Abstract
Introduction
In a previous study of classifying fetuses with cortical formation abnormalities (CFA) with fetal MR, we noticed a cluster of cases with unilateral CFA and complete agenesis of the corpus callosum (ACC). In this study, we provide a detailed morphological analysis of such fetuses using fetal MR to determine if there are indicators (such as the gender of the fetus) that could be used to delineate a genetic substrate of the phenotype in order to inform future studies.
Methods
We have studied 45 fetuses with the unilateral CFA/ACC phenotype and analysed through an expert consensus panel the location and fine detail of the CFA and the associated findings such as associated anomalies, head size, and sex of the fetus.
Results
The frontal lobe was significantly more frequently involved by CFA when compared with other lobes (p < 0.001) but no preference for the left or right hemisphere. CFA most often consisted of excessive/dysmorphic sulcation. The CFA/ACC phenotype was overwhelmingly more frequent in male fetuses (M:F 4.5:1—p < 0.0001). The most frequent associated findings were: ventriculomegaly (16/45 fetuses) and interhemispheric cysts (12/45 cases).
Conclusions
This report highlights the specific phenotype of unilateral CFA/ACC that is much more common in male fetuses. This finding provides a starting point to study possible sex-linked genetic abnormalities that underpin the unilateral CFA/ACC phenotype.
Key Points
• We collected fetuses with unilateral cortical formation abnormality and callosal agenesis.
• That distinctive neuroimaging phenotype has a strong male gender prevalence (over 80%).
• This observation forms the basis of studies about outcomes and genetic substrates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is a growing number of publications that describe the range of appearances of cortical formation anomalies (CFA) in fetuses on in utero and post mortem magnetic resonance (MR) imaging [1,2,3,4,5] and it is well known that CFA are frequently associated with other brain malformations, including complete agenesis of corpus callosum (ACC). Conversely, when complete ACC is detected on prenatal imaging a detailed search should be made for CFA because of the high association and substantial effect on prenatal counselling. In a previous publication, we designed and applied an MR-based classification system for CFA based on laterality, symmetry, and fine detail of the CFA in a cohort of over 350 fetuses [6]. During that study, we noticed a cluster of fetuses with unilateral CFA and ACC.
We have focused on fetuses with complete ACC and unilateral CFA in this study in order to provide a detailed morphological analysis to define the imaging characteristics of such fetuses. CFA are well known to be possibly associated also with partial agenesis or CC hypogenesis; however, the definition of complete ACC is relatively straightforward and not influenced by possible ambiguity related to the high variability of residual segments. The primary aim of the study is to determine if there are indicators (such as the gender of the fetus) that could be used to delineate a genetic substrate of the phenotype in order to inform future studies.
Materials and methods
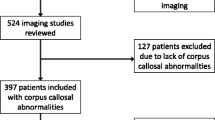
The ethical approach and full methodological details for locating and analysing the original cohort have been described in detail previously [6] but are summarised here. The MR imaging data of fetuses with CFA were collected over an 18-year period (2000–2017) from seven centres in Italy and England, which included approximately 11,000 MR examinations of the fetal brain. All of the studies were performed on 1.5-T scanners and all fetuses had T2-weighted (single-shot FSE) images in the three orthogonal planes (3–5 mm thickness) as a minimum requirement. An expert consensus panel (ECP) was formed to confirm and classify the type of CFA present using the flow diagram developed by the authors in which divided the cases into two main classes based on bilateral or unilateral involvement [6]. The ECP were also asked to record other associated brain abnormalities, including ACC. There were 43 fetuses with ACC and unilateral CFA in the original cohort, and along with two new cases, our current report consists of 45 fetuses with ACC and unilateral CFA. For the present descriptive work, two experts in fetal MR imaging (A.R., C.P.) re-evaluated those 45 studies and provide a more nuanced anatomical description based on lobar location and laterality of the CFA. Other associated anomalies were evaluated including sex of the fetus as from ultrasound, intrauterine MR imaging, fetal karyotyping, autopsy, and post-natal data (at least one criterium available for all cases but one, which was lost at follow-up after unclear pelvic view by ultrasound and MR imaging); head size by biparietal and anterior-posterior diameter measurement according to ref. [7, 8]; and the presence of interhemispheric cysts, ventriculomegaly, and posterior fossa malformations.
Statistical analysis
All analyses were carried out using Statistical Package for Social Science (IBM SPSS Statistics 25). Differences between males and females were probed for categorical and numerical differences. Distributions of discrete and continuous variables were examined for normality using the Shapiro-Wilk test and inspection of histograms. Group differences were tested using either unpaired Student t-test or Mann-Whitney U-test depending if the data appeared to be normally distributed or not. Associations between dichotomous variables and categorical with a level five were studied with contingency tables and tested using the Chi-square test or, alternatively, using the Fisher’s Exact test depending on the frequencies of the contingency tables. When statistical significance was found in contingency tables greater than 2 × 2, pair-wise post hoc tests were performed. Correlation between discrete and numerical variables was studied through the Spearman Correlation Coefficient while dependence between categorical variables was assessed with the Chi-square test. Within-group differences between percentages were assessed by the chi-square test or Fisher’s Exact test, according to the frequencies of the contingency tables. Two-tailed p value < 0.05 was considered statistically significant. p value for post hoc test was adjusted with Bonferroni correction.
Results
Table 1 summarises the main demographic data and the fetal MR imaging findings of the 45 fetuses. The median gestational age (GA) at the time of the MR exam was 22 weeks, interquartile range = 4.25 weeks, Q1 = 21 weeks, Q3 = 25.25 weeks, and full range (20 to 36 weeks).
CFA laterality and lobar involvement
The CFA involved the frontal lobe in 42/45 fetuses (93%) and was confined to the frontal lobe in 18 of those fetuses. The parietal lobe was involved in 25/45 fetuses (56%) including two cases where the CFA involved the parietal lobe only. The temporal lobe was involved much less frequently, in only 3/45 fetuses (7%), and in all of those cases, other lobes were involved. The occipital lobe was involved in only 1/45 fetus (2%), and in that case, the whole hemisphere was involved. The frontal lobe was statistically significantly more frequently involved than other lobes (p < 0.001).
CFA types and fine morphology.
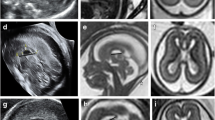
The CFA types according to the scheme reported in ref. 6 were in 17/45 cases C1-type, in 13/45 C2, and in 15/45 C5. The detailed morphology encompassed at least one of the following patterns: multiple tiny excessive invaginations of cortical surface; few (two or three) abnormally major invaginating sulci; irregular fine profile “wart-like”; large tangle of multiple chaotic and deeply invaginating sulci (Figs. 1, 2 and 3). It has to be taken into account that the fine appearance of CFA may be also influenced by the GA [4], since for example, few focally invaginating sulci in a still “lissencephalic” fetus at 21 weeks of age may evolve towards a large tangle of abnormal gyri in a highly gyrificated late third-trimester brain.
A prenatal ss-FSE T2-weighted image from nine exemplificative male cases with CFA located in the right hemisphere is respectively reported (GA in weeks is inserted). Arrows point the CFA location. The frontal lobe alone or in association with parietal lobe is almost always involved. CFA can appear as one of the following: multiple tiny excessive invaginations of cortical surface; few (two or three) abnormally major invaginating sulci (20 and 25 weeks cases example); irregular fine profile “wart-like”; large tangle of multiple chaotic and deeply invaginating sulci
A prenatal ss-FSE T2-weighted image from nine exemplificative male cases with CFA located in the left hemisphere is respectively reported (GA in weeks is inserted). Arrows point to the CFA location. The frontal lobe alone or in association with the parietal lobe is almost always involved. CFA can appear as one of the following: multiple tiny excessive invaginations of cortical surface (23 weeks case example); few (two or three) abnormally major invaginating sulci; irregular fine profile “wart-like”; large tangle of multiple chaotic and deeply invaginating sulci (34 weeks case example)
A ss-FSE T2-wighted prenatal image example from each of the seven male cases with detection of an interhemispheric cyst is respectively reported (GA in weeks is inserted). Arrows point to the unilateral CFA. Asterisks indicate the interhemispheric cyst (in the third case the cyst is not visible on the same section of the CFA). In the last (seventh) case an insert better depicts the CFA
Gender of the fetus
The sex of the fetus was known in 44/45 cases with 36 male and 8 female fetuses indicating a statistically significant excess of male fetuses with ACC and unilateral CFA (male:female = 4.5:1; p < 0.0001—chi-squared test). There were no statistically significant differences between male and female fetuses in terms of GA at MR (p = 0.438) or head size (p = 0.289).
There was no statistical difference between the laterality of the CFA in male fetuses (right hemisphere in 22/36 (61%), left hemisphere in 14/36 (39%)—p = 0.18). CFA lobar location within the hemisphere was in the order of frequency: frontal-parietal (16/36 cases), frontal (16/36 cases), parietal (2/36 cases), temporal (1/36 case), frontal-temporal (1/36 case), and frontal-parieto-temporo-occipital (0/36 cases). The frontal lobe (either in isolation or along with other lobes) was significantly more frequently involved when compared to other locations (p < 0.001). CFA was in the mesial aspect of the hemisphere in 17/36, in the lateral in 2/36, and involved both aspects in 17/36 cases. By pooling together isolated mesial and mesial-lateral extension, they resulted in significantly prevalent (p < 0.001) (Figs. 1, 2 and 3).
Statistical analysis on CFA laterality and lobar involvement in the eight female fetuses could not be performed because of the small number of cases.
Associated intracranial abnormalities
Ventriculomegaly was present in 16/45 fetuses, 15 males and one female (not a statistically significant feature—p = 0.125). Interhemispheric cysts were present in 12/45 cases: 7 males, 4 females, one sex unknown, hence interhemispheric cysts were present in half of the female fetuses but only 16% of male fetuses but this cannot be considered statistically valid because of the small numbers of female fetuses (Fig. 1 supplementary online material). None of the fetal cases in the cohort had clastic lesions using our previously published criteria [6].
Discussion
We have reported a cohort of fetuses with unilateral CFA and ACC, which is very likely to represent a condition (or a small group of conditions) caused by genetic anomalies, because of the absence of any clear imaging signs of acquired pathology and the very high prevalence of males (80%). In those fetuses (particularly in the male fetuses) the unilateral CFA was preferentially located in the frontal lobes, either in isolation or extending into the parietal lobe in its mesial aspect. This quite characteristic and repetitive location of the CFA does not favour the hypothesis of a clastic aetiology which is usually related to more randomly located anomalies; it rather supports the genetic aetiology hypothesis. We cannot currently provide insights into genetic substrate since many genetic anomalies may interact and cause CFA; the striking male gender preponderance might suggest some X-linked entity, at least for part of our cases.
We believe that the identified cohort carries a very low possibility of selection bias: our cases come originally from a MR database of about 11,000 fetal MR examinations gathered from seven international centres [6]. Indeed, the present study population represents the final result of a truly random case collection process, that was prompted by the current trend to perform prenatal ultrasound screening in developed countries at around 20-week gestation. Such screening is usually followed in ACC-suspected cases by an expert ultrasound exam, which in our population, albeit in a minority of cases, identified some unilateral cortical irregularities indeed. Our data may encourage prenatal sonographers to strengthen their scrutiny for associated CFA, especially in male fetuses with ACC. In this regard, the 3D-ultrasound approach may overcome the limitations of the uneven echogenicity between the two hemispheres affecting 2D cross-sectioning. In the vast majority of our population the identification of the CFA was the result of the diagnostic process triggered by ultrasound; therefore, in cases without the ACC “red flag” CFA may go undetected in-utero. Meanwhile, we cannot totally rule out that additional smaller CFA in other locations might have been overlooked by in-utero MR imaging since post-natal imaging or autoptic gold standard was not available in many cases.
While interhemispheric cysts and ventriculomegaly are frequent, findings in ACC and their common pathophysiological basis are quite straightforward; focal CFA are not as frequent and easily explicable as well. It is not clear whether the ACC and the unilateral CFA are associated because of a common pathophysiology mechanism or if the CFA is causally related to the ACC for example by interfering with the normal axonal guidance that is required to form the normal corpus callosum.
The present study concentrates on “complete” ACC and we deliberately did not include cases in which the corpus callosum was formed in part (i.e. “hypogenesis of the corpus callosum”). The rationale for this was that we wanted to focus our analysis on clear-cut, unambiguously defined cases, which is possible for complete ACC because the definition of the entity is relatively straightforward. In contrast, there can be substantial ambiguity about definitions of cases in which some, but not all, of the corpus callosum, is present (hypogenesis of the corpus callosum, dysgenesis of the corpus callosum, callosal thinning, etc.) and this problem has been discussed in the recently published literature [9]. We can report, however, in the cohort of > 350 fetuses with CFA there were 16 fetuses with unilateral CFA and hypogenesis of the corpus callosum, as defined by the original ECP. The gender of those fetuses was known in 11/16 (69%) and a male excess was found (8 males and 3 females). The male preponderance of approximately 2.7:1 in that group is not as high as in the cases of unilateral CFA and ACC (4.5:1) but it still indicates a substantial excess of male fetuses.
Our observation regarding the excess male prevalence is consistent with the small volume of existing publications of case reports/small case series [10,11,12] of unilateral CFA. Meanwhile, in other larger population studies on ACC with or without associated anomalies males result only mildly to moderately more frequently affected than females [13,14,15]. However, it has to be underlined that in the latter larger population studies ACC is frequently associated with any sort of intracranial or somatic anomalies, so that data is quite unspecific. Instead, if we consider the small case series studies with specific association with CFA [10,11,12], such reported cases share their core features more closely with the ones of our cohort. Albeit in those reports, interhemispheric cysts were frequent findings, whilst in our population, interhemispheric cysts were detected in only 12/45 cases overall and proportionally less in male cases (7/36). We may have missed the detection of some interhemispheric cysts, since we relied on fetal MR imaging only and this should be taken into account; still, the large part and at least the majority of our male cohort did not have interhemispheric cysts. Therefore, the hypothesis that an interhemispheric cyst may be involved in halting the normal corpus callosum formation is far from being an exhaustive explanation at least for the majority of our male cases. Ventriculomegaly was present in almost half of the males but it is well-known that some degree of ventriculomegaly is a common feature in cases of isolated ACC.
No significant correlation between morphological features and different gender was found; however, statistical analysis was difficult because of the small number of female fetuses, Regarding the resulting small group of eight female fetuses, it should be mentioned that the possibility that some cases carried Aicardi syndrome is likely, since for example half of them showed clear interhemispheric cysts (Fig. 1 supplementary online material). Due to the small number of females, the comparison with males has low statistical power, even if, as just stated, the proportion of cases with interhemispheric cysts was definitely higher in the females.
There are several limitations to the current study including the retrospective nature and the lack of genetic, histological, and follow-up clinical data. In addition, we do not possess enough material to pursue any statistically valid clinical follow-up assessment on such cohort (mainly due to the high rate of terminations of the pregnancy); however, we provide as an appendix to our report (appendix-supplementary online material) some of the follow-up stories we were able to collect, since we believe that the anectodical cases with the discrepancy between the putative severity of the prenatal imaging picture and the not as severe clinical outcome deserve to be mentioned.
Conclusions
Our report highlights the common MR morphological features in such cases and we propose that such fetuses are very likely to have genetic anomalies and further laboratory-based research is required to define the anomalies and investigate their clinical significance.
Based on our data, when ACC is detected at prenatal imaging in a male fetus, unilateral CFA in the frontal and parietal lobes might be a possible specific additional finding.
Despite the rarity of this “not isolated ACC” condition, the relatively high fraction of cases undergone pregnancy termination in our historical series urges the need for further investigation on pathology or conversely outcome correlates, in order to provide a full rationale counselling.
Abbreviations
- ACC:
-
Agenesis of the corpus callosum
- CFA:
-
Cortical formation abnormalities
- ECP:
-
Expert consensus panel
- GA:
-
Gestational age
- MR:
-
Magnetic resonance
References
Levine D, Barnes PD, Robertson RR, Wong G, Mehta TS (2003) Fast MR imaging of fetal central nervous system abnormalities. Radiology 229:51–61
Griffiths PD, Bradburn M, Campbell MJ, on behalf of the MERIDIAN collaborative group et al (2017) Magnetic resonance imaging to enhance the diagnosis of fetal developmental brain abnormalities in utero (MERIDIAN). Lancet 389:538–546
Griffiths PD, Brackley K, Bradburn M, Connolly D et al (2017) Anatomical subgroup analysis of the MERIDIAN cohort: failed commissuration. Ultrasound Obstet Gynecol 50:753–760
Righini A, Parazzini C, Doneda C et al (2012) Early formative stage of human focal cortical gyration anomalies: fetal MRI. AJNR Am J Roentgenol 198:439–447
Cesaretti C, Spaccini L, Rustico M et al (2014) Prenatal magnetic resonance imaging detection of temporal lobes and hippocampal anomalies in hypochondroplasia. Prenat Diagn 34:1015–1017
Righini A, Genovese M, Parazzini C et al (2020) Cortical formation abnormalities on fetal MR imaging in utero: a proposed classification system trialled on 356 cases from Italian and UK centres. Eur Radiol 30:5250–5260
Conte G, Milani S, Palumbo G et al (2018) Prenatal brain MR imaging: reference linear biometric centiles between 20 and 24 Gestational Weeks. AJNR Am J Neuroradiol 39:963–967
Tilea B, Alberti C, Adamsbaum C et al (2009) Cerebral biometry in fetal magnetic resonance imaging: new reference data. Ultrasound Obstet Gynecol 33:173–181
Mahallatti H, Sotiriadis A, Celestin C et al (2021) Heterogeneity in defining fetal corpus callosum pathology: systematic review. Ultrasound Obstet Gynecol 58:11–18
Uccella S, Accogli A, Tortora D et al (2019) Dissecting the neurological phenotype in children with callosal agenesis, interhemispheric cysts and malformations of cortical development. J Neurol 266:1167–1181
Glenn OA, Quiroz EM, Berman JI, Studholme C, Xu D (2010) Diffusion-weighted imaging in fetuses with unilateral cortical malformations and callosal agenesis. AJNR Am J Neuroradiol 31:1100–1102
Cagneaux M, Lacalm A, Huissoud C et al (2013) Agenesis of the corpus callosum with interhemispheric cyst, associated with aberrant cortical sulci and without underlying cortical dysplasia. Ultrasound Obstet Gynecol 42:603–605
Glass HC, Gary MS, Ma C, Sherr EH (2008) Agenesis of the corpus callosum in california 1983−2003: a population-based study. Am J Med Genet 146A:2495–2500
Szabò N, Gergev G, Kòbor J et al (2011) Corpus callosum anomalies: birth prevalence and clinical spectrum in hungary. Pediatr Neurol 44:420–426
Ballardini E, Marino P, Maietti E, Gianni A, Neville AJ (2018) Prevalence and associated factors for agenesis of corpus callosum in Emilia Romagna (1981–2015). Eur J Med Genet 61:524–530
Funding
Aspects of this work concerning the UK cases were funded by National Institute for Health Research Health Technology Assessment programme (NIHR HTA (09/06/01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Andrea Righini, M.D.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Ethics approvals described by centre
A. UOC di Radiologia e Neuroradiologia Pediatrica, Ospedale dei Bambini V. Buzzi , Milan, Italy and UOC di Neuroradiologia- IRCCS Fondazione Policlinico-Mangiagalli Milan, Italy.
The cases from Milan were recruited as clinical cases with ethical approval for retrospective review of clinical notes and MR images by the “Milano Area B” and “Milano Area C” Ethics Committees, without the need for specific consent from patients (n. 2292/2016 and n. 1394/2015 protocol approval codes respectively).
B. UOC di Neuroradiologia -IRCCS Gaslini Research Children’s Hospital Genoa, Italy.
Local Ethical Committee approval code number 533REG2015.
C. UOC di Neuroradiologia - Spedali Civili di Brescia, Italy, UOC di Neuroradiologia - Azienda Ospedaliera Padovana Padua, Italy, and UOC di Neurologia Pediatrica - IRCCS Ospedale Pediatrico Meyer Firenze, Italy.
For the cases from Brescia, Padua, and Florence (the three centres which provided the minority of cases) the consent form was waived since this was a retrospective analysis of routinely collected anonymized data performed blindly.
D. Academic Unit of Radiology, University of Sheffield, UK.
Most of the women from this centre were recruited from on-going research studies over the recruitment time period and provided informed written consent under the guidance and approval of the Institutional Research Ethics Committee. Those women were not paid for their involvement in the study but travel expenses were offered for themselves and a companion. Relevant review was sought, and approval obtained, from the Institutional Clinical Effectiveness Unit and Research Department in order to allow those cases performed for clinical purposes to be reported in this paper.
Study subjects or cohorts overlap
Statement concerning overlapping content
During the study reported in ref. 6, we noticed a cluster of fetuses with unilateral CFA and ACC. We have focused on fetuses with complete ACC and unilateral CFA in this study in order to provide a detailed morphological analysis to define the imaging characteristics of such fetuses.
Righini A, Genovese M, Parazzini C, et al Cortical formation abnormalities on fetal MR imaging in utero: A proposed classification system trialled on 356 cases from Italian and UK centres. Eur Radiol 2020; 30:5250-5260
Methodology
• retrospective
• cross-sectional study
• multicentre study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 5297 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vola, E.A., Griffiths, P.D., Parazzini, C. et al. Complete agenesis of corpus callosum and unilateral cortical formation anomalies detected on fetal MR imaging: a phenotype strongly associated with the male fetuses. Eur Radiol 33, 2258–2265 (2023). https://doi.org/10.1007/s00330-022-09173-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09173-9