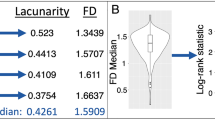
Abstract
Objective
To assess whether 3-dimensional (3D) fractal dimension (FD) and lacunarity features from MRI can predict the meningioma grade.
Methods
This retrospective study included 131 patients with meningiomas (98 low-grade, 33 high-grade) who underwent preoperative MRI with post-contrast T1-weighted imaging. The 3D FD and lacunarity parameters from the enhancing portion of the tumor were extracted by box-counting algorithms. Inter-rater reliability was assessed with the intraclass correlation coefficient (ICC). Additionally, conventional imaging features such as location, heterogeneous enhancement, capsular enhancement, and necrosis were assessed. Independent clinical and imaging risk factors for meningioma grade were investigated using multivariable logistic regression. The discriminative value of the prediction model with and without fractal features was evaluated. The relationship of fractal parameters with the mitosis count and Ki-67 labeling index was also assessed.
Results
The inter-reader reliability was excellent, with ICCs of 0.99 for FD and 0.97 for lacunarity. High-grade meningiomas had higher FD (p < 0.001) and higher lacunarity (p = 0.007) than low-grade meningiomas. In the multivariable logistic regression, the diagnostic performance of the model with clinical and conventional imaging features increased with 3D fractal features for predicting the meningioma grade, with AUCs of 0.78 and 0.84, respectively. The 3D FD showed significant correlations with both mitosis count and Ki-67 labeling index, and lacunarity showed a significant correlation with the Ki-67 labeling index (all p values < 0.05).
Conclusion
The 3D FD and lacunarity are higher in high-grade meningiomas and fractal analysis may be a useful imaging biomarker for predicting the meningioma grade.
Key Points
• Fractal dimension (FD) and lacunarity are the two parameters used in fractal analysis to describe the complexity of a subject and may aid in predicting meningioma grade.
• High-grade meningiomas had a higher fractal dimension and higher lacunarity than low-grade meningiomas, suggesting higher complexity and higher rotational variance.
• The discriminative value of the predictive model using clinical and conventional imaging features improved when combined with 3D fractal features for predicting the meningioma grade.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Meningiomas are the most common primary intracranial neoplasms in adults, comprising 36.7% of all intracranial tumors [1], and approximately 22.0–35.5% are World Health Organization (WHO) grade II or III high-grade meningiomas [2]. Compared with low-grade (benign [WHO grade I]) meningiomas, high-grade (atypical [WHO grade II] or anaplastic [WHO grade III]) tumors have an aggressive biological behavior and an increased risk of recurrence, as well as an increased risk of mortality [3]. The standard management typically involves surgical resection and often adjuvant radiation therapy for high-grade (WHO grades II–III) meningiomas.
Preoperative prediction of the meningioma grade is important because it influences treatment planning, including the surgical resection strategy. Further, according to the recent guideline published by the European Association of Neuro-Oncology [4], incidentally discovered and radiologically presumed meningiomas may be managed solely by observation; thus, the histological grade may not be confirmed in some cases. Clinical information such as age and sex have shown a weak association with the grade [3], and currently, there are no reliable parameters that can predict the tumor grade and associated clinical course [5]. Thus, noninvasive prediction of the meningioma grade may enhance clinical decision-making by providing information on whether observation should be performed. Previous studies have focused on conventional imaging [6, 7], diffusion and perfusion imaging [8,9,10,11], amide proton imaging, and PET to predict the meningioma grade; however, up to now, the value of grading meningioma on imaging has been low [5].
Fractal features are model-based features that characterize the shape complexity of an object over a range of scales using mathematical approaches [12, 13]. They enable quantification of natural objects with high structural complexities that are poorly represented by the conventional Euclidean geometry. Fractal dimension (FD) and lacunarity are the two parameters used in fractal analysis to describe the complexity and distribution of a shape or subject. FD is a non-integer value that describes the intrinsic shape of an object; as the FD increases, the complexity increases [13]. Lacunarity is a geometric measure that represents the degree of gappiness and rotational (or translational) invariance [13]. Several studies have performed fractal analysis on differentiating glioma grade, differentiating glioblastoma from CNS lymphoma, and predicting survival in glioblastoma [14,15,16]. However, to the best of our knowledge, no study has applied fractal analysis for meningioma. Therefore, we hypothesized that 3-dimensional (3D) fractal features could quantify the complexity of meningiomas and subsequently aid in predicting the meningioma grade noninvasively.
Materials and methods
Patient population
Our institutional review board waived the requirement to obtain informed consent from patients for this retrospective study. We retrospectively reviewed meningioma cases in which pathological confirmation and preoperative conventional MRI were performed between June 2009 and March 2018. Exclusion criteria were as follows: (1) patients with a previous history of operation (n = 7), (2) patients with a history of tumor embolization or gamma knife surgery before MRI examination (n = 2), and (3) patients with absence of post-contrast T1-weighted (T1C) images or suboptimal image quality (n = 1). Overall, 131 patients were included in this study (105 women and 26 men; mean age, 57.8 ± 13.0 years).
MRI protocol
Preoperative MRI was performed using a 3.0-T MRI scanner (Achieva, Philips Medical Systems) with an eight-channel sensitivity-encoding head coil. The preoperative MRI protocol included T1-weighted (repetition time [TR]/echo time [TE], 2000/10 ms; field of view, 230 mm; section thickness, 5 mm; matrix, 320 × 198) and T1C images (TR/TE, 1800–2000/10 ms; field of view, 240–250 mm; section thickness, 2 mm; and matrix, 256–312 × 256–312). T1C images were acquired after the administration of 0.1 mL/kg of gadolinium-based contrast material (Gadovist; Bayer).
Pathologic diagnosis
Pathological diagnosis was performed by a neuropathologist, according to the 2016 WHO criteria [12]. Criteria for an atypical meningioma (WHO grade II) comprised 4–19 mitoses per 10 high-power fields, the presence of brain invasion, or the presence of at least three of these features: “sheet-like” growth, hypercellularity, spontaneous necrosis, large and prominent nucleoli, and small cells; criteria for an anaplastic meningioma (WHO grade III) comprised frank anaplasia (histology resembling carcinoma, sarcoma, or melanoma) or elevated mitosis count (> 20 mitoses per 10 high-power fields) [12]. The mitosis count was evaluated using the mitotic marker phosphohistone-H3. The mitosis index was determined by counting the number of unequivocal mitotic figures in 10 consecutive high-power fields (× 400) containing the highest number of mitoses. The Ki-67 labeling index was estimated.
Tumor segmentation
Segmentation was performed by a neuroradiologist (Y.W.P. with 7 years of experience) who was blinded to the clinical information and histopathologic results. ROIs were drawn on each tumor section on T1C images, using a semiautomatic method with an interactive level-set volume of interest and intensity-based algorithms. Gross cystic, hemorrhagic, or necrotic areas were avoided by using conventional T1 and T1C images. To test inter-reader reliability, images from 30 patients were randomly selected and independently segmented by another neuroradiologist (S.S.A. with 14 years of experience). The regions of interests were segmented using a multi-platform, free, open-source software package for visualization and medical image computing (3D slicer, version 4.6.2-1; available at: http://slicer.org).
3D fractal analysis
The 3D FD and lacunarity values were computed from the segmentation annotated by neuroradiologists using the box-counting algorithms [17]. The 3D FD was calculated by the change of the number of boxes that included a part of the 3D binary mask with respect to different box sizes. The 3D lacunarity was calculated by the average of the square of coefficient of variation values of multiple boxes that included a part of the 3D binary mask [18]. Since the optimal box size was unknown, both FD and lacunarity values were computed with different 3D box sizes, ranging from 21 to 27 isotropic voxels. The mean FD and lacunarity values were calculated in each patient. Figure 1 shows the pipeline for fractal analyses. Details of FD and lacunarity calculations are available in the Supplementary Material.
Qualitative evaluation of conventional imaging features
Previously known conventional imaging features for predicting the meningioma grade [5, 7, 19] including the location (skull base vs. non-skull base), capsular enhancement, heterogeneous enhancement, and presence of necrosis were evaluated independently by two neuroradiologists (Y.W.P. and S.S.A.), who were blinded to the clinical information and histopathologic results. Discrepancies were settled through a consensus discussion. A detailed explanation of the definition of imaging findings is available in the Supplementary Material.
Statistical analysis
Baseline characteristics were compared between patients with low-grade and high-grade meningiomas using the chi-square or Fisher exact test for categorical variables, and independent t test or Mann-Whitney U test for continuous variables according to normality. Inter-reader reliability of fractal parameters was assessed by two-way interclass correlation. Interobserver agreement in the imaging evaluation was calculated by using the Cohen kappa index [20]. Univariable and multivariable logistic regression analyses were performed to find the significant clinical and imaging features associated with high-grade meningiomas. Variables of interest in the univariable analysis (p < 0.05) were included in the multivariable models by using the enter method. To determine whether inclusion of 3D fractal features with the predictive model improved the discriminative value, two different models with and without 3D fractal features were assessed based on the receiver operating characteristic curve using c-statistics (the Harrell concordance index). The net reclassification index (NRI) and integrated discrimination improvement (IDI) values of the two models were also calculated to evaluate the increase in the discriminative value [21]. The precision-recall curves were also calculated.
In addition, the correlation between fractal parameters with the mitosis count and the Ki-67 labeling index was evaluated by the Pearson correlation coefficient analysis.
All statistical analyses were performed using the statistical software R (version 3.5.1; R Foundation for Statistical Computing). A p value < 0.05 was considered statistically significant.
Results
The clinical, histopathological, and imaging characteristics of the 131 patients are summarized in Table 1. Ninety-eight patients were pathologically diagnosed with low-grade meningiomas, including 48 (36.6%) transitional, 29 (22.1%) meningothelial, 11 (8.4%) fibroblastic, 4 (3.1%) psammomatous, 3 (2.3%) angiomatous, 2 (1.5%) microcystic, and 1 (0.8%) secretory. Thirty-three patients were pathologically diagnosed as having high-grade meningiomas, with 29 (22.1%) atypical and 4 (3.1%) anaplastic meningiomas. Patients with high-grade meningiomas were significantly older than those with low-grade meningiomas (61.7 vs. 56.5 years, p = 0.047).
Inter-reader reliability of 3D fractal features
The FD showed 1.55 ± 0.81 and 1.56 ± 0.80, and lacunarity showed 4.99 ± 3.0 and 5.1 ± 3.2 in the 30 patients from each reader, respectively. The inter-reader reliability was excellent, with intraclass correlation coefficients of 0.99 (95% confidence interval [CI] 0.98–0.99) for FD and 0.97 (95% CI 0.96–0.98) for lacunarity.
Interobserver agreement of conventional imaging features
The interobserver agreement between the two neuroradiologists was nearly perfect (κ = 0.823) for location, substantial (κ = 0.739) for heterogeneous enhancement, moderate (κ = 0.545) for capsular enhancement, and substantial (κ = 0.695) for necrosis.
Comparison of prediction models for meningioma grades
In the entire group, results of the univariable analysis showed that older age (odds ratio [OR] = 1.034 [95% CI 1.001–1.067], p = 0.049), negative capsular enhancement (OR = 6.6 [95% CI 2.6–16.7], p < 0.001), heterogeneous enhancement (OR = 5.3 [95% CI 2.0–14.0], p = 0.001), necrosis (OR = 3.7 [95% CI 1.6–8.7], p = 0.002), higher FD (OR = 93.6 [95% CI 8.0–1088.5], p < 0.001), and higher lacunarity (OR = 1.9 [95% CI 1.2–3.2], p = 0.008) were associated with high-grade meningiomas. (Boxplots of representation of the fractal dimension and lacunarity according to different meningioma grades are shown in Fig. 2).
In multivariable analysis of the six variables, FD (OR = 184.8 [95% CI 7.5–4565.6], p = 0.001) and lacunarity (OR 2.7 [95% CI 1.4–5.2], p = 0.003) were independent variables for predicting the meningioma grade. In the comparison of the predictive power for high-grade meningiomas in the two multivariable models (model 1 with covariates such as age, negative capsular enhancement, heterogeneous enhancement, and necrosis; model 2 with model 1 with covariates such as age, negative capsular enhancement, heterogeneous enhancement, necrosis, FD, and lacunarity) using c-statistics analysis, model 2 exhibited a better diagnostic performance than model 1 (AUC 0.84 [95% CI 0.75–0.92] vs. 0.78 [95% CI 0.69–0.87]; p = 0.148). Model 2 also exhibited better performance with an NRI of 0.77 (95% CI 0.41–1.13) and IDI of 0.13 (95% CI 0.07–0.20) (Table 2). Figure 3a and b show the ROC curve and precision-recall curve.
Relationship of fractal parameters with the mitosis count and Ki-67 labeling index
FD showed a significant correlation with both the mitosis count (r = 0.192, p = 0.045) and Ki-67 labeling index (r = 0.228, p = 0.010). Lacunarity showed a significant correlation with the Ki-67 labeling index (r = 0.230, p = 0.009), but not with the mitosis count (r = 0.109, p = 0.256). Scatter plots are shown in Supplementary Fig. 1.
Discussion
The present study emphasizes the potential applications of 3D fractal analysis for the differentiation and characterization of different grades of meningiomas. The results of multivariable regression analysis with clinical and conventional imaging features and 3D fractal features as covariates revealed only 3D fractal features as being independently associated with high-grade meningiomas. The diagnostic improvement of the predictive model upon inclusion of 3D fractal features was also confirmed by the NRI and IDI, which exhibited values greater than zero. The ability to differentiate between low-grade and high-grade meningiomas before treatment has potentially profound clinical utility. In routine clinical settings, surgery is usually recommended for patients with neurologic symptoms, large tumors, and associated cerebral edema [4]. However, patients with high-grade meningiomas could benefit from early resection even in the absence of these clinical and radiological findings.
The FD was significantly higher for high-grade meningiomas than for low-grade meningiomas, suggesting that high-grade meningiomas exhibit a more complicated texture pattern on MRI than low-grade meningiomas. Supporting our results, a previous study has also stated that irregular tumor border was correlated with high-grade meningioma [7]. Previous studies have shown that meningioma with a high proliferative potential may exhibit highly heterogeneous distributions of proliferating cells in the tumor, and this heterogeneity may produce irregular shapes [6, 22]. Our study shows that quantitative assessment of the complexity is useful in grading meningioma. Additionally, the lacunarity of high-grade meningiomas was significantly higher than that of low-grade meningiomas, suggesting that the necrosis or cystic change visualized as gaps in the tumor lesion may increase its rotational variance. In other words, we indirectly quantified the amount of necrosis or cystic change via lacunarity. Fractal analysis is a measure of the complexity of structures and complex geometric patterns. Fractal features have the potential to become a powerful and useful tool; they are relatively stable and less susceptible to imaging noise than other texture features [23], and could be used for longitudinal assessment in a single patient [24]. For non-Euclidean objects, FD usually exceeds topological dimension and is a non-integer, whereas for ordinary shapes, it is equal to the traditional Euclidean dimension. Therefore, FD as an index of statistical complexity has been applied in several fields of tumor study [14, 15]. Previous studies on brain tumors have mostly focused on 2D fractal analyses using imaging software with an averaging of parameter values on several contiguous axial images [14,15,16]; however, 2D analyses have limited value because a 2D image cannot exhibit true fractal behavior of a 3D object [25]. The 2D analysis may not be fully representative of the tumor as a whole and supports the case for investigating 3D analysis techniques. The 3D fractal analyses are known to provide values with larger magnitude, which reflects the volume assessed.
The 3D fractal features were also correlated with the mitosis count and Ki-67 labeling index. The mitosis count and Ki-67 labeling index are important tools in addition to routine histological evaluation; these proliferation indices were correlated with an increased risk of recurrence and are known to be important prognostic factors in cases of meningiomas [26, 27]. Higher FD was correlated with a higher mitosis count and Ki-67 index, and higher lacunarity was correlated with a higher Ki-67 index, suggesting that a more complicated pattern and increased rotational variance is correlated with proliferation activity.
Previous studies have reported that conventional imaging findings such as non-skull base location, unclear tumor-brain interface, heterogeneous enhancement, or presence of necrosis/hemorrhage were associated with the probability of a high-grade meningioma [7, 28,29,30]. Our study showed similar results; however, the inter-rater agreement ranged from a kappa value from moderate to nearly perfect, suggesting the subjectivity of visual assessment, whereas the fractal analysis of meningiomas showed excellent inter-rater reliability. Moreover, the power of many of these studies has been limited by both a small sample size and the significant overlapping in imaging features of low-grade and high-grade meningiomas. On the other hand, recent studies showed the potential of radiomics features not only on glioma grading or molecular differentiation [31, 32] but also for differentiating the meningioma grade [30, 33], but fractal features have not been routinely implemented in the previous radiomics studies on meningiomas. Moreover, radiomics studies are prone to overfitting due to high dimensionality, and the large number of features requires intensive computational resources [34]. The multiplicity of data can result in high probability of a false-positive rate [34]. In our study, fractal parameters showed high inter-rater reliability, and we used a conventional T1C sequence rather than advanced imaging techniques, suggesting a more feasible methodology. Advanced sequences such as DWI and perfusion studies have shown contradictory results in grading meningioma [8,9,10,11, 35], and the practical usage of amide proton imaging or PET is limited in clinical setting although promising results have been shown [36, 37].
Our study has several limitations. First, our study was based on a single institution and retrospectively collected dataset, with a limited sample size. Further study with a larger number of patients is needed to validate our results. Second, there is currently a lack of standardization in the fractal analysis algorithms. However, we used mean values from the box-counting method because it is the most commonly used algorithm to assess fractal parameters of natural objects in biomedical research.
In conclusion, 3D FD and lacunarity are higher in high-grade meningiomas and fractal analysis may be a useful imaging biomarker for predicting the meningioma grade.
Abbreviations
- 2D:
-
2-Dimensional
- 3D:
-
3-Dimensional
- FD:
-
Fractal dimension
- ICC:
-
Intraclass correlation coefficient
- IDI:
-
Integrated discrimination improvement
- NRI:
-
Net reclassification index
- OR:
-
Odds ratio
- TE:
-
Echo time
- TIC:
-
T1-weighted
- TR:
-
repetition time
- WHO:
-
World Health Organization
References
Ostrom QT, Gittleman H, Xu J et al (2016) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol 18:v1–v75
Willis J, Smith C, Ironside J, Erridge S, Whittle I, Everington D (2005) The accuracy of meningioma grading: a 10-year retrospective audit. Neuropathol Appl Neurobiol 31:141–149
Kshettry VR, Ostrom QT, Kruchko C, Al-Mefty O, Barnett GH, Barnholtz-Sloan JS (2015) Descriptive epidemiology of World Health Organization grades II and III intracranial meningiomas in the United States. Neuro Oncol 17:1166–1173
Goldbrunner R, Minniti G, Preusser M et al (2016) EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol 17:e383–e391
Nowosielski M, Galldiks N, Iglseder S et al (2017) Diagnostic challenges in meningioma. Neuro Oncol 19:1588–1598
Nakasu S, Nakasu Y, Nakajima M, Matsuda M, Handa J (1999) Preoperative identification of meningiomas that are highly likely to recur. J Neurosurg 90:455–462
Kawahara Y, Nakada M, Hayashi Y et al (2012) Prediction of high-grade meningioma by preoperative MRI assessment. J Neurooncol 108:147–152
Santelli L, Ramondo G, Della Puppa A et al (2010) Diffusion-weighted imaging does not predict histological grading in meningiomas. Acta Neurochir (Wien) 152:1315–1319
Nagar V, Ye J, Ng W et al (2008) Diffusion-weighted MR imaging: diagnosing atypical or malignant meningiomas and detecting tumor dedifferentiation. AJNR Am J Neuroradiol 29:1147–1152
Zhang H, Rödiger LA, Shen T, Miao J, Oudkerk M (2008) Preoperative subtyping of meningiomas by perfusion MR imaging. Neuroradiology 50:835
Azizyan A, Eboli P, Drazin D, Mirocha J, Maya MM, Bannykh S (2014, 2014) Differentiation of benign angiomatous and microcystic meningiomas with extensive peritumoral edema from high grade meningiomas with aid of diffusion weighted MRI. Biomed Res Int
Lee G, Lee HY, Park H et al (2017) Radiomics and its emerging role in lung cancer research, imaging biomarkers and clinical management: state of the art. Eur J Radiol 86:297–307
Lennon FE, Cianci GC, Cipriani NA et al (2015) Lung cancer—a fractal viewpoint. Nat Rev Clin Oncol 12:664
Liu S, Wang Y, Xu K et al (2017) Relationship between necrotic patterns in glioblastoma and patient survival: fractal dimension and lacunarity analyses using magnetic resonance imaging. Sci Rep 7:8302
Liu S, Fan X, Zhang C et al (2019) MR imaging based fractal analysis for differentiating primary CNS lymphoma and glioblastoma. Eur Radiol 29:1348–1354
Smitha K, Gupta A, Jayasree R (2015) Fractal analysis: fractal dimension and lacunarity from MR images for differentiating the grades of glioma. Phys Med Biol 60:6937
Falconer K (2004) Fractal geometry: mathematical foundations and applications. Wiley, New York
Plotnick RE, Gardner RH, O’Neill RV (1993) Lacunarity indices as measures of landscape texture. Landsc Ecol 8:201–211
Kane AJ, Sughrue ME, Rutkowski MJ et al (2011) Anatomic location is a risk factor for atypical and malignant meningiomas. Cancer 117:1272–1278
Park JE, Han K, Sung YS et al (2017) Selection and reporting of statistical methods to assess reliability of a diagnostic test: conformity to recommended methods in a peer-reviewed journal. Korean J Radiol 18:888–897
Pencina MJ, D’Agostino RB Sr, Demler OV (2012) Novel metrics for evaluating improvement in discrimination: net reclassification and integrated discrimination improvement for normal variables and nested models. Stat Med 31:101–113
Siegers H, Zuber P, Hamou M, Van Melle G, Tribolet ND (1989) The implications of the heterogeneous distribution of Ki-67 labelled cells in meningiomas. Br J Neurosurg 3:101–107
Al-Kadi OS (2010) Assessment of texture measures susceptibility to noise in conventional and contrast enhanced computed tomography lung tumour images. Comput Med Imaging Graph 34:494–503
Karemore G, Nielsen M (2009) Fractal dimension and lacunarity analysis of mammographic patterns in assessing breast cancer risk related to hrt treated population: a longitudinal and cross-sectional study. Proc. SPIE 7260, Medical Imaging 2009: Computer-Aided Diagnosis. https://doi.org/10.1117/12.813699
Sanghera B, Banerjee D, Khan A et al (2012) Reproducibility of 2D and 3D fractal analysis techniques for the assessment of spatial heterogeneity of regional blood flow in rectal cancer. Radiology 263:865–873
Kim Y-J, Ketter R, Steudel W-I, Feiden W (2007) Prognostic significance of the mitotic index using the mitosis marker anti–phosphohistone H3 in meningiomas. Am J Clin Pathol 128:118–125
Takahashi JA, Ueba T, Hashimoto N, Nakashima Y, Katsuki N (2004) The combination of mitotic and Ki-67 indices as a useful method for predicting short-term recurrence of meningiomas. Surg Neurol 61:149–155
Hale AT, Wang L, Strother MK, Chambless LB (2018) Differentiating meningioma grade by imaging features on magnetic resonance imaging. J Clin Neurosci 48:71–75
Lin B-J, Chou K-N, Kao H-W et al (2014) Correlation between magnetic resonance imaging grading and pathological grading in meningioma. J Neurosurg 121:1201–1208
Coroller TP, Bi WL, Huynh E et al (2017) Radiographic prediction of meningioma grade by semantic and radiomic features. PLoS One 12:e0187908
Park YW, Choi YS, Ahn SS, Chang JH, Kim SH, Lee S-K (2019) Radiomics MRI phenotyping with machine learning to predict the grade of lower-grade gliomas: a study focused on nonenhancing tumors. Korean J Radiol 20:1381–1389
Park YW, Han K, Ahn SS et al (2018) Prediction of IDH1-mutation and 1p/19q-codeletion status using preoperative MR imaging phenotypes in lower grade gliomas. AJNR Am J Neuroradiol 39:37–42
Park YW, Oh J, You SC et al (2019) Radiomics and machine learning may accurately predict the grade and histological subtype in meningiomas using conventional and diffusion tensor imaging. Eur Radiol 29:4068–4076
Park JE, Park SY, Kim HJ, Kim HS (2019) Reproducibility and generalizability in radiomics modeling: possible strategies in radiologic and statistical perspectives. Korean J Radiol 20:1124–1137
Toh C-H, Castillo M, Wong A-C et al (2008) Differentiation between classic and atypical meningiomas with use of diffusion tensor imaging. AJNR Am J Neuroradiol 29:1630–1635
Han K, Choi YS, Lee S-K et al (2018) Amide proton transfer imaging for differentiation of benign and atypical meningiomas. Eur Radiol 28:331–339
Cornelius JF, Stoffels G, Filß C et al (2015) Uptake and tracer kinetics of O-(2-18 F-fluoroethyl)-L-tyrosine in meningiomas: preliminary results. Eur J Nucl Med Mol Imaging 42:459–467
Funding
This research received funding from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, Information and Communication Technologies & Future Planning (2017R1D1A1B03030440). This work was supported under the framework of international cooperation program managed by National Research Foundation of Korea (NRF-2018K2A9A2A06020642).
This research received funding from the Korean Society for Neuro-Oncology.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Professor Seung-Koo Lee, MD, PhD, from Yonsei University College of Medicine (slee@yuhs.ac).
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise (K.H, a biostatistician with 10 years of experience in biostatistics).
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Diagnostic or prognostic study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 103 kb)
Rights and permissions
About this article
Cite this article
Park, Y.W., Kim, S., Ahn, S.S. et al. Magnetic resonance imaging–based 3-dimensional fractal dimension and lacunarity analyses may predict the meningioma grade. Eur Radiol 30, 4615–4622 (2020). https://doi.org/10.1007/s00330-020-06788-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06788-8