Abstract
Objective
To investigate the value of histogram analysis of T1 mapping and diffusion-weighted imaging (DWI) in predicting the grade, subtype, and proliferative activity of meningioma.
Methods
This prospective study comprised 69 meningioma patients who underwent preoperative MRI including T1 mapping and DWI. The histogram metrics, including mean, median, maximum, minimum, 10th percentiles (C10), 90th percentiles (C90), kurtosis, skewness, and variance, of T1 and apparent diffusion coefficient (ADC) values were extracted from the whole tumour and peritumoural oedema using FeAture Explorer. The Mann-Whitney U test was used for comparison between low- and high-grade tumours. Receiver operating characteristic (ROC) curve and logistic regression analyses were performed to identify the differential diagnostic performance. The Kruskal-Wallis test was used to further classify meningioma subtypes. Spearman’s rank correlation coefficients were calculated to analyse the correlations between histogram parameters and Ki-67 expression.
Results
High-grade meningiomas showed significantly higher mean, maximum, C90, and variance of T1 (p = 0.001–0.009), lower minimum, and C10 of ADC (p = 0.013–0.028), compared to low-grade meningiomas. For all histogram parameters, the highest individual distinctive power was T1 C90 with an AUC of 0.805. The best diagnostic accuracy was obtained by combining the T1 C90 and ADC C10 with an AUC of 0.864. The histogram parameters differentiated 4/6 pairs of subtype pairs. Significant correlations were identified between Ki-67 and histogram parameters of T1 (C90, mean) and ADC (C10, kurtosis, variance).
Conclusion
T1 and ADC histogram parameters may represent an in vivo biomarker for predicting the grade, subtype, and proliferative activity of meningioma.
Key Points
• The histogram parameter based on T1 mapping and DWI is useful to preoperatively evaluate the grade, subtype, and proliferative activity of meningioma.
• The combination of T1 C90 and ADC C10 showed the best performance for differentiating low- and high-grade meningiomas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Meningiomas are considered one of the most frequent neoplasms of the central nervous system (CNS), representing 20–36% of all primary brain tumours with an increasing incidence [1]. The 2016 World Health Organization (WHO) classifies meningiomas into three grades pathologically: grade I are low-grade meningiomas (LGMs), while grades II and III are high-grade meningiomas (HGMs) [2]. LGMs and HGMs greatly differ with respect to biological behaviour. LGMs are benign slow-growing lesions and can achieve a good prognosis after surgical resection. In contrast, HGMs possess a higher rate of recurrence and shorter survival times [3–5]. Hence, patients with HGMs generally should receive adjuvant radiotherapy to reduce the possibility of tumour recurrence [6]. Additionally, the tumour subtype is another important factor in determining treatment outcome. Fibroblastic subtypes exhibit a hard consistency, making the resection of the tumour difficult, especially for those located at the skull base [7]. Moreover, proliferative activity is also an indicator of patient prognosis [8]. Antigen Ki-67 is a proliferation-related nuclear protein that indicates the risk of tumour progression and recurrence [9]. Therefore, predicting meningioma grade, subtype, and Ki-67 expression preoperatively would be helpful to direct treatment planning, follow-up, and progression.
Conventional MRI can be used to diagnose a meningioma but has a limited diagnostic value in determining meningioma grade, subtype, and predicting proliferative behaviour [10–12]. T1 mapping is a novel diagnostic technique, mainly used in cardiovascular imaging. T1 mapping could virtualise the original longitudinal magnetisation, which is determined by how rapidly the proton rebalances its spin after being excited by the radiofrequency pulse. Contrast-enhanced T1 mapping is mainly used to calculate the fraction of extracellular volume. Gadolinium agents are distributed in the whole extracellular space and shorten the T1 relaxation time, which is directly proportional to the local gadolinium concentration [13]. Native T1 mapping shows a similar performance to contrast-enhanced T1 mapping in the detection of the proportion of extracellular compartment and underlying pathophysiological processes in soft tissues [14–16]. Thus, T1 mapping measurements may serve as a quantitative approach to biological properties [17, 18]. Unlike T1 mapping, DWI reflects the restriction degree of the random diffusion of water molecules. ADC value obtained by DWI correlated with the tissue cellularity, which may be helpful in characterising the tumour microenvironment [19–21]. Previous studies have explored the role of ADC value in meningioma grading, but there are inconsistencies among studies [22–27]. One possible explanation for the inconsistencies is that they have used different measuring methods, in which ADC values were obtained by either ROI analysis or whole tumour evaluation. Hence, the inherent heterogeneity of tumours will lead to controversial results. Histogram analysis is an objective and reproducible method based on the tissue volume, providing more abundant information on tumour characteristics, especially when the intensity distribution is not normal [28–32]. The changes in histogram shape, asymmetry, and variation reflect the change in tumour structure, physiology, molecules, and metabolism [30]. This method has shown potential for predicting tumour grade and differential diagnosis [33–35].
As two quantitative imaging techniques, T1 mapping can reflect the extracellular structure of the tumour, while DWI can quantify tumour cellularity. Therefore, we hypothesised that a combination of T1 mapping and DWI could better evaluate the histopathological microstructures of tumours. We aimed to determine the utility of these two techniques in the grades and subtypes of meningiomas by histogram analysis. In addition, we analysed the association between histogram parameters with the Ki-67 labelling index (LI).
Materials and methods
Patients
The present study was approved by the local institutional review boards. We prospectively included 93 consecutive patients with suspected meningiomas in our hospital from August 2020 to August 2021, according to the following inclusion criteria: (I) performed preoperative brain MRI including T1 mapping and DWI; and (II) received surgical removal in our hospital; (III) surgical pathology confirmed meningioma based on the 2016 WHO classification [2]. Seventy-four patients were confirmed as meningiomas pathologically. The exclusion criteria were as follows: (I) patients had undergone radiotherapy, chemotherapy, or surgery; and (II) insufficient quality of images. Of these, 2 patients were excluded because of relevant treatment, and 3 patients were excluded because of severe motion artefacts. The final cohort consisted of 69 patients, with 57 LGMs and 12 HGMs included. Patient characteristics between them are summarised in Table 1. The patient flowchart is shown in Fig. S1.
Data acquisition
All patients underwent the same preoperative MRI using a 3.0-T GE MRI system (Discovery 750w, General Electric Healthcare) with a 24-channel head coil. MRI sequence included pre-contrast, spin-echo T1-weighted images, fluid-attenuated inversion recovery (FLAIR) T2-weighted images, axial gradient recalled echo (GRE), T2*-weighted images, axial diffusion-weighted imaging (DWI), T1 mapping, and post-contrast axial 3D T1-weighted fast-spoiled gradient recalled (FSPGR) images. The gadolinium-diethylenetriamine contrast agent (0.1 mmol/kg of body weight) was injected intravenously with a flow rate of 2 ml/s to perform post-contrast T1-weighted imaging, and imaging parameters were as follows: FOV = 240 mm × 240 mm; matrix = 256 × 256; slice thickness = 1 mm; number of slices = 150; TR/TE = 7.3/3.0 ms; acquisition time = 214 s.
DWI images were performed using a multi-slice, spin-echo planar imaging (EPI) pulse sequence. Scan parameters were as follows: TR/TE = 3000/70 ms; NEX = 4; FOV = 240 mm × 240 mm; matrix = 160 × 160; slice thickness = 5mm; intersection gap = 1.5 mm; acquisition time = 42 s. The b-values were 0 and 1000 s/mm2 with three orthogonal directions.
T1 mapping images were acquired using a multiple inversion recovery fast spin echo (IR-FSE) sequence. The detailed acquisition parameters were as follows: FOV = 240 mm × 240 mm; matrix = 240 × 192; slice thickness = 4 mm; intersection gap = 1 mm; number of slices = 30; TR/TE = 1600/27 ms; TI = 50/300/500/800/1300 ms acquisition time = 252 s.
MR image processing and histogram analysis
The final T1 maps were calculated using a custom MATLAB (MathWorks) code based on a reduced-dimension nonlinear least squares method proposed previously [36]. For each lesion, ROI was semiautomatically delineated using the ITK-SNAP program (version 4.6.1, University of Pennsylvania, www.itksnap.org), by two independent radiologists (with 15 and 10 years of experience in neuroradiology, respectively), who were blinded to the pathology. By referring to conventional MR images, the ROIs over the solid portion of the tumours were delineated on axial contrast-enhanced T1WI, excluding necrosis, cystic areas, calcification, and haemorrhage as much as possible, and ROIs for the peritumoural oedema were drawn on axial FLAIR T2WI. All imaging slices were obtained to compose the whole lesion, then automatically projected onto T1 mapping images using a coregistration tool in SPM8 (Wellcome Centre for Human Neuroimaging, http://www.fl.ion.ucl.ac.uk/spm/). Then the data were evaluated with an open-source software package named FeAture Explorer (FAE; https://github.com/salan668/FAE). The final 3D information from all voxels was calculated to generate the corresponding histogram. The following metrics were obtained from each histogram: (i) mean, median, maximum, minimum, percentiles (10th, 90th); (ii) kurtosis, skewness, and variance. In addition, ADC maps were generated from DW images obtained with 1000s/mm2 b values. The corresponding ADC histogram metrics were extracted using the same method as T1 values.
Pathological analysis
The pathological diagnosis of meningioma was made by a neuropathologist with 10 years of experience in neuropathology. Tumour grades and subtypes were determined according to the WHO Central Nervous System Tumour Classification 2016. Additionally, Ki-67 expression was estimated by immunohistochemical staining. The Ki-67 LI was determined by the percentage of positive tumour nuclei labelled with the Ki-67 antibody, the area with the highest density of immunoreactive cells was selected for scoring.
Statistical analysis
All data analysis was performed with IBM SPSS Statistics (Version 23.0, IBM Corp) and Medcalc (Version 11.1.1.0) for Windows. The inter-observer and intra-observer agreements of all histogram parameters were evaluated with the intraclass correlation coefficient (ICC): 0.00–0.20, poor correlation; 0.21–0.40, fair correlation; 0.41–0.60, moderate correlation; 0.61–0.80, good correlation; and 0.81–1.00, excellent correlation. A nonparametric Mann-Whitney U test was performed to test the differences in histogram parameters between LGMs and HGMs. For statistically significant parameters, binary logistic regression analysis was used to obtain a combined variable, and ROC curves were conducted to evaluate their diagnostic abilities in differentiating LGMs from HGMs. The corresponding area under the ROC curves (AUCs), with the 95% confidence interval (CI), was calculated to determine the optimal cut-off values for each histogram metric in the grading of meningioma. The diagnostic sensitivity and specificity of the value were also computed. The optimal threshold was selected by the maximum Youden index. The DeLong method was used to analyse the significance of the difference between the AUCs. In addition, the Kruskal-Wallis test was used to classify meningioma subtypes, and multiple comparisons were performed by the Bonferroni method. Spearman’s rank correlation was performed to evaluate the associations between histogram parameters and the Ki-67 index. P values less than 0.05 were considered statistically significant.
Results
Of 69 patients in this study, 57 were classified as LGMs (WHO grade I), including 29 with meningothelial meningiomas, 17 with transitional meningiomas, 11 with fibroblastic meningiomas; 12 were confirmed with HGMs (WHO grade II/III), including 11 with atypical meningiomas and 1 with anaplastic meningioma.
Comparisons of histogram parameters between low- and high-grade meningiomas
The histogram parameters of T1 and ADC maps for LGMs and HGMs are listed in Tables 2 and S1. According to ICC, moderate to excellent intra- and inter-reader agreement was obtained for all measurements of T1 (ICC, 0.785–0.988) and ADC (ICC, 0.760–0.996) maps. Table S2 showed intra- and inter-observer variability in all histogram parameters of meningiomas. The mean, maximum, variance, and C90 of T1 in tumours were significantly higher in HGMs than those in LGMs (p = 0.001–0.009), particularly C90. In addition, the minimum, maximum, variance, and C10 of ADC also showed a significant difference (p = 0.013–0.028). In peritumoural oedema, variance and C90 of T1, mean and C10 of ADC were significantly higher in HGMs than those in LGMs (p = 0.036–0.049). Representative cases with LGMs and HGMs for parametric maps are shown in Figs. 1 and 2.
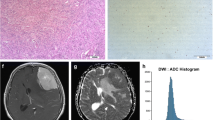
A 47-year-old woman with grade I fibroblastic meningioma (low-grade) in left occipital-temporal convexity. The mass demonstrated hyperintensity on the T1 map (a) and isointensity on the ADC map (b) (the corresponding histogram on the right). The lesion shows hyperintensity on the T2WI (c), homogeneous enhancement on the postcontrast T1WI (d), and slightly hyperintensity on the DWI (e). Pathological analysis (f) confirmed WHO grade I fibroblastic meningioma (hematoxylin and eosin, ×100). Ki-67 labelling index was 4% (g)
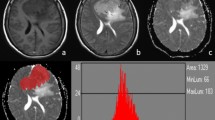
A 62-year-old woman with grade II atypical meningioma (high-grade) in left parafalx cerebri. The mass demonstrated hyperintensity on the T1 map (a) and hypointensity on the ADC map (b) (the corresponding histogram on the right). The lesion shows hyperintensity on the T2WI (c), homogeneous enhancement on the postcontrast T1WI (d), and hyperintensity on the DWI (e). Pathological analysis (f) confirmed WHO grade II atypical meningioma (hematoxylin and eosin, ×100). Ki-67 labelling index was 15% (g)
Evaluation of the diagnostic performance of histogram parameters for differentiating LGMs and HGMs
The results of ROC analyses of the significant histogram parameters are presented in Tables 3 and S3 and Figs. 3 and S2. The histogram parameters of T1 and ADC in tumours differentiated HGMs and LGMs with AUCs of 0.740–0.805 and 0.703–0.730, respectively. The histogram parameters of T1 and ADC in peritumoural oedema differentiated HGMs and LGMs with AUCs of 0.717–0.731 and 0.717–0.729, respectively. The DeLong test showed that there was no obvious statistical significance between the combination and other histogram parameters (p > 0.05).
T1 C90 in tumours has a higher diagnostic value among T1 individual indices, with the AUC of 0.805; the sensitivity, specificity, and accuracy were 0.667, 0.842, and 0.816, respectively. In addition, ADC parameters exhibited lower diagnostic value, compared to T1 parameters. Among all histogram parameters, the combination of T1 C90 and ADC C10 in tumours showed the highest areas under the ROC curves of 0.864, with a sensitivity of 0.750, specificity of 0.872, and accuracy of 0.855 in the diagnosis.
Correlation of histogram parameters with Ki-67 LI
The Ki-67 LI was available for 59 patients. It was significantly higher in HGMs (7, range 2–15) than that in LGMs (2, range 0.5–10, p < 0.001) as expected. Table 4 showed the Spearman correlation coefficients between all histogram parameters and the Ki-67 index. Significant correlations between the Ki-67 LI and the following histogram parameters were identified: C90, mean of T1; kurtosis, C10 and variance of ADC (all p < 0.05). The histogram parameters, particularly the T1 C90 (r = 0.304, p = 0.019) and the variance of ADC (r = 0.351, p = 0.006) showed significant positive correlations with Ki-67 expression, while the C10 of ADC was negatively correlated (r = −0.294, p = 0.024) with Ki-67 LI.
Histogram parameters for the identification of four common subtypes of meningioma
Due to the small sample size, one anaplastic meningioma was not included in the statistics. The histogram parameters of the four common meningioma subtypes are shown in Tables S4 and S5 and Figs. 4 and S3. Among 6 possible pairwise comparisons of meningioma subtypes, tumour histogram parameters differentiated 4/6 pairs and oedema histogram parameters differentiated 2/6 pairs. Histogram parameters of combined tumour and oedema were able to distinguish 4/6 pairs.
Discussion
Preoperative prediction of meningioma histological features and biological behaviour is clinically important. This study demonstrated that histogram parameters based on T1 mapping and DWI correlated significantly with the meningioma grade, subtype, and proliferative status.
T1 mapping is an advanced MRI modality and enables to quantify and virtualise T1 relaxation time, which is an intrinsic and fundamental property of natural matter [14–16]. This sequence has mainly been used in cardiovascular imaging at present. In recent research, T1 mapping has been extended to other body regions. Some scholars pointed out that T1 relaxation measurement might provide crucial information on gliomas, which was not visible in contrast-weighted imaging [37, 38]. Among other studies, quantitative T1 mapping has been demonstrated to monitor the response to treatment in brain tumour patients [39, 40], and postcontrast T1 mapping is optimal for differentiating radionecrosis from tumour recurrence in brain metastasis [41]. It has also been shown that the histogram analysis of T1 relaxation time can sensitively detect widespread abnormalities in the normal-appearing brain tissue in early multiple sclerosis (MS), due to the histogram peak position of MS was significantly higher than that of the control subjects [42–44]. Furthermore, T1 imaging has been reported as a potential imaging method for early identification of the pathophysiological state of brain tissue in acute stroke compared to DWI [45]. However, no studies have reported the histogram analysis of T1 mapping in meningioma grades and subtypes. In the present study, we analysed the value of T1 mapping techniques in the grading of meningiomas. Compared to LGMs, HGMs had a significantly different mean, maximum, variance, and C90 of T1 values. Histopathologically, malignant meningiomas are characterised by active mitosis, increased proliferation, and ratio of nucleus/cytoplasm, which are associated with aggressive behaviour [46–48]. The degradation of extracellular matrix (ECM) derived from T1 mapping is a critical step in tumour invasion [49, 50]. The abundance of ECM proteins such as collagen was shown to correlate well with native T1 values [51]. In this sense, the differences in specific T1 values between LGMs and HGMs are reasonable. Furthermore, T1 values can be considered complex variables, reflecting tissue properties besides the ECM [14, 52]. Therefore, quantitative T1 values are also influenced by nucleolar differences between low-grade and high-grade meningiomas. Likewise, meningioma subtypes have different T1 values due to their histopathology. The number of cells, the viscosity of water, and extracellular fibre composition could influence T1 value. The higher T1 values accompany higher water content and rarer fibres. Compared with meningothelial meningiomas, fibroblastic meningiomas are rich in collagen fibres and atypical meningioma cells are closely arranged. Therefore, T1 median could distinguish these three subtypes. However, more research on T1 mapping for brain tumours is needed in the future.
DWI could evaluate the restriction degree of diffusion of water molecules and has become a routine brain MR sequence [53]. ADC values are obtained from DWI and appear to correlate with tumour cellularity. However, the usefulness of DWI in evaluating the grade of meningiomas is controversial. Due to the spatial distribution and histopathological heterogeneity, ADC values obtained from local ROIs could not represent the overall characteristics of the whole tumour entity [54, 55]. Therefore, we evaluate the whole tumour based on histogram analysis, which can obtain more objective and valuable information. In our study, we found that the maximum and variance of ADC values of LGMs were significantly lower than those of HGMs, in line with the former study [27]. These results suggest that ADC maximum reflects uneven distribution of proliferation cells and micronecrosis in high-grade meningiomas [56, 57]. However, contrary to their study, our study showed no significant difference in ADC kurtosis between the two groups. Histopathologically, ADC kurtosis is related to tumour heterogeneity [30, 58]. Necrosis, cystic degeneration, haemorrhage, and fibrosis in tumours may affect ADC values and spatial distribution of ADC histograms. Differences in patient cohorts may lead to different results. Furthermore, the diagnostic performance of ADC kurtosis might be not high in meningioma grading, which caused controversy in different studies, some showing effectiveness, others the opposite [27, 59, 60]. Manisha et al [61] reported that the variance of ADC in HGMs was higher than those of LGMs, consistent with our results. Besides, the minimum and C10 of ADC values of solid regions in LGMs were significantly higher than those in HGMs. One possible reason was that increased mitosis, enlarged nuclei, and reduction of extracellular space led to water diffusion restriction of HGMs, resulting in a reduced ADC value [48, 62]. Wenle He et al [63] identified that ADC minimum and ADC C5 were significantly lower in LGMs compared to HGMs, which was similar to our research. Additionally, Surov et al [59] revealed that mean and 10th, 25th, and 75th percentiles of ADC were significantly lower in HGMs than in LGMs. In contrast, Lu et al [64] found that the mean ADC value could not differentiate grades of meningiomas. Consistent with the latter report, our study showed no significant difference in mean, but for C10. These inconsistent results may be due to different scanning parameters, sample sizes, and experimental methods in different research [63, 65]. For subtype analysis, we demonstrated that ADC variance could help distinguish meningothelial meningiomas from fibroblastic subtype. By contrast, previous studies suggested no significant difference in mean ADC values among low-grade subtypes [26, 66]. Notably, this positive finding may be attributed to the use of histogram analysis improves the diagnostic performance of ADC in identifying subtypes of meningioma.
Another valuable finding was that the diagnostic efficiency of the combination of T1 mapping and DWI was better than that of anyone alone, with the AUC and cut-off value being 0.864 and 0.24 mm2/s, indicating that combining index could be used as an effective imaging biomarker in predicting histological grades of meningiomas. Of note, the diagnostic efficiency of T1 performed better than that of ADC, which also proves the clinical value of T1 mapping technique in meningioma grading.
Skewness and kurtosis parameters, which are used to measure the peakedness and asymmetry of histogram distribution, are two important indexes for differentiating brain tumours, providing useful information for intratumoural spatial heterogeneity [21, 67, 68]. Kurtosis reflects the peak value of the distribution, while skewness represents the asymmetry of probability. They could be used to reflect differences in tumour microstructure and function [30]. In this study, both two indexes showed no significant difference between low-grade and high-grade meningiomas. This is mainly because both are solid tumours with hypervascular components [63]. In addition, the kurtosis and skewness are also influenced by the tail structure of the histogram [69]. The similarity of skewness and kurtosis between LGMs and HGMs may be due to the obvious overlap between intratumoural micronecrosis and cystic changes. However, it is still difficult to explain these characteristics from a biological point of view, and more research is needed in the future to prove the relationship between these characteristics and tumour biology.
In this study, we further investigated the association between T1 and ADC parameters with the Ki-67 proliferation marker. T1 (mean, C90) and ADC (variance) were positively correlated with a higher Ki-67 index, while C10 and kurtosis of ADC have a significantly negative correlation with the Ki-67 index, indicating that a more heterogeneous microenvironment could be evaluated by histogram parameters to predict the proliferation activity of meningiomas [70]. In addition, Shun Zhang et al [27] reported that the mean, C25, and C50 of ADC showed significant correlations with the proliferation index, which was inconsistent with our results. The different results may be attributed to differences in scanning parameters and histological subtypes included in different studies.
Our study had several limitations. First, this is a single-centre study, and selection bias was inevitable. In addition, as the procedure of DWI is not standardised in different institutions, different scanning parameters, and post-processing methods will affect ADC values [71]. A multicentre, multi-scanner study using the same protocol is needed to verify our results. Second, the sample size was relatively small, especially regarding high-grade meningiomas, leading to a larger margin of error. Finally, although larger areas of necrosis were manually excluded, a few forms of micronecrosis regions below MRI resolution may not be eliminated in the ROI and thus influence T1 measurements.
In conclusion, T1 and ADC histogram features may represent an in vivo imaging biomarker for differentiating meningiomas grades and subtypes. The combination of T1 C90 and ADC C10 showed the highest diagnostic performance for grading tumours. Besides, these features showed great potential in evaluating the proliferation activity of meningiomas. This multiparametric approach may provide a new approach for selecting therapeutic strategies for meningioma patients.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUCs:
-
Area under the receiver operating characteristic curves
- C10:
-
10th percentile
- C90:
-
90th percentile
- CI:
-
Confidence interval
- CNS:
-
Central nervous system
- DWI:
-
Diffusion-weighted imaging
- ECM:
-
Extracellular matrix
- FSPGR:
-
Fast-spoiled gradient recalled
- GRE:
-
Gradient recalled echo
- HGMs:
-
High-grade meningiomas
- ICC:
-
Intraclass correlation coefficient
- IR-FSE:
-
Inversion recovery fast spin echo
- LGMs:
-
Low-grade meningiomas
- LI:
-
Labelling index
- MS:
-
Multiple sclerosis
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- WHO:
-
World Health Organization
References
Shibuya M (2015) Pathology and molecular genetics of meningioma: recent advances. Neurol Med Chir (Tokyo) 55:14–27
Louis D, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820
Riemenschneider MJ, Perry A, Reifenberger G (2006) Histological classification and molecular genetics of meningiomas. Lancet Neurol 5:1045–1054
Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS (2020) Corrigendum to: CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol. https://doi.org/10.1093/neuonc/noaa269
Banan R, Abbetmeier-Basse M, Hong B et al (2021) The prognostic significance of clinicopathological features in meningiomas: microscopic brain invasion can predict patient outcome in otherwise benign meningiomas. Neuropathol Appl Neurobiol. https://doi.org/10.1111/nan.12700
Goldbrunner R, Stavrinou P, Jenkinson M et al (2021) EANO guideline on the diagnosis and management of meningiomas. Neuro Oncol 23:1821–1834
Kashimura H, Inoue T, Ogasawara K et al (2007) Prediction of meningioma consistency using fractional anisotropy value measured by magnetic resonance imaging. J Neurosurg 107:784–787
Swiderska-Chadaj Z, Markiewicz T, Grala B, Lorent M (2016) Content-based analysis of Ki-67 stained meningioma specimens for automatic hot-spot selection. Diagn Pathol 11:93
Marciscano A, Stemmer-Rachamimov A, Niemierko A et al (2016) Benign meningiomas (WHO Grade I) with atypical histological features: correlation of histopathological features with clinical outcomes. J Neurosurg 124:106–114
Lin L, Xue Y, Duan Q et al (2019) Grading meningiomas using mono-exponential, bi-exponential and stretched exponential model-based diffusion-weighted MR imaging. Clin Radiol 74:651.e615–651.e623
Lin L, Bhawana R, Xue Y et al (2018) Comparative analysis of diffusional kurtosis imaging, diffusion tensor imaging, and diffusion-weighted imaging in grading and assessing cellular proliferation of meningiomas. AJNR Am J Neuroradiol 39:1032–1038
Yu H, Wen X, Wu P et al (2019) Can amide proton transfer-weighted imaging differentiate tumor grade and predict Ki-67 proliferation status of meningioma? Eur Radiol 29:5298–5306
Parsai C, O'Hanlon R, Prasad S, Mohiaddin R (2012) Diagnostic and prognostic value of cardiovascular magnetic resonance in non-ischaemic cardiomyopathies. J Cardiovasc Magnet Resonance : Official J Soc Cardiovasc Magnet Resonance 14:54
Everett RJ, Stirrat CG, Semple SI, Newby DE, Dweck MR, Mirsadraee S (2016) Assessment of myocardial fibrosis with T1 mapping MRI. Clin Radiol 71:768–778
Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S (2016) Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson 18:89
Aherne E, Chow K, Carr J (2020) Cardiac T1 mapping: techniques and applications. J Magn Reson Imaging 51:1336–1356
Fernandes JL, Rochitte CE (2015) T1 mapping: technique and applications. Magn Reson Imaging Clin N Am 23:25–34
Child N, Suna G, Dabir D et al (2018) Comparison of MOLLI, shMOLLLI, and SASHA in discrimination between health and disease and relationship with histologically derived collagen volume fraction. Eur Heart J Cardiovasc Imaging 19:768–776
Ramsahye H, He H, Feng X, Li S, Xiong J (2013) Central neurocytoma: radiological and clinico-pathological findings in 18 patients and one additional MRS case. J Neuroradiol 40:101–111
Svolos P, Tsolaki E, Kapsalaki E et al (2013) Investigating brain tumor differentiation with diffusion and perfusion metrics at 3T MRI using pattern recognition techniques. Magn Reson Imaging 31:1567–1577
El-Ali A, Agarwal V, Thomas A, Hamilton R, Filippi C (2019) Clinical metric for differentiating intracranial hemangiopericytomas from meningiomas using diffusion weighted MRI. Clin Imaging 54:1–5
Atalay B, Ediz S, Ozbay N (2020) Apparent diffusion coefficient in predicting the preoperative grade of meningiomas. J Coll Physicians Surg Pak 30:1126–1132
Meyer H, Wienke A, Surov A (2020) ADC values of benign and high grade meningiomas and associations with tumor cellularity and proliferation - a systematic review and meta-analysis. J Neurol Sci 415:116975
Xiaoai K, Qing Z, Lei H, Junlin Z (2020) Differentiating microcystic meningioma from atypical meningioma using diffusion-weighted imaging. Neuroradiology 62:601–607
Bozdağ M, Er A, Ekmekçi S (2021) Association of apparent diffusion coefficient with Ki-67 proliferation index, progesterone-receptor status and various histopathological parameters, and its utility in predicting the high grade in meningiomas. Acta Radiol 62:401–413
Yiping L, Kawai S, Jianbo W, Li L, Daoying G, Bo Y (2017) Evaluation parameters between intra-voxel incoherent motion and diffusion-weighted imaging in grading and differentiating histological subtypes of meningioma: a prospective pilot study. J Neurol Sci 372:60–69
Zhang S, Chiang G, Knapp J et al (2020) Grading meningiomas utilizing multiparametric MRI with inclusion of susceptibility weighted imaging and quantitative susceptibility mapping. J Neuroradiol = J Neuroradiol 47:272–277
Ahn SJ, Choi SH, Kim YJ et al (2012) Histogram analysis of apparent diffusion coefficient map of standard and high B-value diffusion MR imaging in head and neck squamous cell carcinoma: a correlation study with histological grade. Acad Radiol 19:1233–1240
Wang S, Kim S, Zhang Y et al (2012) Determination of grade and subtype of meningiomas by using histogram analysis of diffusion-tensor imaging metrics. Radiology 262:584–592
Just N (2014) Improving tumour heterogeneity MRI assessment with histograms. Br J Cancer 111:2205–2213
Liu HS, Chiang SW, Chung HW et al (2018) Histogram analysis of T2*-based pharmacokinetic imaging in cerebral glioma grading. Comput Methods Prog Biomed 155:19–27
Murayama K, Nishiyama Y, Hirose Y et al (2018) Differentiating between central nervous system lymphoma and high-grade glioma using dynamic susceptibility contrast and dynamic contrast-enhanced MR imaging with histogram analysis. Magn Reson Med Sci 17:42–49
Liu P, Chen L, Wang QX et al (2020) Histogram analysis of T2 mapping for detecting early involvement of extraocular muscles in patients with thyroid-associated ophthalmopathy. Sci Rep 10:19445
Li D, Cui Y, Hou L et al (2021) Diffusion kurtosis imaging-derived histogram metrics for prediction of resistance to neoadjuvant chemoradiotherapy in rectal adenocarcinoma: preliminary findings. Eur J Radiol 144:109963
Xie T, Zhao Q, Fu C, Grimm R, Gu Y, Peng W (2021) Improved value of whole-lesion histogram analysis on DCE parametric maps for diagnosing small breast cancer (≤ 1 cm). Eur Radiol. https://doi.org/10.1007/s00330-021-08244-7
Barral J, Gudmundson E, Stikov N, Etezadi-Amoli M, Stoica P, Nishimura D (2010) A robust methodology for in vivo T1 mapping. Magn Reson Med 64:1057–1067
Just M, Thelen M (1988) Tissue characterization with T1, T2, and proton density values: results in 160 patients with brain tumors. Radiology 169:779–785
Komiyama M, Yagura H, Baba M et al (1987) MR imaging: possibility of tissue characterization of brain tumors using T1 and T2 values. AJNR Am J Neuroradiol 8:65–70
Larsson C, Kleppestø M, Grothe I, Vardal J, Bjørnerud A (2015) T1 in high-grade glioma and the influence of different measurement strategies on parameter estimations in DCE-MRI. J Magn Reson Imaging 42:97–104
Andersen C, Astrup J, Gyldensted C (1994) Quantitative MR analysis of glucocorticoid effects on peritumoural oedema associated with intracranial meningiomas and metastases. J Comput Assist Tomogr 18:509–518
Wang B, Zhang Y, Zhao B et al (2018) Postcontrast T1 mapping for differential diagnosis of recurrence and radionecrosis after gamma knife radiosurgery for brain metastasis. AJNR Am J Neuroradiol 39:1025–1031
Vrenken H, Geurts J, Knol D et al (2006) Whole-brain T1 mapping in multiple sclerosis: global changes of normal-appearing gray and white matter. Radiology 240:811–820
van Walderveen M, van Schijndel R, Pouwels P, Polman C, Barkhof F (2003) Multislice T1 relaxation time measurements in the brain using IR-EPI: reproducibility, normal values, and histogram analysis in patients with multiple sclerosis. J Magn Reson Imaging 18:656–664
Griffin C, Dehmeshki J, Chard D et al (2002) T1 histograms of normal-appearing brain tissue are abnormal in early relapsing-remitting multiple sclerosis. Mult Scler 8:211–216
Tan Y, Xu J, Chen R et al (2018) Use of T relaxation time in rotating frame (T ρ) and apparent diffusion coefficient to estimate cerebral stroke evolution. J Magnet Resonance Imaging : JMRI 48:1247–1254
Ayerbe J, Lobato RD, de la Cruz J et al (1999) Risk factors predicting recurrence in patients operated on for intracranialmeningioma. A multivariate analysis. Acta Neurochir (Wien) 141:921–932
Takahashi JA, Ueba T, Hashimoto N, Nakashima Y, Katsuki N (2004) The combination of mitotic and Ki-67 indices as a useful method for predicting short-term recurrence of meningiomas. Surg Neurol 61(149-155):discussion 155-146
Nagar VA, Ye JR, Ng WH et al (2008) Diffusion-weighted MR imaging: diagnosing atypical or malignant meningiomas and detecting tumor dedifferentiation. AJNR Am J Neuroradiol 29:1147–1152
Okuducu AF, Zils U, Michaelis SA, Michaelides S, von Deimling A (2006) Ets-1 is up-regulated together with its target gene products matrix metalloproteinase-2 and matrix metalloproteinase-9 in atypical and anaplastic meningiomas. Histopathology 48:836–845
Piechnik SK, Jerosch-Herold M (2018) Myocardial T1 mapping and extracellular volume quantification: an overview of technical and biological confounders. Int J Card Imaging 34:3–14
Adams LC, Ralla B, Jurmeister P et al (2019) Native T1 mapping as an in vivo biomarker for the identification of higher-grade renal cell carcinoma: correlation with histopathological findings. Invest Radiol 54:118–128
Ma R, Geng Y, Gan L et al (2021) Quantitative T1 mapping MRI for the assessment of extraocular muscle fibrosis in thyroid-associated ophthalmopathy. Endocrine. https://doi.org/10.1007/s12020-021-02873-0
Yamasaki F, Kurisu K, Satoh K et al (2005) Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology 235:985–991
Surov A, Gottschling S, Mawrin C et al (2015) Diffusion-weighted imaging in meningioma: prediction of tumor grade and association with histopathological parameters. Transl Oncol 8:517–523
Sanverdi SE, Ozgen B, Oguz KK et al (2012) Is diffusion-weighted imaging useful in grading and differentiating histopathological subtypes of meningiomas? Eur J Radiol 81:2389–2395
Park YW, Kim S, Ahn SS et al (2020) Magnetic resonance imaging-based 3-dimensional fractal dimension and lacunarity analyses may predict the meningioma grade. Eur Radiol 30:4615–4622
Hsu C, Pai C, Kao H, Hsueh C, Hsu W, Lo C (2010) Do aggressive imaging features correlate with advanced histopathological grade in meningiomas? J Clin Neurosci 17:584–587
Choi Y, Kim S, Youn I, Kang B, Park W, Lee A (2017) Rim sign and histogram analysis of apparent diffusion coefficient values on diffusion-weighted MRI in triple-negative breast cancer: comparison with ER-positive subtype. PLoS One 12:e0177903
Surov A, Ginat D, Lim T et al (2018) Histogram analysis parameters apparent diffusion coefficient for distinguishing high and low-grade meningiomas: a multicenter study. Transl Oncol 11:1074–1079
Gihr G, Horvath-Rizea D, Garnov N et al (2018) Diffusion profiling via a histogram approach distinguishes low-grade from high-grade meningiomas, can reflect the respective proliferative potential and progesterone receptor status. Mol Imaging Biol 20:632–640
Bohara M, Nakajo M, Kamimura K et al (2020) Histological grade of meningioma: prediction by intravoxel incoherent motion histogram parameters. Acad Radiol 27:342–353
Lu S, Kim S, Kim N, Kim H, Choi C, Lim Y (2015) Histogram analysis of apparent diffusion coefficient maps for differentiating primary CNS lymphomas from tumefactive demyelinating lesions. AJR Am J Roentgenol 204:827–834
He W, Xiao X, Li X et al (2019) Whole-tumor histogram analysis of apparent diffusion coefficient in differentiating intracranial solitary fibrous tumor/hemangiopericytoma from angiomatous meningioma. Eur J Radiol 112:186–191
Lu Y, Liu L, Luan S, Xiong J, Geng D, Yin B (2019) The diagnostic value of texture analysis in predicting WHO grades of meningiomas based on ADC maps: an attempt using decision tree and decision forest. Eur Radiol 29:1318–1328
Li X, Miao Y, Han L et al (2019) Meningioma grading using conventional MRI histogram analysis based on 3D tumor measurement. Eur J Radiol 110:45–53
Pond J, Morgan T, Hatanpaa K, Yetkin Z, Mickey B, Mendelsohn D (2015) Chordoid meningioma: differentiating a rare World Health Organization grade II tumor from other meningioma histologic subtypes using MRI. AJNR Am J Neuroradiol 36:1253–1258
King AD, Chow KK, Yu KH et al (2013) Head and neck squamous cell carcinoma: diagnostic performance of diffusion-weighted MR imaging for the prediction of treatment response. Radiology 266:531–538
Cho GY, Moy L, Kim SG et al (2016) Evaluation of breast cancer using intravoxel incoherent motion (IVIM) histogram analysis: comparison with malignant status, histological subtype, and molecular prognostic factors. Eur Radiol 26:2547–2558
Westfall PH (2014) Kurtosis as Peakedness, 1905 - 2014. R.I.P. Am Stat 68:191-195
Del Gobbo A, Pellegrinelli A, Gaudioso G et al (2016) Analysis of NSCLC tumour heterogeneity, proliferative and 18F-FDG PET indices reveals Ki67 prognostic role in adenocarcinomas. Histopathology 68:746–751
Becker A, Wagner M, Wurnig M, Boss A (2017) Diffusion-weighted imaging of the abdomen: impact of b-values on texture analysis features. NMR Biomed https://doi.org/10.1002/nbm.3669
Acknowledgements
We acknowledge PuYeh Wu from GE Healthcare for the technical support.
Funding
This study has received funding from the Joint Funds for the Innovation of Science and Technology, Fujian province (Grant number: 2018Y9044) and Fujian Provincial Health Technology Project (grant number: 2020GGA039).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Lin Lin, MD, PhD, Fujian Medical University Union Hospital.
Conflict of interest
One of the authors of this manuscript (PuYeh Wu) is an employee of GE Healthcare. The remaining authors declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 314 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, T., Jiang, R., Zheng, L. et al. T1 and ADC histogram parameters may be an in vivo biomarker for predicting the grade, subtype, and proliferative activity of meningioma. Eur Radiol 33, 258–269 (2023). https://doi.org/10.1007/s00330-022-09026-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09026-5