction was done using iterative reconstruction (Ordered Subset Expectation Maximum) algorithm. Transaxial, coronal, and sagittal images were obtained after reconstruction. Spatial resolution after reconstruction was 5 mm at FWHM.
Data analysis
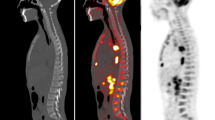
Two experienced nuclear medicine physicians, blinded to the result of the BMB, read the F-18 FDG PET/CT scan and interpreted as positive or negative for BMI. PET/CT was interpreted as positive for BMI in the presence of (a) isolated/multiple focal uptake in the bone marrow more than the liver uptake and/or (b) diffuse heterogeneous marrow involvement with sites of intense focal involvement. PET/CT was interpreted negative for BMI in the presence of diffuse homogenous marrow involvement with uptake less than or equal to liver. The representative positive and negative images are shown in Fig. 1.
F-18 FDG PET/CT images. a, b Maximum intensity projection (MIP) and transaxial fused PET/CT images at the level of liver of a patient showing diffuse marrow uptake with intensity equal to liver suggestive of negative PET scan for BMI. c, d MIP and transaxial-fused PET/CT images of another patient showing multiple foci of tracer uptake in the skeleton without diffuse marrow uptake which was considered as positive for BMI; however, BMB taken from another site was negative. e, f MIP and transaxial fused PET/CT images of a patient showing diffuse marrow uptake with multiple foci of tracer uptake which was also considered as positive for BMI involvement with BMB showing BMI
Ideally, a definitive validation of true positivity would require histologic examination of PET-positive lesions. However, due to practical and ethical constraints, bone biopsies could not be performed in addition to standard BMBs. PET/CT was considered true positive in cases where any one of the following criteria was present: (a) positive BMB if the focal tracer uptake in PET/CT and site of BMB are same, (b) presence of morphologic changes on CT evaluation corresponding to site of uptake in PET, (c) disappearance of lesions on follow-up PET/CT after chemotherapy. The rest of the cases positive for BMI on PET/CT were considered as false positive. PET/CT was considered as false negative if BMB and/or CT was positive and PET/CT was negative for BMI. A negative PET/CT was considered as true negative, if both BMB and CT were negative for BMI. The positive predictive value (PPV) was calculated as the number of true positives (test-positive patients who fulfilled the composite criteria elaborated) divided by the total number of test positives. The negative predictive value (NPV) was calculated as the number of true negatives divided by the total number of test negatives patients.
Results
BMB was not performed in 7 patients due to localized disease. PET/CT was negative for BMI in all these 7 patients. Further, all these patients were disease free at 6-month follow-up. Of the remaining 31 patients in whom the BMB was performed, PET/CT was reported as negative for BMI in 24 patients. Seven of these 24 patients showed diffuse FDG uptake in the bone marrow with no change in the follow-up scan post-chemotherapy, and were interpreted as negative for BMI as per the interpretation criteria. Of these PET/CT negative cases, one patient was positive for BMI on BMB, while 23/24 (96 %) patients had negative BMB and/or CT results. Thus, PET/CT was true negative in 23 patients and false negative in 1 patient for BMI.
PET/CT was reported as positive for BMI in 7 patients as per the composite criteria elaborated in methods of the study. Out of these seven patients, 1 patient had stage II disease, 3 patients had stage III disease and 3 patients had stage IV disease excluding BMI in the staging. BMB was positive in 4/7 (57 %) patients. In the other 3 patients who were F-18 FDG PET/CT positive but BMB negative, iliac crest (site of the BMB) was not involved on PET/CT. An interim and/or end-therapy F-18 FDG PET/CT examination was used to assess response to chemotherapy. This follow-up PET/CT showed complete resolution of FDG avidity in the bone marrow in 6 patients. One patient was lost to follow-up. Morphological changes in CT part of PET/CT were present in 4 of 7 patients on retrospective evaluation, including one patient who was lost to follow-up. The median follow-up was 9 months (range 5–16 months). One patient was lost to follow-up. All other patients were in complete remission (CR) at last follow-up (Table 2). A representative scan of a patient with negative BMB but showing focal enhanced FDG uptake in right scapula (a, b, d) and sacrum (a, c, e) bones is depicted in Fig. 2.
F-18 FDG PET/CT images. a Maximum intensity projection (MIP), b coronal fused PET/CT, c sagittal fused PET/CT, d transaxial fused PET/CT at scapular level and e transaxial fused PET/CT at sacral level of a 7-year-old boy with a negative bone marrow biopsy showing focal enhanced F-18 FDG uptake in right scapula (a, b, d) and sacrum (a, c, e) bones
The sensitivity, specificity, NPV and PPV of FDG PET/CT for evaluation of BMI were 87.5, 100, 96 and 100 %, respectively. The sensitivity, specificity, NPV and PPV of BMB for BMI were 62.5, 100, 88.4 and 100 %, respectively.
Discussion
Accurate assessment of bone marrow plays a crucial role in staging lymphoma, because of the fact that BMI can upstage the disease to stage IV. BMI in HL patients is close to 5 %, whereas in pediatric HL after bilateral BMB it is almost 9 % [17–19]. In our study, BMI was diagnosed using BMB in 13 % of patients, which is consistent with the incidence reported in previous studies.
BMB, which is considered as the current gold standard for evaluating BMIs, is generally safe but is not a risk-free procedure as adverse events (hemorrhage, infection, etc.) have been reported. Moreover, BMI is heterogeneous in aggressive lymphomas. In advanced stage lymphomas, a single iliac crest BMB identified BMI in 5–34 % of patients, whereas bilateral iliac crest biopsies increase detection by another 10–20 % [20, 21]. In the present study, F-18 FDG PET/CT diagnosed BMI in 18 % of the patients, reinforcing the fact that BMI is heterogeneous.
Abundant literature is available on the value of F-18 FDG PET/CT in the evaluation of BMI in adult lymphoma patients [10–16]. However, only a few studies in literature exist examining the sensitivity of F-18 FDG PET/CT for BMI in a homogenous population of HL. Pakos et al. [22] in a meta-analysis of five studies with a total of 191 patients of HL reported positive BMB in 11 patients. The estimated sensitivity of F-18 FDG PET/CT was 76 %. F-18 FDG PET/CT had sensitivity of 87.5 % in the present study, which is consistent with the meta-analysis of 32 studies by Wu et al. [23] in which PET/CT had the highest pooled sensitivity of 91.6 % compared to MRI. Of note, in this meta-analysis, PET/CT was done in only 5 studies and PET/CT was compared with BMB in NHL patients in two studies, mixed population of NHL and HL in 2 studies [11, 24] and pure HL patients in only one study [10].
A previous study from the same centre compared BMB and F-18 FDG PET/CT to assess BMI in 97 lymphoma in adult patients (HL: 20 patients) [16]. PET/CT was 100 % sensitive and 86 % specific for evaluation of BMI in the HL patients. Moulin-Romsee et al. [10] retrospectively reviewed 83 newly diagnosed HL patients who had received contrast-enhanced CT, BMB and F-18 FDG PET/CT. All patients with bone marrow and/or bone lesions at conventional staging were positive on F-18 FDG PET/CT scan. F-18 FDG PET/CT was positive for BMI in additional 10.7 % patients that were undetected by BMB. In this study, the mean patient age was 30.7 years (range 7–82 years). Cheng et al. [17] examined the role of F-18 FDG PET/CT in the initial evaluation of BMI in pediatric lymphoma patients including 31 HL and 23 NHL patients. PET/CT showed BMI in 2/31 (6.5 %) additional patients with HL compared to the result of BMB. In our study, F-18 FDG PET/CT was positive for BMI in 3/38 (8 %) additional patients not detected by BMB.
In a recent study, Purz et al. [25] compared the BMB and F-18 FDG PET/CT for the diagnosis of BMI in 175 pediatrics patients with HL stage greater than IIA and concluded that F-18 FDG PET may safely be substituted for a BMB in routine staging procedure in pediatric HL. F-18 FDG PET/CT positive but BMB negative findings for BMI occur in 22 % of patients. This higher F-18 FDG PET positivity may be due to the fact that the study included only those patients in whom staging BMB and F-18 FDG PET/CT were conducted, which may have introduced patient selection bias. Moreover, only 4 % of patients with stage greater than IIA were BMB positive for BMI and even patient with PET/CT positivity for bilateral iliac crest involvement was negative on BMB. They showed that PET/CT findings would have lead to change in stage in 11 % of patients and change in treatment in 5.7 % of patients.
It is important to note that in the present study, diffuse homogenous bone marrow uptake without focal bone marrow uptake in all 7 patients was negative for BM infiltration on BMB. Diffuse homogeneous bone marrow uptake was not considered as positive for BMI in this study. Salaun et al. [26] retrospectively analyzed 106 patients who underwent F-18 FDG PET/CT for initial staging of HL and concluded that an increased bone marrow uptake could more likely be due to inflammation than BMI and only the presence of bone foci should be analyzed as bone involvement on a visual F-18 FDG PET/CT interpretation. Our study again supports this finding. Thus, in the absence of definite isolated or multifocal uptake with or without diffuse homogenous uptake, F-18 FDG PET/CT studies should be considered as negative. In all three F-18 FDG PET/CT positive but BMB negative cases, the iliac crest had no F-18 FDG avidity, reinforcing the fact that F-18 FDG PET/CT positivity but BMB negativity for BMI is mostly due to heterogeneous bone marrow infiltration.
The limitations of this study are small sample size and non-performance of diagnostic-guided BMB from the F-18 FDG avid bone/BM lesion in 7 patients where PET/CT was positive for BMI. But the intense focal uptake and disappearance of all lesions following therapy may support the specificity of lymphoma lesions.
In conclusion, on the basis of our findings in pediatric HL, F-18 FDG PET/CT may be used at initial staging of pediatric Hodgkin’s lymphoma as it uncovers unsuspected BMI and BMB may be omitted in patients with PET-positive BMI.
References
Oberlin O. Hodgkin’s disease. In: Voûte PA, Kalifa C, Barrett A, editors. Cancer in children. Clinical management. 4th ed. New York: Oxford University; 1998. p. 137–53.
Lanzkowski P. Hodgkin’s disease. In: Lanzkowski P, editor. Manual of pediatric hematology and oncology. 3rd ed. San Diego: Academic; 1999. p. 413–43.
Lukes R, Butler J, Hicks E. Natural history of Hodgkin’s disease as related to its pathological picture. Cancer. 1966;19:317–44.
Eghbali H, Raemaekers J, Carde P. EORTC Lymphoma Group. The EORTC strategy in the treatment of Hodgkin’s lymphoma. Eur J Haematol Suppl. 2005;66:135–40.
Connors JM. State-of-the-art therapeutics: Hodgkin’s lymphoma. J Clin Oncol. 2005;23:6400–8.
Diehl V, Fuchs M. Early, intermediate and advanced Hodgkin’s lymphoma: modern treatment strategies. Ann Oncol. 2007;18(suppl 9):71–9.
Favier O, Heutte N, Stamatoullas-Bastard A, Carde P, Van’t Veer MB, Aleman BM, et al. Survival after Hodgkin lymphoma: causes of death and excess mortality in patients treated in 8 consecutive trials. Cancer. 2009;115:1680–91.
Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res. 1971;31:1860–1.
Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol 1989;7:1630–1636. Erratum in: J Clin Oncol 1990;8:1602.
Moulin-Romsee G, Hindié E, Cuenca X, Brice P, Decaudin D, Bénamor M, et al. (18)F-FDG PET/CT bone/bone marrow findings in Hodgkin’s lymphoma may circumvent the use of bone marrow trephine biopsy at diagnosis staging. Eur J Nucl Med Mol Imaging. 2010;37:1095–105.
Schaefer NG, Strobel K, Taverna C, Hany TF. Bone involvement in patients with lymphoma: the role of FDG-PET/CT. Eur J Nucl Med Mol Imaging. 2007;34:60–7.
Fuster D, Chiang S, Andreadis C, Guan L, Zhuang H, Schuster S, et al. Can [18F]fluorodeoxyglucose positron emission tomography imaging complement biopsy results from the iliac crest for the detection of bone marrow involvement in patients with malignant lymphoma? Nucl Med Commun. 2006;27:11–5.
Pelosi E, Pregno P, Penna D, Deandreis D, Chiappella A, Limerutti G, et al. Role of whole-body [18F] fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) and conventional techniques in the staging of patients with Hodgkin and aggressive non Hodgkin lymphoma. Radiol Med. 2008;113:578–90.
Ribrag V, Vanel D, Leboulleux S, Lumbroso J, Couanet D, Bonniaud G, et al. Prospective study of bone marrow infiltration in aggressive lymphoma by three independent methods: whole-body MRI, PET/CT and bone marrow biopsy. Eur J Radiol. 2008;66:325–31.
Chen YK, Yeh CL, Tsui CC, Liang JA, Chen JH, Kao CH. F-18 FDG PET for evaluation of bone marrow involvement in non-Hodgkin lymphoma: a meta-analysis. Clin Nucl Med. 2011;36:553–9.
Mittal BR, Manohar K, Malhotra P, Das R, Kashyap R, Bhattacharya A, et al. Can fluorodeoxyglucose positron emission tomography/computed tomography avoid negative iliac crest biopsies in evaluation of marrow involvement by lymphoma at time of initial staging? Leuk Lymphoma. 2011;52:2111–6.
Cheng G, Chen W, Chamroonrat W, Torigian DA, Zhuang H, Alavi A. Biopsy versus FDG PET/CT in the initial evaluation of bone marrow involvement in pediatric lymphoma patients. Eur J Nucl Med Mol Imaging. 2011;38:1469–76.
Brusamolino E, Bacigalupo A, Barosi G, Biti G, Gobbi PG, Levis A, et al. Classical Hodgkin’s lymphoma in adults: guidelines of the Italian Society of Hematology, the Italian Society of Experimental Hematology, and the Italian Group for Bone Marrow Transplantation on initial work-up, management, and follow-up. Haematologica. 2009;94:550–65.
Hines-Thomas MR, Howard SC, Hudson MM, Krasin MJ, Kaste SC, Shulkin BL, et al. Utility of bone marrow biopsy at diagnosis in pediatric Hodgkin’s lymphoma. Haematologica. 2010;95:1691–6.
Wang J, Weiss LM, Chang KL, Slovak ML, Gaal K, Forman SJ, et al. Diagnostic utility of bilateral bone marrow examination: significance of morphologic and ancillary technique study in malignant. Cancer. 2002;94:1522–31.
Brunning RD, Bloomfield CD, McKenna RW, Peterson LA. Bilateral trephine bone marrow biopsies in lymphoma and other neoplastic diseases. Ann Intern Med. 1975;82:365–6.
Pakos EE, Fotopoulos AD, Ioannidis JP. 18F-FDG PET for evaluation of bone marrow infiltration in staging of lymphoma: a meta-analysis. J Nucl Med. 2005;46:958–63.
Wu LM, Chen FY, Jiang XX, Gu HY, Yin Y, Xu JR. (18)F-FDG PET, combined FDG-PET/CT and MRI for evaluation of bone marrow infiltration in staging of lymphoma: a systematic review and meta-analysis. Eur J Radiol. 2012;81:303–11.
Ngeow JY, Quek RH, Ng DC, Hee SW, Tao M, Lim LC, et al. High SUV uptake on FDG-PET/CT predicts for an aggressive B-cell lymphoma in a prospective study of primary FDG-PET/CT staging in lymphoma. Ann Oncol. 2009;20:1543–7.
Purz S, Mauz-Körholz C, Körholz D, Hasenclever D, Krausse A, Sorge I, et al. [18F]fluorodeoxyglucose positron emission tomography for detection of bone marrow involvement in children and adolescents with Hodgkin’s lymphoma. J Clin Oncol. 2011;29:3523–8.
Salaun PY, Gastinne T, Bodet-Milin C, Campion L, Cambefort P, Moreau A, et al. Analysis of 18F-FDG PET diffuse bone marrow uptake and splenic uptake in staging of Hodgkin’s lymphoma: a reflection of disease infiltration or just inflammation? Eur J Nucl Med Mol Imaging. 2009;36:1813–21.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agrawal, K., Mittal, B.R., Bansal, D. et al. Role of F-18 FDG PET/CT in assessing bone marrow involvement in pediatric Hodgkin’s lymphoma. Ann Nucl Med 27, 146–151 (2013). https://doi.org/10.1007/s12149-012-0665-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-012-0665-5