Abstract
Purpose
Presurgical grading, estimation of growth kinetics, and other prognostic factors are becoming increasingly important for selecting the best therapeutic approach for meningioma patients. Diffusion-weighted imaging (DWI) provides microstructural information and reflects tumor biology. A novel DWI approach, histogram profiling of apparent diffusion coefficient (ADC) volumes, provides more distinct information than conventional DWI. Therefore, our study investigated whether ADC histogram profiling distinguishes low-grade from high-grade lesions and reflects Ki-67 expression and progesterone receptor status.
Procedures
Pretreatment ADC volumes of 37 meningioma patients (28 low-grade, 9 high-grade) were used for histogram profiling. WHO grade, Ki-67 expression, and progesterone receptor status were evaluated. Comparative and correlative statistics investigating the association between histogram profiling and neuropathology were performed.
Results
The entire ADC profile (p10, p25, p75, p90, mean, median) was significantly lower in high-grade versus low-grade meningiomas. The lower percentiles, mean, and modus showed significant correlations with Ki-67 expression. Skewness and entropy of the ADC volumes were significantly associated with progesterone receptor status and Ki-67 expression. ROC analysis revealed entropy to be the most accurate parameter distinguishing low-grade from high-grade meningiomas.
Conclusions
ADC histogram profiling provides a distinct set of parameters, which help differentiate low-grade versus high-grade meningiomas. Also, histogram metrics correlate significantly with histological surrogates of the respective proliferative potential. More specifically, entropy revealed to be the most promising imaging biomarker for presurgical grading. Both, entropy and skewness were significantly associated with progesterone receptor status and Ki-67 expression and therefore should be investigated further as predictors for prognostically relevant tumor biological features. Since absolute ADC values vary between MRI scanners of different vendors and field strengths, their use is more limited in the presurgical setting.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Meningiomas are the most frequently diagnosed primary brain tumors in adults. According to the last report from the Central Brain Tumor Registry of the United States (CBTRUS), they comprise about 36.6 % of all intracranial masses [1]. The WHO classification system of CNS tumors, which is mainly based on histopathological features and molecular pathways, divides meningiomas into three groups—benign (grade I), atypical (grade II), and anaplastic (grade III) [2]. Earlier studies reported that approximately 80–90 % of all cases are slowly growing, low-grade meningiomas (WHO I), associated with lower recurrence rates after surgery [3] and better overall prognosis compared to high-grade meningiomas (WHO grade II and III) [4]. More recent studies reported higher proportions of 10–15 % for atypical and 1–3 % for anaplastic meningiomas [5, 6]. Other authors suggested even higher rates of WHO grade II/III meningiomas [7, 8]. However, besides the comparatively invasive group of grade II and III meningiomas, also benign meningiomas exhibit unfavorable long-term outcomes in middle-aged and elderly patients [9].
The only curative approach for meningiomas is complete resection inclusive of the infiltrated pachymeninx, which is associated with substantial surgical morbidity and mortality [10, 11]. Considering the facts that (i) a significant proportion of all meningiomas remains asymptomatic and is discovered incidentally on neuroimaging studies or at autopsy [12] and (ii) especially elderly patients, who may not even live long enough to experience tumor-related impairment, are more prone to surgical complications [13]; a conservative approach may represent the best strategy in some cases [14]. As a consequence, preoperative imaging of meningiomas, especially focusing on the prediction of tumor grade, growth kinetics, and further prognostically relevant biomarkers are becoming increasingly important to distinguish patients who benefit from extensive surgery from those better treated conservatively.
So far, evaluation of macroscopic tumor characteristics by magnetic resonance imaging (MRI) still remains the limited standard approach in the clinical presurgical setting [14]. Regarding the respective anatomical MRI sequences (T1-weighted, T2-weighted, and gadolinium-enhanced post contrast imaging), few studies postulated the potential diagnostic value of simple morphological features like signal heterogeneity, tumor shape, and tumor-brain interface or more complex texture and shape analyses to differentiate between low-grade and high-grade meningiomas [15,16,17]. Contrarily, other investigators did not find significant differences between low-grade and high-grade meningiomas when comparing conventional MRI signal intensities [18].
A complementary technique in MRI—better reflecting tissue architecture on a microscopical scale than the conventional sequences—is diffusion-weighted imaging (DWI) [19]. DWI uses opposed gradients to measure the random “Gaussian” translocation of hydrogen nuclei within biological tissues, where mostly cell membranes act as natural boundaries and cause a tissue-specific diffusion pattern [20]. This pattern is visualized on the respective apparent diffusion coefficient (ADC) maps. ADC values, obtained via manually drawn, solitary regions of interest (ROI), have been shown to differ significantly between low-grade and high-grade meningiomas [21, 22]. Furthermore, an inverse correlation between ADC values and Ki-67 expression, representing the proliferative potential of the tumor, was reported previously [22]. However, other studies in the field do not corroborate these findings [23, 24].
So far, studies investigating the complexity of the whole tumor diffusion pattern of meningiomas in correlation to histopathological properties are lacking. Therefore, the aim of this study was to evaluate whether whole tumor histogram profiling in preoperative ADC maps hast the potential to (i) sufficiently reflect the prognostically relevant Ki-67 expression, representing the growth kinetics of the lesions in question, (ii) to differentiate progesterone receptor-negative and -positive tumors, and (iii) to distinguish between low-grade and high-grade meningiomas.
Methods
Ethics Approval
The study was approved by the ethics committee of the medical council of Baden-Württemberg (Ethik-Kommission Landesärztekammer Baden-Württemberg, F-2017-047).
Patients Collective
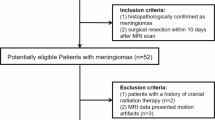
The institutional radiological information system (RIS) was searched for patients with the diagnosis meningioma. Fifty-six patients were identified between 01/2012 and 08/2017; all of whom had surgery in our hospital with at least partial removal of the tumor and subsequent neuropathological workup. Only patients who received pretreatment MRI scans with sufficient DWI were included. MRI examinations of patients indicating hemorrhage or significant calcifications were excluded, since these conditions severely influence quantification, and hence, produce incorrect ADC values. Therefore, only 37 patients (30 females, 7 males; ranging from 40 to 87 years with a mean age of 61.4 years) were included in our retrospective analysis. Twenty-eight cases were low-grade meningiomas and nine cases were high-grade meningiomas.
MRI Specifics
For all patients, MRI of the brain was performed using a 1.5-T device (MAGNETOM Aera, Tx/Rx CP head coil, Siemens, Erlangen, Germany). The imaging protocol included the following sequences: (1) axial T2-weighted (T2w) turbo spin echo (TSE) sequence (TR/TE: 4000/69, flip angle: 150°, slice thickness: 4 mm, acquisition matrix: 200 × 222, field of view: 100 mm); (2) axial T1-weighted (T1w) turbo spin echo (TSE) sequences (TR/TE 765/9.5, flip angle 150°, slice thickness 5 mm, acquisition matrix 200 × 222, field of view 100 mm) prior and post intravenous application of contrast medium (Gadobutrol, Gadovist®, Bayer Schering Pharma, Leverkusen, Germany); and (3) axial DWI (EPI sequence; TR/TE 5400/69, flip angle 180°, slice thickness 4 mm, acquisition matrix 200 × 222, field of view 100 mm) with b values of 0 and 1000 s/mm2. ADC maps were generated automatically by the implemented software package.
All images were available in digital form and analyzed by two experienced radiologists (DHR, SS) without knowledge of the histopathological diagnosis on a PACS workstation (Impax EE R20 XII).
Histogram Profiling of ADC Maps
ADC maps and T1-weighted post contrast images were exported from our institutional archive in DICOM format via the aforementioned AGFA PACS. Using a custom-made DICOM image analysis tool (programmed by N.G. using Matlab, The Mathworks, Natick, MA), whole lesion histogram profiling was performed as follows: T1-weighted post contrast images were loaded into a graphical user interface (GUI) to tag the contrast-enhancing tumor of each patient in all respective MRI sections. All regions of interest were then automatically co-registered with the corresponding ADC maps, and the whole lesion histogram profile was consecutively calculated, providing the following set of parameters: ADCmean, ADCmin, ADCmax, ADCp10, ADCp25, ADCp75, ADCp90, ADCmodus, ADCmedian, skewness, kurtosis, and entropy.
Neuropathology
All tumor specimens were used for neuro-histological confirmation of the diagnosis. A 5-μm section of each tumor was stained by H&E and two further sections were employed for Ki-67—and progesterone receptor immunohistochemistry to determine the proliferation rate [25] and the progesterone receptor status of each tumor, as previously reported [26]. The histopathological images were digitalized with a Jenalumar microscope, carrying a 4.2 digital camera (Zeiss, Jena, Germany). Thereupon, Ki-67 index was quantified using the ImageJ particles tool as described previously [25].
Statistical Analysis
Statistical analysis including graphics creation was performed using SPSS 23.0 (SPSS Inc., Chicago, IL, USA). In a first step, DWI data and histopathological information were investigated using descriptive statistics. In a second step, data was tested for Gaussian distribution using the Kolmogorov-Smirnov test. A t test was performed to compare evaluated, normally distributed parameters of DWI histogram profiling between low-grade and high-grade meningiomas. Also, normally distributed DWI histogram profiling parameters between progesterone receptor-positive and negative meningiomas were compared using unpaired t test. Mann-Whitney U test was performed to compare parameters exhibiting a non-Gaussian distribution between low-grade and high-grade meningiomas as well as between progesterone receptor-positive and -negative meningiomas. Finally, correlation analysis for normally distributed parameters was performed using Pearson correlation coefficient. In case of non-Gaussian distribution, the Spearman-rho rank-order correlation was calculated. p values < 0.05 were taken to indicate statistical significance in all instances. Finally, to assess the accuracy of ADC volume histogram profiling, receiver operating characteristic (ROC) curve analysis was performed and the respective area under the curve (AUC) was calculated. For optimal discrimination between low-grade and high-grade meningiomas, only the parameter exhibiting AUC values with excellent accuracy (AUC 90–1) was used for calculation of Youden’s Index.
Results
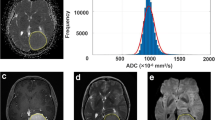
Examples of cranial MRI from patients with low-grade (upper case) and high-grade meningiomas (lower case) are demonstrated in (Fig. 1). Corresponding whole tumor ADC histograms are also given in the respective line of images.
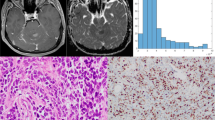
a Representative T1 weighted post contrast image. b The co-registered ADC map. c The corresponding whole tumor ADC histogram. d H&E staining and e Ki-67 immunohistochemistry from a low-grade and (f–j) high-grade meningioma. f Representative T1 weighted post contrast image. g The co-registered ADC map. h The corresponding whole tumor ADC histogram. i H&E staining. j Ki-67 immunohistochemistry from a high-grade meningioma. For the first case (low-grade meningioma), a proliferation index of 5 % was calculated. In the second case (high-grade meningioma), proliferation index was 10 %. The scale bars represent 1 mm in each image.
The results of the descriptive analysis on DWI data and histopathological information are summarized in Table 1. The Kolmogorov-Smirnov test revealed Gaussian distribution for ADCmean, ADCp10, ADCp25, ADCp75, ADCp90, ADCmodus, ADCmedian, entropy, and Ki-67 (p all < 0.05). Non-Gaussian distribution was determined for ADCmin, ADCmax, kurtosis, and skewness.
As a consequence, (unpaired) t test was used to compare Ki-67, ADCmean, ADCp10, ADCp25, ADCp75, ADCp90, ADCmodus, ADCmedian, and entropy between low-grade and high-grade as well as between progesterone receptor-positive and progesterone receptor-negative meningiomas. Mann-Whitney U test was used to compare ADCmin, ADCmax, skewness, and kurtosis between low-grade and high-grade as well as between progesterone receptor-negative and progesterone receptor-positive meningiomas. In brief, significant differences between low-grade and high-grade meningiomas were identified for the following set of DWI histogram profile parameters: ADCmean, ADCp10, ADCp25, ADCp75, ADCp90, ADCmedian, and entropy (all p < 0.05). Mean values of all ADC fractions (e.g., percentiles, mean, median, modus) were significantly lower in the high-grade meningioma group, whereas entropy of the DWI histogram profile was significantly greater in high-grade compared to low-grade meningiomas. Differences in Ki-67 expression, representing the actively proliferating tumor fraction, also achieved statistical significance, being increased in the high-grade group. Furthermore, significant differences between progesterone receptor-negative and progesterone receptor-positive meningiomas were identified for skewness and Ki-67 expression. Skewness and Ki-67 expression were both significantly greater in the progesterone receptor-negative group compared to the progesterone receptor-positive group. For reasons of comprehensibility and clarity, results of the comparative statistical analysis are summarized in Tables 2 and 3. Figure 2a–d shows differences (low-grade versus high-grade) in ADCpercentiles, Fig. 2e, f shows differences (low-grade versus high-grade) in ADCmean and ADCmedian values, Fig. 2g compares entropy values of low-grade versus high-grade meningiomas, and Fig. 2h compares skewness of progesterone receptor-negative and progesterone receptor-positive meningiomas.
Pearson’s correlation coefficient was used to investigate the association between ADCmean, ADCp10, ADCp25, ADCp75, ADCp90, ADCmodus, ADCmedian, entropy, and Ki-67. The Spearman-rho rank-order correlation was calculated to investigate the association between ADCmin, ADCmax, skewness, kurtosis, and Ki-67. Significant correlations (p < 0.05) between Ki-67 and the following DWI histogram profile parameters were identified: ADCp10, ADCp25, ADCmedian, ADCmodus, and entropy. The complete results of the correlative analysis are summarized in (Table 4). Figure 2i shows a scatter plot graphically demonstrating the association of entropy and Ki-67, the set of parameters with the strongest correlation (r = 0.518, p = 0.001).
Finally, AUC values were calculated for each of the evaluated parameters exhibiting statistically significant differences between low-grade and high-grade meningiomas. The following values were obtained (CI confidence interval): ADCmean (AUC = 0.067, [CI 0.000–0.150], p < 0.001), ADCp10 (AUC = 0.067, [CI 0.000–0.170], p < 0.001), ADCp25 (AUC = 0.062, [CI 0.000–0.159], p < 0.001), ADCp75 (AUC = 0.087, [CI 0.000–0.180], p < 0.001), ADCp90 (AUC = 0.139, [CI 0.015–0.263], p = 0.001), ADCmedian (AUC = 0.075, [CI 0.000–0.164], p < 0.001), and entropy (AUC = 0.919, [CI 0.804–1.000], p < 0.001). Hence, the only parameter showing an excellent level of accuracy was entropy, the corresponding ROC curve is displayed in Fig. 3. For cutpoint evaluation, Youden’s Index was calculated and gave the following result: entropy values of 3.805 and greater strongly indicate high-grade meningioma (sensitivity 0.889, specificity 0.821).
Discussion
To the best of our knowledge, this is the first study using a whole tumor ADC histogram approach that shows statistically significant differences between low-grade and high-grade meningiomas. In line with the findings of Nagar et al. and Surov et al. [21, 22], significantly reduced water diffusibility in the extracellular compartment, predominantly as a consequence of more densely packed tumor cells [27] and increased intra-tumoral fibrosis or widespread collagen formation [28], was demonstrated in grade II/III meningiomas compared to grade I meningiomas. In detail, whole tumor ADCp10, ADCp25, ADCp75, ADCp90, ADCmean, and ADCmedian values were harmonically decreased in high-grade versus low-grade meningiomas. The fact that significant, unidirectional differences were identified for all ADC percentiles, ADCmean, and ADCmedian values, representing the entire continuum of the diffusion profile, indicates that variations in the tumor microstructure are rather homogeneously distributed among the respective WHO grades. This finding matches the well-known phenomenon of a more heterogeneous contrast enhancement in high-grade meningiomas versus the rather homogeneous enhancement pattern in low-grade meningiomas [15, 16]. In conclusion, tumor micro-architecture appears to be comparatively uniform within the respective WHO grades and is reflected by corresponding characteristic ADC profiles.
Furthermore, entropy of the entire ADC tumor volume was the most accurate parameter distinguishing low-grade from high-grade meningiomas. The entropy of an ADC volume is a quantity describing the degree of randomness of ADC values within the investigated volume of interest and has been used as a measure of tumor heterogeneity in previous works [29]. In our collective, high entropy values, using a cutpoint value of 3.805, were strongly predictive for high-grade meningiomas with excellent accuracy according to the ROC analysis. This finding exemplarily corresponds to the results of Rosenkrantz et al. and Suo et al., who demonstrated significantly higher ADC entropy values in malignant lesions of the prostate and breast compared to benign lesions [30, 31]. Our results further accord to the results of Foroutan and coworkers, who were able to demonstrate significant changes in ADC entropy values of cancer cells treated with cyto-reductive agents [32]. Therefore, in addition to the homogeneous decrease of the whole ADC spectrum, increased values of ADC entropy in meningiomas strongly indicate a more dynamic and invasive tumor process and should be considered a prognostically relevant imaging biomarker.
Additionally, significant correlations between Ki-67 expression, the lower ADC percentiles (p10 and p25), ADCmodus, and ADCmedian were demonstrated. These findings are in line with several previously published reports, exemplarily a study on primary CNS lymphomas, which was able to show that different ADC fractions are inversely associated with Ki-67 expression, representing the actively proliferating portion of the neoplasm [25]. Also, in the context of the above-discussed aspects, decreased water diffusibility in the extracellular compartment secondary to increased tumor cellularity is comprehensibly correlated with increased cell proliferation.
Further on, second order characteristics of the ADC profile—kurtosis, skewness, and entropy—were correlated with Ki-67 expression. Interestingly, the strongest correlation between all ADC profile parameters and the histopathological equivalent for growth kinetic in our study was identified for entropy of the ADC volume, indicating that increased ADC entropy, and hence, greater tumor heterogeneity, is directly associated with an enhanced growth potential of the respective meningioma. This finding is corroborated by the work of Ryu et al., who reported analogous findings in low-grade and high-grade gliomas [33].
As reported previously, a positive progesterone receptor status in meningiomas is associated with better outcomes [34]. Therefore, we investigated whether parameters of ADC profiling are associated with the progesterone receptor status. Interestingly, skewness of the ADC volume histogram (and Ki-67 expression) was significantly increased in the progesterone receptor-negative group compared to the progesterone receptor-positive group. This concurs with the findings of Hsu et al. and Pravdenkova et al. [34] and indicates that skewness of ADC volumes in meningiomas may reflect a distinct aspect of the specific tumor biology, which is not accounted for by the remaining parameters of diffusion profiling.
Our study suffers from some limitations. Firstly, it is only of retrospective nature and based on a small patients’ collective. Secondly, only data from a singular 1.5-T MRI system was available. Further investigations at higher field strength are necessary to validate the findings of our study and to compare results between different scanners. Since ADC values vary between MRI scanners of different vendors [25, 35], special focus should then be placed on the histogram parameters’ skewness, kurtosis, and entropy, which do not depend on field strength and sequence specifics. Thirdly, only two b values (0 and 1000 s/mm2) were available for ADC calculation; therefore, small vessel perfusion may have an impact on ADC values in our cohort.
Conclusions
ADC histogram profiling provides a distinct set of parameters, which help differentiate low-grade versus high-grade meningiomas. Also, histogram metrics correlate significantly with histological surrogates of the respective proliferative potential. More specifically, entropy revealed to be the most promising imaging biomarker for presurgical grading. Both, entropy and skewness were significantly associated with progesterone receptor status and Ki-67 expression and therefore should be investigated further as predictors for prognostically relevant tumor biological features. Since absolute ADC values vary between MRI scanners of different vendors and field strengths, their use is more limited in the presurgical setting.
References
Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2016) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro-Oncology 18(suppl_5):v1–v75. https://doi.org/10.1093/neuonc/now207
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Böker DK, Meurer H, Gullotta F (1985) Recurring intracranial meningiomas. Evaluation of some factors predisposing for tumor recurrence. J Neurosurg Sci 29:11–17
Marosi C, Hassler M, Roessler K et al (2008) Meningioma. Crit Rev Oncol Hematol 67:153–171
Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM (2005) Epidemiology of intracranial meningioma. Neurosurgery 57(6):1088–1095. https://doi.org/10.1227/01.NEU.0000188281.91351.B9
Kshettry VR, Ostrom QT, Kruchko C, al –Mefty O, Barnett GH, Barnholtz–Sloan JS (2015) Descriptive epidemiology of World Health Organization grades II and III intracranial meningiomas in the United States. Neuro-Oncology 17(8):1166–1173. https://doi.org/10.1093/neuonc/nov069
Willis J, Smith C, Ironside JW et al (2005) The accuracy of meningioma grading: a 10-year retrospective audit. Neuropathol Appl Neurobiol 31:141–149
Pearson BE, Markert JM, Fisher WS, Guthrie BL, Fiveash JB, Palmer CA, Riley K (2008) Hitting a moving target: evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus 24(5):E3. https://doi.org/10.3171/FOC/2008/24/5/E3
van Alkemade H, de Leau M, Dieleman EMT et al (2012) Impaired survival and long-term neurological problems in benign meningioma. Neuro-Oncology 14:658–666
Meixensberger J, Meister T, Janka M et al (1996) Factors influencing morbidity and mortality after cranial meningioma surgery—a multivariate analysis. In: Modern Neurosurgery of Meningiomas and Pituitary Adenomas. Springer Vienna, Vienna, pp 99–101. https://doi.org/10.1007/978-3-7091-9450-8_27
Black P, Kathiresan S, Chung W (1998) Meningioma surgery in the elderly: a case-control study assessing morbidity and mortality. Acta Neurochir 140:1013–1017
Vernooij MW, Ikram MA, Tanghe HL, Vincent AJPE, Hofman A, Krestin GP, Niessen WJ, Breteler MMB, van der Lugt A (2007) Incidental findings on brain MRI in the general population. N Engl J Med 357(18):1821–1828. https://doi.org/10.1056/NEJMoa070972
Steinberger J, Bronheim RS, Vempati P, Oermann EK, Ladner TR, Lee NJ, Kothari P, Caridi JM, Shrivastava RK (2017) Morbidity and mortality of meningioma resection increases in octogenarians. World Neurosurg 109:e16–e23. https://doi.org/10.1016/j.wneu.2017.09.021
Goldbrunner R, Minniti G, Preusser M et al (2016) Review EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol 17:e383–e391
Kawahara Y, Nakada M, Hayashi Y et al (2012) Prediction of high-grade meningioma by preoperative MRI assessment. J Neuro-Oncol 108:147–152
Lin B-J, Chou K-N, Kao H-W, Lin C, Tsai WC, Feng SW, Lee MS, Hueng DY (2014) Correlation between magnetic resonance imaging grading and pathological grading in meningioma. J Neurosurg 121(5):1201–1208. https://doi.org/10.3171/2014.7.JNS132359
Yan P-F, Yan L, Hu T-T et al (2017) The potential value of preoperative MRI texture and shape analysis in grading meningiomas: a preliminary investigation. TRANON 10:570–577
Schob S, Frydrychowicz C, Gawlitza M et al (2016) Signal intensities in preoperative MRI do not reflect proliferative activity in meningioma. Transl Oncol 9:274–279
Le Bihan D (2013) Apparent diffusion coefficient and beyond: what diffusion MR imaging can tell us about tissue structure. Radiology 268(2):318–322. https://doi.org/10.1148/radiol.13130420
Schob S, Surov A, Wienke A et al (2016) Correlation between aquaporin 4 expression and different DWI parameters in grade I meningioma. Mol Imaging Biol 19(1):138–142
Nagar VA, Ye JR, Ng WH, Chan YH, Hui F, Lee CK, Lim CCT (2008) Diffusion-weighted MR imaging: diagnosing atypical or malignant meningiomas and detecting tumor dedifferentiation. AJNR Am J Neuroradiol 29(6):1147–1152. https://doi.org/10.3174/ajnr.A0996
Surov A, Gottschling S, Mawrin C et al (2015) Diffusion-weighted imaging in meningioma: prediction of tumor grade and association with histopathological parameters. TRANON 8:517–523
Ginat DT, Mangla R, Yeaney G, Wang HZ (2010) Correlation of diffusion and perfusion MRI with Ki-67 in high-grade meningiomas. Am J Roentgenol 195(6):1391–1395. https://doi.org/10.2214/AJR.10.4531
Sanverdi SE, Ozgen B, Oguz KK et al (2012) Is diffusion-weighted imaging useful in grading and differentiating histopathological subtypes of meningiomas? Eur J Radiol 81:2389–2395
Schob S, Meyer J, Gawlitza M et al (2016) Diffusion-weighted MRI reflects proliferative activity in primary CNS lymphoma. PLoS One 11(8):e0161386. https://doi.org/10.1371/journal.pone.0161386
Surov A, Caysa H, Wienke A et al (2015) Correlation between different ADC fractions, cell count, Ki-67, total nucleic areas and average nucleic areas in meningothelial meningiomas. Anticancer Res 35:6841–6846
Chen L, Liu M, Bao J et al (2013) The correlation between apparent diffusion coefficient and tumor cellularity in patients: a meta-analysis. PLoS One 8:e79008. https://doi.org/10.1371/journal.pone.0079008.s001
Backer-Grøndahl T, Moen BH, Torp SH (2012) The histopathological spectrum of human meningiomas. Int J Clin Exp Pathol 5(3):231–242
Schob S, Meyer HJ, Pazaitis N, Schramm D, Bremicker K, Exner M, Höhn AK, Garnov N, Surov A (2017) ADC histogram analysis of cervical cancer aids detecting lymphatic metastases—a preliminary study. Mol Imaging Biol 19(6):953–962. https://doi.org/10.1007/s11307-017-1073-y
Rosenkrantz AB (2013) Histogram-based apparent diffusion coefficient analysis: an emerging tool for cervical cancer characterization? Am J Roentgenol 200(2):311–313. https://doi.org/10.2214/AJR.12.9926
Suo S, Zhang K, Cao M et al (2016) Characterization of breast masses as benign or malignant at 3.0T MRI with whole-lesion histogram analysis of the apparent diffusion coefficient. J Magn Reson Imaging 43:894–902
Foroutan P, Kreahling JM, Morse DL, Grove O, Lloyd MC, Reed D, Raghavan M, Altiok S, Martinez GV, Gillies RJ (2013) Diffusion MRI and novel texture analysis in osteosarcoma xenotransplants predicts response to anti-checkpoint therapy. PLoS One 8(12):e82875. https://doi.org/10.1371/journal.pone.0082875
Ryu YJ, Choi SH, Park SJ, Yun TJ, Kim JH, Sohn CH (2014) Glioma: application of whole-tumor texture analysis of diffusion-weighted imaging for the evaluation of tumor heterogeneity. PLoS One 9(9):e108335. https://doi.org/10.1371/journal.pone.0108335
Hsu DW, Efird JT, Hedley-Whyte ET (1997) Progesterone and estrogen receptors in meningiomas: prognostic considerations. J Neurosurg 86(1):113–120. https://doi.org/10.3171/jns.1997.86.1.0113
Sasaki M, Yamada K, Watanabe Y et al (2008) Variability in absolute apparent diffusion coefficient values across different platforms may be substantial: a multivendor, multi-institutional comparison study. Radiology 249:624–630
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the ethics committee of the medical council of Baden-Württemberg (Ethik-Kommission Landesärztekammer Baden-Württemberg, F-2017-047).
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding Information
This study acknowledges funding via the Clinician-Scientist-Program of the medical faculty of the University Hospital Leipzig.
Rights and permissions
About this article
Cite this article
Gihr, G.A., Horvath-Rizea, D., Garnov, N. et al. Diffusion Profiling via a Histogram Approach Distinguishes Low-grade from High-grade Meningiomas, Can Reflect the Respective Proliferative Potential and Progesterone Receptor Status. Mol Imaging Biol 20, 632–640 (2018). https://doi.org/10.1007/s11307-018-1166-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-018-1166-2