Abstract
Objective
To formulate a classification system for foetal cortical formation abnormalities (CFAs) based on in utero magnetic resonance (iuMR) appearances and trial it in 356 cases.
Methods
This retrospective study included all cases of foetal CFA diagnosed between 2000 and 2017 from seven centres in Italy and UK. All of the studies were reviewed by a panel of paediatric neuroradiologists experienced in iuMR with the aid of an algorithm designed to categorise the abnormalities.
Results
Consensus expert review confirmed 356 foetuses with CFA and the first level of classification distinguished bilateral CFA (229/356–64%) from unilateral CFA (127/356–36%) cases with sub-classification of the bilateral cases into asymmetric (65/356–18%) and symmetric (164/356–46%) involvement. There was a statistically significant excess of foetuses with small head size, e.g. 17% of the cohort had a bi-parietal diameter < 3rd centile. There was a small but statistically significant excess of males in the cohort. Further categorisation was made on fine anatomical structure.
Conclusions
It is often not possible to classify foetal CFA using the principles and nomenclature used in paediatric neuroradiology. We have created a classification system for foetal CFA based on the analysis of 356 cases and believe that this will assist future research designed to correlate ante-natal and post-natal imaging features and understand the clinical sequelae of CFA described in utero.
Key Points
• We describe a morphological classification system of foetal brain cortical formation abnormalities that can be used in clinical practice.
• This classification system can be used in future research studies to evaluate the long-term imaging and clinical outcomes of foetal brain cortical formation abnormalities in 17- to 38-week gestational age range.
• The practical value of the work is in providing a framework and language to look for imaging clues that may differentiate between different CFA in further studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In utero MR (iuMR) imaging has become a routine part of the ante-natal assessment of foetal brain abnormalities because of proven improvements in diagnostic accuracy and positive contributions to clinical management [1,2,3,4,5]. However, some foetal neuropathologies, such as cortical formation abnormalities (CFAs), are difficult (or impossible) to detect on ultrasonography and present substantial challenges on iuMR imaging. The diagnostic accuracy for detecting foetal CFA with iuMR is not known and the challenges are likely to be more pronounced in less mature (i.e., second trimester) foetuses [6]; indeed, some CFAs do not become apparent until after birth. A further problem is the classification of CFA when a definite abnormality is shown on iuMR imaging. Many radiologists who report those studies are familiar with the classification of paediatric CFA on post-natal neuroimaging, which are well described in the published literature [7, 8].
In addition, it is often not possible to arrive at a definitive nosological diagnosis of a CFA ante-natally, which limits the information available for prognostication. Furthermore, there is currently no systematic classification of foetal CFA and little is known about the correlation between the different morphological types of foetal CFA with aetiology or the long-term clinical sequelae. It has also been reported that CFAs show different features at different stages of pregnancy [9,10,11,12] and this further complicates the clinical scenario. Establishing a morphological classification of foetal CFA should, therefore, have a positive impact on research and clinical management.
The purpose of this study is to produce a morphological classification system for foetal CFA and to trial it on a large cohort of foetuses with CFA. Such a classification system would be valuable to radiologists reporting such cases clinically and will pave the way to future studies into the aetiology of CFA and their long-term neurodevelopmental sequelae.
Materials and methods
Subjects
We performed a retrospective analysis of iuMR imaging obtained over an 18-year period (2000–2017) from six regional centres in Italy and one in England. Ethical approval was obtained separately at all collaborating sites. Each neuroradiology site lead searched their institution’s databases to locate iuMR studies of foetal brain examinations that included comments such as distortion of the cortical surface, abnormal sulcation/gyration for gestational age, trans-mantle cleft, unilateral or bilateral abnormal volume increase of cerebral lobes, global distortion of shape of one or both cerebral hemisphere. Those screening terms were chosen because they were more inclusive, allowing of more CFA cases than definite entities such as polymicrogyria, lissencephaly and schizencephaly. Cases of abnormal primary neurulation (e.g. anencephaly) and failed ventral induction (holoprosencephaly) were not included. Foetuses with developmental brain abnormalities, such as agenesis of the corpus callosum, brainstem or Dandy-Walker malformations, were included only if a CFA was present as well. Cases with acute-subacute signs of extensive brain haemorrhage deranging the global brain shape were excluded, as well as those with remarkable brain surface deformation due to congenital neoplasms or large vascular malformations (i.e. dural sinus malformation). We explain the use of previously reported material in appendix ESM 1 (Electronic Supplementary Material).
iuMR imaging and analysis
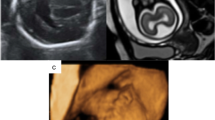
iuMR imaging protocols were not standardised across the recruiting centres due to the retrospective nature of the study and the use of different MR scanners. All studies, however, were performed at 1.5 Tesla using abdominal or cardiac coils and with at least three-planar single-shot fast spin-echo T2-weighted sequences (TE range 80–180 ms), slice thickness between 3 and 5 mm (the latter mostly in foetuses older than 30 week gestation) and in-plane resolution between 1.1 and 1.3 mm2. The site leads selected 4–10 representative images from the study for review by an expert panel, which consisted of at least four from a pool of six paediatric neuroradiologists (with more than 10-year experience in iuMR (A.R., C.P., M.S., L.P., F.T, P.D.G)) from other centres; i.e. neuroradiologists did not review his/her own cases. Information including gestational age at iuMR study, the centile measurement of bi-parietal diameter from iuMR [13, 14] and a list of any other brain abnormalities was available. The reviewers could review the entire study if they required.
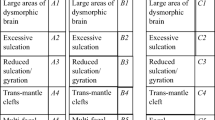
The expert panel categorised the type of abnormality present using a flow diagram devised by two of the authors (A.R. and P.D.G.) (Fig. 1). Primary classification produced three groups based on laterality of CFA; group A—bilateral, asymmetric CFA; group B—bilateral, symmetric CFA; and group C—unilateral CFA. Seventeen categories (five from group A, six from group B and six from group C) were then defined from fine anatomical features as shown in Figs. 2, 3 and 4: group A, bilateral asymmetric—A1, large areas of dysmorphic brain; A2, excessive sulcation/gyration; A3, shallow opercula; A4, trans-mantle clefts; A5, multi-focal ‘bites’. Group B, bilateral symmetric: B1, large areas of dysmorphic brain; B2, excessive sulcation/gyration; B3, reduced sulcation/gyration; B4, trans-mantle cleft; B5, multi-focal ‘bites’; B6, cobblestone cortex. Group C, unilateral: C1, large area of dysmorphic brain; C2, excessive sulcation/gyration; C3, poor sulcation/gyration; C4, trans-mantle cleft; C5, focal distortion; C6, enlarged hemisphere. In cases where more than one feature was present, the panel was asked to define the most prominent anatomical abnormality and provide a free-text description of the other features. The reviewers specified the location of the abnormality in cases of abnormal sulcation (A2, A3, B2, B3, C2 and C3). The head size and sex distribution of the complete cohort, groups and categories was assessed using 95% confidence intervals.
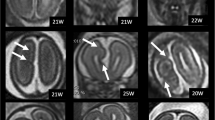
Examples of group A—bilateral asymmetric CFA as defined in this paper. Dysmorphic condition was defined as the clear abnormal distortion of profile of one or more lobes on both cortical and ventricular surface side, when present as dominant finding. Excessive gyration/sulcation was defined as the number of gyri/sulci of one or more lobes was higher than expected for gestational age, when present as dominant finding. Definitions of the other categories are self-explanatory. A1 (a)—axial and coronal images of a foetus (33 gestational weeks) with bilateral, asymmetric areas of dysmorphic brain (arrows). Other features recognised in this case were hypoplasia of the brainstem and cerebellum (not shown). A2 (b)—axial and coronal images of a foetus (23 gestational weeks) with bilateral asymmetric excessive sulcation/gyration (arrows). Cerebellar hypoplasia was also present. A3 (c)—axial and coronal images of a foetus (23 gestational weeks) with bilateral asymmetric shallow operculi (arrows). The foetus had severe hypogenesis of corpus callosum and unilateral mild ventriculomegaly. A4 (d)—axial and coronal images of a foetus (33 gestational weeks) with bilateral asymmetric trans-mantle clefts (arrows). The foetus had ventriculomegaly and was microcephalic. A5 (e)—coronal and sagittal images of a foetus (25 gestational weeks) with bilateral asymmetric multi-focal “bites” (arrows)
Examples of group B—bilateral symmetric CFA as defined in this paper. B1 (a)—axial and coronal images of a foetus (21 gestational weeks) with bilateral, symmetric areas of dysmorphic brain (arrows). Agenesis of the corpus callosum was also present. B2 (b)—axial and coronal images of a foetus (21 gestational weeks) with bilateral, symmetric excessive sulcation/gyration (arrows). B3 (c)—axial and coronal images of a foetus (30 gestational weeks) with bilateral, symmetric reduced sulcation/gyration (arrows). The foetus was microcephalic. B4 (d)—axial and coronal images of a foetus (27 gestational weeks) with bilateral, symmetric trans-mantle cleft (arrows). B5—there were no cases of bilateral symmetric multi-focal “bites” in the cohort. B6 (e)—axial and coronal images of a foetus (31 gestational weeks) with bilateral symmetric cobblestone cortex (arrows). Microphthalmia, abnormal brainstem (not shown) and ventriculomegaly were also present
Examples of group C—unilateral CFA as defined in this paper. C1 (a)—axial images of a foetus (25 gestational weeks) with a unilateral area of dysmorphic brain (arrows). There is abnormal low T2-weighted signal in the affected (left) hemisphere. The foetus also had agenesis of the corpus callosum and interhemispheric cyst. C2 (b)—axial and coronal images of a foetus (32 gestational weeks) with unilateral excessive sulcation/gyration (numerous, small gyri) in part of the left hemisphere (arrows). C3 (c)—axial and coronal images of a foetus (35 gestational weeks) with unilateral poor sulcation/gyration of the left hemisphere (arrows), that is hypoplastic. Unevenly diffuse reduced signal within white matter is present. The foetus also had a Dandy-Walker malformation (not shown). C4 (d)—axial and coronal images of a foetus (21 gestational weeks) with unilateral trans-mantle cleft in the left hemisphere (arrows). The cavum of septum pellucidum is absent. C5 (e)—axial and coronal images of a foetus (18 gestational weeks) with unilateral focal distortion in the form of an early abnormal sulcus in the left hemisphere (arrows). This foetus also had hypoplasia of the corpus callosum. C6 (f)—axial and coronal images of a foetus (26 gestational weeks) with unilateral enlarged right hemisphere (arrows), which is also generally malformed
Analysis was supplemented by comment on abnormalities of the transient layers; assessment of brain mantle layering anomalies was focused mainly on the absence of clear demarcation between intermediate zone and subplate, as compared with expected normal features by reference (15). Examples are reported in figure ESM 1: too indistinct/disrupted for gestational age, too prominent for gestational age, abnormal enlargement of the germinal matrix, especially in ganglionic eminence region (with/without cavitations), or areas of abnormal low T2-weighted signal in the hemisphere (generalised or focal). Abnormality of the contour of the ventricular system was recorded as irregular/dysmorphic resulting from distortion of the adjacent brain (e.g. secondary to schizencephaly and features in keeping with subependymal heterotopias). Periventricular nodular heterotopia was assessed as associated finding and not among primary classification criteria for two reasons: firstly, it may not affect the cortical surface, secondly the well-known low sensitivity of iuMR for such findings [16]. The panel was asked to comment if they thought the CFA was due to a developmental or acquired cause or if it was not possible to speculate on the aetiology. The findings reported in table ESM 2 were considered signs of possible acquired aetiology.
The sex of the foetus was recorded after delivery or termination of pregnancy wherever possible.
When a foetus had more than one iuMR study, the panel evaluated the one performed at younger age and we will describe the foetuses with two studies in a future publication.
Results
Our cohort came from iuMR studies of 11,100 foetuses and 374 of those were reviewed by the panel. Eighteen out of 374 (4.8%) were excluded because the panel did not think a CFA was present. Most of the excluded cases involved foetuses with agenesis/hypogenesis of the corpus callosum in which an associated CFA had been described on the clinical report but was not confirmed by the panel. Three of the excluded foetuses that had a second iuMR study later in pregnancy that did reveal a CFA and will be described in a subsequent report. Three hundred fifty-six foetuses with CFA are reported in this paper as shown in ESM 1 and the gestational ages at iuMR imaging is presented in Fig. 5 but summarised here. One hundred seventy-two out of 356 (48%) had iuMR between 17–23 gw and 184/356 (52%) at ≥ 24 gw. The descriptions of the CFA type provided by the consensus panel are shown in Fig. 6. Bilateral CFA (groups A and B) were present in 229/356 cases (64%). Bilateral asymmetric abnormalities (group A) were described in 65/356 cases (18%) and bilateral symmetric abnormalities (group B) in 164/356 (46%). Unilateral CFAs (group C) were present in 127/356 cases (36%). The panel concluded that the CFA was probably due to a developmental anomaly in 257/356 (72%), probably an acquired lesion in 56/356 (16%) foetuses and that it was not possible to speculate on the aetiology in 43/356 (12%). The figures for the bilateral CFA (groups A and B) were similar to the entire cohort but there were more speculative acquired lesions (20%) and ‘not known’ cases (16%) in the unilateral CFA cases (group C).
A breakdown of the cohort in terms of skull size by centile group is given in Table 1. There was a statistically significant excess of foetuses with small head size across the cohort, with 40% having bi-parietal diameter ≤ 10th centile and 17% with head size < 3rd centile (95% CI, 13–21%). The small head size in the cohort was primarily due to a large excess of foetuses with small heads in group B—bilateral symmetrical CFA cases (62% of foetuses had head size below < 10th centile). There was a small, but statistically significant, preponderance of males in the cohort as shown in ESM 1. There were no intracranial abnormalities other than CFA in 74/356 (21%) foetuses, ventriculomegaly along with CFA was present in 86/356 (24%) and structural brain abnormalities other than CFA were present in 196/356 (55%). The most frequent associated brain abnormalities involved the corpus callosum 127/356 (36%—agenesis 83, hypoplasia 44) and cerebellar malformations 60/356 (17%). Note that 19 foetuses had corpus callosum and cerebellar malformations.
Specific categories of cortical formation abnormalities
A1—bilateral, asymmetric areas of dysmorphic brain
This was the largest group A category (43/65–66% of the bilateral, asymmetric CFA cases). There was an excess of foetuses with head size ≤ 10th centile in this category (15/43–35%, expected 10%) but only one foetus had head size ≤ 3rd centile. Two foetuses had head size ≥ 90th centile. No other intracranial abnormalities were present in 3/43 (7%), ventriculomegaly only was found in 4/43 (9%) and other structural brain abnormalities were found in 36/43 (84%). Abnormality of the transient layers occurred in 34/43 (79%) foetuses, the most frequently indistinct or disrupted transient layers (20/43–47%), whilst abnormally prominent transient layers were present in 4/43 (9%). The germinal matrix was abnormally large in ganglionic eminence region (with/without cavitation) in 3/43 (7%) and there was abnormal reduced T2-weighted signal in the cerebral hemispheres in 7/43 (16%).
B3—bilateral symmetric reduced sulcation/gyration
This was the largest group B category (91/164–55% of bilateral, symmetric CFA cases). There was an excess of foetuses with head size ≤ 10th centile in this category (64/91–70%, expected 10%) and 37/91 (41%) had a head size ≤ 3rd centile, expected 3%). Two foetuses had a head size ≥ 90th centile. No other intracranial abnormalities were shown in 29/91 (32%), ventriculomegaly only was present in 24/91 (26%) and other structural brain abnormalities were found in 38/91 (42%). In 47/91 of B3 who had ‘poor opercularisation’, the implication being the abnormality was confined to the Sylvian fissure-operculum. All of those cases occurred in foetuses ≤ 25 gestational weeks at iuMR time. In 44/91 cases, the abnormality was described as ‘sulcation abnormality’ implying delayed sulcation at sites other than the Sylvian fissure (although the Sylvian fissure-operculum may be involved as well). Those cases occurred in more mature foetuses, all but three were 25 gestational weeks or later. Abnormality of the transient layers occurred in 70/91 (77%) foetuses. The most frequent abnormality described was indistinct or disrupted transient layers (34/91–37%), whilst abnormally prominent transient layers were present in 17/91 (19%). The germinal matrix was abnormally large in ganglionic eminence region (with/without cavitation) in 17/91 (19%) and there was abnormal reduced T2-weighted signal in the cerebral hemispheres in 2/91 (2%).
C5—unilateral focal distortion
This was the largest group C category (43/127–34% of the unilateral CFA cases). The head sizes in this category approximated to a normal distribution with 37/43 (86%) described as having ‘normal’ head size (10–90th centile–—expected 80%). Two foetuses had a head size ≤ 10th centile and one had a head size ≥ 90th centile. No other intracranial abnormalities were shown in 15/43 (35%), ventriculomegaly only was found in 4/43 (9%) and other structural brain abnormalities were found in 24/43 (56%). The type of focal distortion was defined further as ‘early and/or abnormal sulcus’ in 32/41, ‘bite’ in 7/41 and ‘wart-like’ in 4/41 as described in an earlier publication [9] (see also figure ESM 2 for examples and definitions). There were no foetuses with unilateral ‘saw-tooth’ focal distortions. Abnormality of the transient layers occurred in 32/43 (74%) of foetuses with the most frequent abnormality described was indistinct transient layers (25/43–58%), whilst abnormally prominent transient layers were present in 1/43 (2%). The germinal matrix of ganglionic eminence region was abnormally large (with/without cavitation) in 1/43 (2%) and there was abnormal reduced T2-weighted signal in the affected cerebral hemisphere in 5/43 (12%).
Cases from some of the smaller categories of CFA are presented in ESM 2.
Discussion
We have produced a classification system for foetal CFA based on iuMR features and applied it to 356 cases as determined by a panel of experienced paediatric neuroradiologists. We stress that outcome reference diagnoses were not available for the entire cohort so no formal comparison with post-natal MR has been attempted but is planned for the future. The purpose of the study was to describe foetal CFA using well-established radiological approaches but this is difficult because of the rarity of the condition. As such, we needed to perform a retrospective study pooling data from seven regional centres over an 18-year period.
A potentially relevant finding was the excess of foetuses with CFA and small head size. There is no universally accepted definition of foetal microcephaly but if we use the threshold of < 3rd centile, we have shown that there are nearly six times more than expected in the cohort (17%). A major contribution comes from group B, bilateral symmetrical CFA, which had nearly an eleven-fold increase in microcephaly (32%). On superficial review, there appears to be a minor excess (5%) of foetuses with macrocephaly (≥ 97th centile) but this is due to the number of cases from category C6 (hemimegalencephaly). It is also important to note that other structural brain abnormalities were present in the majority of cases 55% with failure of commissuration the most prevalent associated brain pathology.
The diagnosis of CFA in paediatric and adolescent populations has improved substantially recently due to a number of factors. The quality and access to MR imaging has improved but it is also important to recognize the role of publications that provide frameworks for classification. It is less clear if that approach in children can be applied to foetal cases. Understanding the diagnostic capability and impact of iuMR in the field of foetal CFA is still in its early stages, but there have been some important contributions to the literature. Glenn et al reported 31 young children with CFA confirmed on post-natal imaging (13 with polymicrogyria, 15 with periventricular heterotopia and three with schizencephaly) and then reviewed the associated foetal MR studies [17]. They showed that iuMR had exceptionally high ‘sensitivity’ and ‘specificity’ for detecting polymicrogyria and schizencephaly but heterotopia presented more of a problem (sensitivity 73%, specificity 92%).
The likely timings of the events that underpin CFA lead us to predict that some will have comparable features when imaged ante-natally and compared with post-natal imaging. Hemimegalencephaly is a case in point where unilateral, abnormal over-production of neurons and glia has manifested many of its long-term features (enlarged, dysmorphic hemisphere with an extensive CFA) by the time iuMR studies are commenced (around 20 gestational week). The rarity of hemimegalencephaly makes formal assessment of the diagnostic accuracy of iuMR imaging virtually impossible, but a recent publication suggests that the diagnosis of hemimegalencephaly is straightforward on iuMR [18]. Similarly, early experience of imaging schizencephaly with iuMR is relatively straightforward [14, 15]. The diagnosis of many other forms of foetal CFA relies on more subtle ante-natal features including recognition of abnormal surface features (e.g. too few or too many sulci/gyri for gestational age and/or disturbances of the ‘transient layers’) [9, 10, 19,20,21,22,23,24,25,26]. For example, a foetus with symmetric reduction in cortical sulci/gyri may go on to show lissencephaly, some severity of pachygyria or polymicrogyria post-natally. The practical value of the work described in this paper is in providing a framework and language to look for imaging clues that may differentiate between those pathologies. By way of example, a foetus shown to have an A1 CFA may be at increased risk of having a tubulin anomaly and will direct genetic investigations.
Our study has several limitations, the main one being lack of outcome reference diagnoses and lack of comparison with post-natal MR imaging, although we hope to address this in the future. In addition, the judgement of symmetry in bilateral CFA may have been hampered by non-orthogonal images, although the expert panel always made their assessment on images in three planes. Moreover, thicker (5 mm) slices were used in more mature foetuses so the risk of partial volume effects was reduced.
In conclusion, we have devised and trialled a classification system for foetal CFA abnormalities. We have shown a statistically significant excess of male foetuses with CFA, corroborating post-natal data about male prevalence in cortical anomalies [24]. Other brain abnormalities are present in the majority of cases and a high proportion of foetuses have a small head size. Future assessments of the cases presented here will include detailing the change in appearance of CFA during pregnancy by reporting the 64 cases from the cohort that had a second follow-up iuMR study.
Abbreviations
- CFA:
-
Cortical formation abnormalities
- iuMR:
-
In utero magnetic resonance
References
Mundy L, Hiller J, Braunack-Mayer A, Merlin T (2007) MRI for the detection of foetal abnormalities. Adelaide Health Technology Assessment, Adelaide
Levine D, Barnes PD, Robertson RR, Wong G, Mehta TS (2003) Fast MR imaging of fetal central nervous system abnormalities. Radiology 229(1):51–61
Rossi AC, Prefumo F (2014) Additional value of fetal magnetic resonance imaging in the prenatal diagnosis of central nervous system anomalies: a systematic review of the literature. Ultrasound Obstet Gynecol 44(4):388–393
Van Doorn M, Oude Rengerink K, Newsum EA, Reneman L, Majoie CB, Pajkrt E (2015) Added value of fetal MRI in foetuses with suspected brain abnormalities on neurosonography: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 23:1–13
Griffiths PD, Bradburn M, Campbell MJ et al (2017) Use of MRI in the diagnosis of fetal brain abnormalities in utero (MERIDIAN): a multicentre, prospective cohort study. Lancet 389:538–546
Williams F, Griffiths PD (2017) In utero MR imaging in foetuses at high risk of lissencephaly. Br J Radiol 90(1072):20160902
Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB (2012) A developmental and genetic classification for malformations of cortical development: update 2012. Brain 135(5):1348–1369
Barkovich AJ, Raybaud CA (2012) Congenital malformations of the brain and skull. In: Barkovich AJ, Raybaud CA (eds) Pediatric Neuroimaging, 6th edn. Lippincott Williams and Wilkins, Philadelphia, chapter 5, pp 367–568
Righini A, Zirpoli S, Mrakic F, Parazzini C, Pogliani L, Triulzi F (2004) Early prenatal MR imaging diagnosis of polymicrogyria. AJNR Am J Neuroradiol 25(2):343–346
Glenn OA, Norton ME, Goldstein RB, Barkovich AJ (2005) Prenatal diagnosis of polymicrogyria by fetal magnetic resonance imaging in monochorionic co-twin death. J Ultrasound Med 24(6):711–716
Glenn OA, Barkovich AJ (2006) Magnetic resonance imaging of the fetal brain and spine:an increasingly important tool in prenatal diagnosis -Part 2. AJNR Am J Neuroradiol 27(9):1807–1814
Righini A, Parazzini C, Doneda C et al (2012) Early formative stage of human focal cortical gyration anomalies:fetal MRI. AJR Am J Roentgenol 198(2):439–447
Conte G, Milani S, Palumbo G et al (2018) Prenatal brain MR imaging: reference linear biometric centiles between 20 and 24 gestational weeks. AJNR Am J Neuroradiol 39(5):963–967
Tilea B, Alberti C, Adamsbaum C et al (2009) Cerebral biometry in fetal magnetic resonance imaging: new reference data. Ultrasound Obstet Gynecol 33(2):173–181
Kostović I, Judas M, Rados M, Hrabac P (2002) Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex 12(5):536–544
Conte G, Parazzini C, Falanga G et al (2016) Diagnostic value of prenatal MR imaging in the detection of brain malformations in fetuses before the 26th week of gestational age. AJNR Am J Neuroradiol 37(5):946–951
Glenn OA, Cuneo AA, Barkovich AJ, Hashemi Z, Bartha AI, Xu D (2012) Malformations of cortical development: diagnostic accuracy of fetal MR imaging. Radiology 263(3):843–855
Williams F, Griffiths PD (2014) The diagnosis of hemimegalencephaly using in utero MRI. Clin Radiol 69(6):291–297
Glenn OA, Quiroz EM, Berman JI, Studholme C, Xu D (2010) Diffusion-weighted imaging in fetuses with unilateral cortical malformations and callosal agenesis. AJNR Am J Neuroradiol 31(6):1100–1102
Levine D, Barnes PD (1999) Cortical maturation in normal and abnormal fetuses as assessed with prenatal MR imaging. Radiology 210(3):751–758
Larroche JC, Girard N, Narcy F, Fallet C (1994) Abnormal cortical plate (polymicrogyria), heterotopias and brain damage in monozygous twins. Biol Neonate 65(6):343–352
Lerman-Sagie T, Leibovitz Z (2016) Malformations of cortical development: from postnatal to fetal imaging. Can J Neurol Sci 43(5):611–618
Frassoni C, Avagliano L, Inverardi F et al (2016) Familial precocious fetal abnormal cortical sulcation. Neuropediatrics 47(4):253–258
Leventer RJ, Jansen A, Daniela T et al (2010) Clinical and imaging heterogeneity of polymicrogyria: a study of 328 patients. Brain 133:1415–1427
Fogliarini C, Chaumoitre K, Chapon F et al (2005) Assessment of cortical maturation with prenatal MRI: part II: abnormalities of cortical maturation. Eur Radiol 15(9):1781–1789
Righini A, Frassoni C, Inverardi F et al (2013) Bilateral cavitations of ganglionic eminence: a fetal MR imaging sign of halted brain development. AJNR Am J Neuroradiol 34(9):1841–1845
Funding
Aspects of this work concerning the UK cases were funded by the National Institute for Health Research Health Technology Assessment programme (NIHR HTA (09/06/01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Andrea Righini M.D.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and Biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Ethics approvals described by centre:
-
A)
UOC di Radiologia e Neuroradiologia Pediatrica, Ospedale dei Bambini V. Buzzi, Milan, Italy and UOC di Neuroradiologia-IRCCS Fondazione Policlinico-Mangiagalli Milan, Italy.
The cases from Milan were recruited as clinical cases with ethical approval for retrospective review of clinical notes and MR images by the ‘Milano Area B’ and ‘Milano Area C’ Ethics Committees, without the need for specific consent from patients (n. 2292/2016 and n. 1394/2015 protocol approval codes respectively).
-
B)
UOC di Neuroradiologia-IRCCS Gaslini Research Children’s Hospital Genoa, Italy.
Local Ethical Committee approval code number 533REG2015.
-
C)
UOC di Neuroradiologia-Spedali Civili di Brescia, Italy, UOC di Neuroradiologia-Azienda Ospedaliera Padovana Padua, Italy, and UOC di Neurologia Pediatrica-IRCCS Ospedale Pediatrico Meyer Firenze, Italy.
For the cases from Brescia, Padua and Florence (the three centres which provided the minority of cases), the consent form was waived since this was a retrospective analysis of routinely collected anonymized data performed blindly.
-
D)
Academic unit of Radiology, University of Sheffield, UK.
Most of the women from this centre were recruited from ongoing research studies over the recruitment time period and provided informed written consent under the guidance and approval of the Institutional Research Ethics Committee. Those women were not paid for their involvement in the study but travel expenses were offered for themselves and a companion. Relevant review was sought, and approval obtained, from the Institutional Clinical Effectiveness Unit and Research Department in order to allow those cases performed for clinical purposes to be reported in this paper.
Study subjects or cohorts overlap
Statement concerning overlapping content
-
a)
Ten of the cases included in this paper were recruited as part of a study to the benefit of performing iuMR imaging in cases of isolated ventriculomegaly diagnosed by ultrasonography and have reported elsewhere (see AJNR Am J Neuroradiol 2010, 31: 106–111). There was no detailed information about the morphology of the cortical formation abnormalities and no attempt at classification. That paper showed images from one of the cases reported in the current paper (Figure 3 of the 2010 paper) but that case has not been used as an illustration in the present study.
-
b)
Fifty-eight of the cases included in this paper were recruited as part of the MERIDIAN study and reported (along with 512 other cases) as a cohort in The Lancet (see reference [1] in the present paper). That paper did not discuss cortical formation abnormalities as a separate entity and no images of the current cases were used in that publication. A sub-group analysis paper arising from the MERIDIAN study reported on cases of failed commissuration (see Ultrasound Obstet Gynecol 2017; 50: 753–76) and seven of the 58 cases from (reference [1] in the present paper) were presented in tabular form (Table 2 of Ultrasound Obstet Gynecol 2017, 50:753–76), although there was no detailed information about the morphology of the cortical formation abnormalities and no attempt at classification. That paper used images from one of the cases reported in the current paper (figure 2 of reference Ultrasound Obstet Gynecol 2017; 50: 753–76) but that case has not been used as an illustration in the present study.
-
c)
Thirty of the cases included in this paper were reported on American Journal of Roentgenology by Righini et al (see reference [12] in the present paper). It was basically a report on early stages of polymicrogyria development at iuMRI with some histology correlates, meanwhile we aimed to describe the features of the vast majority of CFA encountered in clinical imaging. Focal polymicrogyria cases were only a fraction of our global cohort, and we showed that they can be expected to be mostly unilateral anomalies (C5 much more numerous than A5/B5). The very few bilateral ‘saw-tooth’ appearance cases of that previous series fell into B2-symmetric excessive sulcation category of the present more general classification. Moreover, our cohort covered the entire range of gestational ages usually studied by iuMR. None of the cases reported in that previous paper appeared as exemplificative figure in the present article.
-
d)
A case included in the present paper was reported as Case report of early development of focal polymicrogyria (see reference [9] in the present paper). This was one of the very first examples in the literature aiming at just demonstrating that iuMRI has the potential of detecting focal cortical anomalies even before 25 weeks of gestation. None of the images of that case was among the exemplificative images used for the present publication.
-
e)
A case included in the present paper was reported as Case report of early abnormal sulcation in hypochondroplasia as detectable by iuMR (see Prenat. Diagnosis 2014, 34: 1015–1017). This was one of the very first examples in the literature aiming at just demonstrating that in hypochondroplasia brain cortical anomalies (B2-symmetric bilateral excessive sulcations of our categorisation) can be detected at early foetal stage. None of the images of that case was among the exemplificative images used for the present publication.
Methodology
• retrospective
• cross-sectional study
• multicentre study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 266 kb)
Rights and permissions
About this article
Cite this article
Righini, A., Genovese, M., Parazzini, C. et al. Cortical formation abnormalities on foetal MR imaging: a proposed classification system trialled on 356 cases from Italian and UK centres. Eur Radiol 30, 5250–5260 (2020). https://doi.org/10.1007/s00330-020-06899-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06899-2