Abstract
Objectives
Percutaneous radiofrequency ablation (RFA) is one of the curative treatments for hepatocellular carcinoma (HCC), but local tumor progression (LTP) has been a main limitation of RFA. This study aims to evaluate the LTP of percutaneous no-touch RFA (NtRFA) for HCC ≤ 5 cm and compare with conventional RFA (intratumoral puncture) through a systematic review and meta-analysis.
Methods
MEDLINE, EMBASE, and Cochrane Library were searched for studies on percutaneous NtRFA for HCC ≤ 5 cm. The pooled proportions of the overall and cumulative incidence rates at 1, 2, and 3 years for LTP after NtRFA were assessed using a random-effects model. For studies comparing NtRFA with conventional RFA, relative risks (RR) and hazard ratios (HR) were meta-analytically pooled with LTP as the outcome.
Results
Twelve studies with 900 patients were included. The pooled overall rate of LTP after NtRFA was 6% (95% CI, 4–8%). The pooled 1-, 2-, and 3-year cumulative incidence rates of LTP were 3% (95% CI, 2–5%), 5% (95% CI, 3– 9%), and 8% (95% CI, 6–11%), respectively. Compared to conventional RFA, the pooled RR and HR of LTP were 0.26 (95% CI, 0.16–0.41) and 0.28 (95% CI, 0.11–0.70), respectively (both p < 0.01). Subgroup analysis including only randomized controlled studies also showed better local tumor control of NtRFA with HR of 0.13 (95% CI, 0.14–0.42).
Conclusions
Percutaneous NtRFA is an effective treatment for HCC ≤ 5 cm with an overall LTP rate of 6% and provides lower LTP compared with conventional RFA.
Key Points
• The pooled 1-, 2-, and 3-year cumulative incidence rates of local tumor progression after no-touch radiofrequency ablation for HCC ≤ 5 cm were 3% (95% CI, 2–5%), 5% (95% CI, 3–9%), and 8% (95% CI, 6–11%).
• No-touch radiofrequency ablation had significantly lower rates of local tumor progression compared to conventional radiofrequency ablation (hazard ratio, 0.28; 95% CI, 0.11–0.70; relative risk, 0.26; 95% CI, 0.16–0.41; p < 0.01, respectively).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Percutaneous radiofrequency ablation (RFA) is one of the curative treatment options for early-stage hepatocellular carcinoma (HCC), providing comparable overall survival with the potential benefits obtained from repeat RFA and less aggressive deterioration on liver function compared to hepatic resection [1,2,3]. Conventional RFA has been performed by placing RF electrodes intratumorally to maximize thermal energy delivery to the target tumor. However, local tumor progression (LTP) has been one of the main limitations of conventional RFA with reported risk factors such as satellite nodules [4] or microvascular invasion [5], which may be untreated by insufficient ablative margins [6], track seeding along the electrode insertion route [7], increased intratumoral pressure during ablation which may facilitate tumor spread [8], or decrease of RFA temperature when a tumor is located in the vicinity of blood flow (so-called heat-sink effect) [9]. In fact, the 3-year and 5-year cumulative incidence rates of LTP after conventional RFA for single HCC smaller than 3 cm were 28.5% and 32.1%, respectively [10]. Although subsequent interventions in patients with LTP provide comparable overall survival outcome to those without LTP, there is still a clinically unmet need to reduce LTP [3].
In this regard, no-touch RFA (NtRFA) has recently been introduced and investigated in clinical practice, to improve local tumor control. NtRFA is performed by inserting multiple electrodes outside the tumors to achieve ablation with sufficient margins and to avoid violation of the tumor itself to prevent track seeding or intratumoral pressure increase. Previously, the proof of concept of better local tumor control of NtRFA compared to conventional RFA was substantiated in both in vivo [11] and histopathological study [12]. Several clinical studies with NtRFA with multi-bipolar electrodes have been published providing LTP rates as low as 4% at 3 years [13,14,15,16] and lower LTP rates [17,18,19] compared to conventional RFA with monopolar electrodes. Two recent studies using the same RFA system and electrodes also showed similar trends of lower LTP in NtRFA than in conventional RFA [20, 21]. In addition, another retrospective study by Kawamura et al [19] reported that NtRFA showed significantly lower intra-subsegmental recurrence than conventional RFA, although the cumulative LTP rates were not significantly different between ablation procedures.
The clinical application of NtRFA has been slow despite satisfactory local tumor control rates in the literature [13,14,15,16,17,18,19,20,21,22,23,24]. This is partly because of the heterogeneous study population, different RF energy delivery modes (i.e., multi-bipolar or monopolar), and the small number of patients in prior studies. Therefore, this study aimed to systematically review the literature and perform a meta-analysis of LTP after percutaneous NtRFA for HCC equal to or less than 5 cm, and to compare it with conventional RFA.
Materials and methods
We performed this meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines [25]. This study was not registered on PROSPERO (International Prospective Register of Systematic Reviews).
Literature search
We conducted systematic search in MEDLINE, EMBASE, and Cochrane Library databases up to May 9, 2022, to find relevant original articles reporting on per-patient or per-lesion LTP after NtRFA for HCC. The following keywords and their synonyms were included in the search terms: (“no touch” OR nontouch OR bipolar OR multipolar) AND (“radiofrequency ablation” OR RFA) AND recurrence AND (“hepatocellular carcinoma” OR “hepatic cancer” OR hepatoma OR HCC). The references of the included articles were screened to identify other eligible studies.
Inclusion criteria
The inclusion criteria according to the Population, Intervention, Comparison, Outcomes and Study criteria for studies in the meta-analysis were as follows [26]: (1) studies comprising patients (P) diagnosed with HCC (either by pathology or by non-invasive imaging criteria) ≤ 5 cm who underwent percutaneous NtRFA for curative purposes; (2) studies with NtRFA as an index test (I); (3) studies with conventional RFA as comparison (C) if available; (4) studies with outcome (O) as the overall or cumulative incidence rates of LTP; and (5) studies with study type (S) as an original article.
Exclusion criteria
The exclusion criteria were as follows: (1) studies with sample size less than 10 patients; (2) studies with publication type other than original articles; (3) studies with insufficient data for evaluating the overall or cumulative incidence rates for LTP after NtRFA; and (4) studies with complete population and data overlap. One author (T.H.K.) performed the literature search and study selection, which was double-checked by another author (J.M.L.).
Data extraction and quality assessment
Data from the included studies regarding demographic and clinical characteristics, overall rates, and cumulative incidence rates at years 1, 2, and 3 of LTP after NtRFA were retrieved using a standardized form. If studies provided comparison data of LTP rates between conventional RFA and NtRFA, the data were also recorded for further analysis.
Two reviewers independently assessed the methodological quality of the selected studies by using the Quality in Prognostic Studies (QUIPS) tool [27]. Disagreements were resolved by consensus from the two reviewers.
Data synthesis and analyses
The primary outcome for the current meta-analysis was pooled proportions of the overall and cumulative incidence rates at years 1, 2, and 3 of the LTP after NtRFA. We separately calculated per-lesion and per-patient LTP rates, and combined both to synthesize the overall LTP rates. The pooled proportions and 95% confidence intervals (CIs) were calculated using the DerSimonian-Laird random-effects model, and weights were determined using the inverse variance method [28].
The second outcome was to compare the LTP rates between conventional RFA and NtRFA based on the risk ratios (RR) and hazard ratios (HR) with 95% CIs extracted, respectively. For studies that only provided the Kaplan-Meier curves without HR from univariate Cox regression analysis, their HRs and 95% CIs were indirectly calculated using Engauge Digitizer (version 10.4; http://markummitchell.github.io/engauge-digitizer/), based on the methodology proposed by Guyot et al [29]. Heterogeneity among studies was assessed using Higgins I2, which describes the percentage of variation across studies that is due to heterogeneity rather than chance, with significant heterogeneity considered present when I2 was > 50% [30]. For the pooled proportions, publication bias was visually assessed by using funnel plots, and statistical significance was evaluated using the Egger test [31]. A meta-regression analysis using the following covariates was performed to identify the sources of heterogeneity: study design (retrospective vs. prospective), mean or median HCC size (≤ 2 cm vs. > 2 cm), HCC number (single vs. multiple), inclusion of recurrent HCC (vs. only including treatment-naive HCC), continent (Asia vs. Western), and RFA mode (multi-bipolar vs. multiple monopolar).
All statistical analyses were performed using the “meta” and “metafor” packages of the R statistical software (version 3.6.0, The R Foundation for Statistical Computing).
Results
Literature search
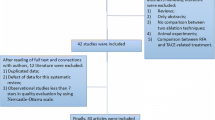
The initial systematic search identified 295 studies. After the removal of 100 duplicates, 195 articles were screened based on their titles and abstracts. Subsequent full-text review of 23 potentially eligible studies excluded 11 studies. Ultimately, 12 studies evaluating LTP after NtRFA in HCC ≤ 5 cm were included [13,14,15,16,17,18,19,20,21,22,23,24]. The study selection process is illustrated in Fig. 1.
Characteristics of included studies
The patient, study, and RFA characteristics are summarized in Table 1. The number of patients and HCC treated with NtRFA ranged from 15 to 181 and 17 to 132, respectively. In total, 900 patients and 639 HCC treated with NtRFA were included in our meta-analysis, reflecting two studies only reported the number of patients and not the number of treated HCC [18, 22]. Initial HCC diagnosis was based on pathology in one study [14], non-invasive imaging criteria in six studies [13, 16, 17, 21,22,23], or both in five studies [15, 18,19,20, 24]. Six studies included single HCC [14, 16, 17, 19, 23, 24], and the other six studies included multiple HCC, with maximum number up to 3 [13, 15, 18, 20,21,22]. The median or mean size of HCC was less than 2 cm in six studies [17, 19,20,21,22,23] and larger than 2 cm in six studies [13,14,15,16, 18, 24]. Seven studies included only patients with treatment-naive HCC [13,14,15,16,17,18, 24], whereas five studies also included patients’ recurrent HCC from previous treatments [19,20,21,22,23]. Seven studies were retrospective [13,14,15, 17,18,19, 24], three prospective [16, 22, 23], and two randomized controlled trials [20, 21]. Regarding the NtRFA mode, three studies utilized multiple switching monopolar [14, 20, 23], whereas the other nine studies used multi-bipolar [13, 15,16,17,18,19, 21, 22, 24]. Six studies compared LTP with conventional RFA [17,18,19,20,21,22]. Operator numbers and experience varied across the studies.
Quality assessment
The risk of bias evaluation using the QUIPS tool is summarized in Fig. 2. For the study participant domain, four studies showed a moderate risk as they shared a partial overlapping population [13, 15, 18, 24]. A low risk of bias was observed in all studies for the study attrition and study confounding domains. Regarding the prognostic factor measurement domain, high risk of bias was present in one study as it included two patients who underwent NtRFA under laparoscopic approach [24]. For the outcome measurement domain, all studies showed a moderate risk of bias because LTP was determined by knowing that patients underwent NtRFA. For the statistical analysis and reporting domain, two studies showed a moderate risk of bias as they included both LTP and primary RFA failure in the cumulative incidence calculation [13, 18].
Local tumor progression
The definition of LTP was almost identical throughout the 12 studies, 11 studies [13, 15,16,17,18,19,20,21,22,23,24] defining recurrence abutting the ablation zone and one study [14] additionally including recurrence within 2 cm from the ablation zone. The pooled proportion of LTP is summarized in Fig. 3A. The LTP rate was available as per-lesion analysis in nine studies, and as per-patient analysis in three studies, ranging from 2 to 11%. The pooled proportion of LTP was 6% (95% CI, 4–9%), 5% (95% CI, 2–9%), and 6% (95% CI 4–8%) in per-lesion, per-patient, and overall analysis, respectively. In the funnel plots and the Egger test, significant publication bias was observed (p < 0.01) (Supplementary Figure 1). The pooled 1-, 2-, and 3-year cumulative incidence rates of LTP were 3% (95% CI, 2–5%), 5% (95% CI, 3–9%), and 8% (95% CI, 6–11%), respectively (Fig. 3B). Heterogeneity among studies was not observed in the pooled proportions of the overall rate and the 1- and 3-year cumulative incidence rates (I2, 15–29%) and were moderate only in the 2-year cumulative incidence rate (I2 = 51%).
The results of the meta-regression analysis performed to explore the possible sources of heterogeneity are summarized in Table 2. The analysis revealed that studies with prospective design showed significantly lower rates of LTP (2.4%; 95% CI, 1.1–5.1%) than those with retrospective design (6.9%; 95% CI, 5.1–9.5%; p = 0.01). Other covariates, including HCC characteristics (mean/median size cutoff of 2 cm, number of HCC, or inclusion of recurrent HCC), study population (Asia vs. Western), and RFA mode (multi-bipolar vs. multiple monopolar) did not differ significantly across the studies.
Local tumor progression comparison between no-touch radiofrequency ablation and conventional radiofrequency ablation
Figure 4 summarizes the pooled analysis for LTP comparison between NtRFA and conventional RFA from studies which provided relevant data [17,18,19,20,21,22]. NtRFA had significantly lower rates of LTP compared to conventional RFA (HR, 0.28; 95% CI, 0.11–0.70; RR, 0.26; 95% CI, 0.16–0.41; p < 0.01 respectively). Subgroup analysis including only studies with randomized controlled design also showed better local tumor control of NtRFA with HR of 0.13 [95% CI, 0.04–0.42] (Fig. 4C) [20, 21].
Discussion
This is the first meta-analysis focusing on local tumor control of NtRFA for HCC ≤ 5 cm, which proved to be effective with an overall LTP rate of 6% and cumulative incidence LTP rates of 3%, 5%, and 8% at 1, 2, and 3 years, respectively. Furthermore, NtRFA showed better control of LTP than conventional RFA, with four times more likelihood of LTP occurring in conventional RFA than in NtRFA. Based on our study results, NtRFA is an effective treatment for achieving satisfactory LTP for HCC and may be preferentially used for better local tumor control than conventional RFA.
The efficacy of local tumor control in conventional RFA has been investigated in numerous studies. For instance, in a large-scale retrospective study with 1305 patients with 1502 early-stage HCC over 12 years of enrollment period [2], the incidence rate of LTP was 19.4% with 1- and 3-year cumulative rates of 9.7% and 27.0%, respectively. Another retrospective study of 301 patients with solitary HCC less than 3 cm over 15 years of enrollment period [32] reported overall LTP rates of 16.6% and 20.1% in HCC > 2 cm. The reported results from conventional RFA were approximately three times higher than the pooled overall rates and cumulative incidence rates of LTP after NtRFA from our meta-analysis. Furthermore, we reidentified the better local tumor control of NtRFA compared to conventional RFA in our meta-analysis, with pooled HR and RR of 0.28 and 0.26, respectively. It is noteworthy that subgroup analysis including only studies with randomized controlled design showed a similar trend of better local tumor control of NtRFA with HR of 0.13. The lower incidence of LTP after NtRFA may be explained by several factors. First, during NtRFA, multiple electrodes inserted outside the tumor margins initiate ablation from the periphery to centripetally to the target tumor, which enables sufficient ablation margins to be achieved. Second, coagulation of tumor feeding and draining vessels in the periphery of the target tumor occur in the early period of ablation, which may block the dispersal of tumor cells into the drainage bloodstream. Furthermore, contrary to conventional RFA, NtRFA does not violate the tumor capsule or elevate intratumoral pressure during ablation which are well-known leading factors for recurrence in surgery and conventional RFA, respectively [33, 34]. In line with this, lower rates of intra-subsegmental recurrence of NtRFA have also been highlighted ranging from 2.9 to 5.5% [15, 19], compared to 13.2 to 19.2 % for conventional RFA [19, 35]. Finally, as more than one electrode are used in NtRFA, in treating HCC located near large vessels where the heat-sink effect precludes efficient treatment, one of the electrodes can be placed at the abutting vessels to deliver optimal energy to maximize the local efficacy [15, 18].
In the meta-regression analysis, studies stratified by the cutoff of 2 cm for the mean or median tumor size did not show significant difference in overall LTP rates (p = 0.13). Although not all studies in the current meta-analysis targeted HCC larger than 3 cm, the high efficacy of NtRFA even in larger tumors between 3 and 5 cm may be attributed to this insignificance. For instance, in the retrospective study by Seror et al [15], which included HCC ≤ 5 cm treated by NtRFA, tumor larger than 3 cm was not a predictive factor for LTP. Furthermore, a lower rate of LTP of 9.3% after NtRFA compared to 20% after conventional RFA for HCC larger than 3 cm may substantiate the capacity of NtRFA to successfully treat HCC larger than 2 cm [18, 36]. At the same time, albeit without statistical significance, given that overall LTPs after NtRFA for HCC larger than 2 cm are twice as high as those for HCC less than 2 cm (6.7% vs. 3.3%) and technical difficulty in performing NtRFA for HCC larger than 3 cm, we may not have reached a statistical significance from a small number of included studies. Second, the, RFA mode (i.e., multi-bipolar vs. multiple monopolar) in NtRFA was not a significant factor for LTP. Although multi-bipolar has been reported to induce a larger ablation volume compared to multiple monopolar [37], the mode may not affect as in the real clinical situation as operators manipulate to achieve optimal ablation. Lastly, prospective studies showed a lower overall LTP rate than retrospective studies (2.4% vs. 6.9%). We speculate that at least in part, predetermined focus with strict eligibility criteria to achieve complete ablation in prospective design may have contributed to lower LTP.
This study has some limitations. First, the number of included studies was relatively small (n = 12). This may stem from the fact that NtRFA was introduced recently in the last decade and has not been as extensively studied as conventional RFA. Caution is also warranted as publication bias was present in the funnel plot and Egger’s test. Second, although we performed subgroup analysis based on the tumor size with a cutoff of 2 cm, subgroup analysis based on the different tumor sizes (i.e., < 3 cm versus 3–5 cm) or RFA characteristics that may influence the technical success and subsequent LTP rate such as visibility during the ablation, or application of adjuvant maneuvers such as artificial ascites or fusion imaging, was not feasible due to the unavailability of information in the included studies. Third, as we mainly focused on the LTP of NtRFA, clinical aspects of NtRFA, such as technical feasibility according to tumor location, learning curve to achieve satisfactory treatment efficacy, or postprocedural liver failure from larger ablation margins could not be dealt in depth.
In conclusion, NtRFA showed effective local tumor control in the treatment of HCC ≤ 5 cm, with lower LTP compared to conventional RFA. Therefore, NtRFA should be considered an effective therapeutic option with the intent to cure for HCC.
Abbreviations
- CIs:
-
Confidence intervals
- HCC:
-
Hepatocellular carcinoma
- HR:
-
Hazard ratio
- LTP:
-
Local tumor progression
- NtRFA:
-
No-touch radiofrequency ablation
- QUIPS:
-
Quality in prognostic studies tool
- RFA:
-
Radiofrequency ablation
- RR:
-
Risk ratio
References
Feng K, Yan J, Li X et al (2012) A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 57:794–802. https://doi.org/10.1016/j.jhep.2012.05.007
Kim YS, Lim HK, Rhim H et al (2013) Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol 58:89–97. https://doi.org/10.1016/j.jhep.2012.09.020
Lee DH, Lee JM, Lee JY et al (2014) Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 270:900–909. https://doi.org/10.1148/radiol.13130940
Brillet PY, Paradis V, Brancatelli G et al (2006) Percutaneous radiofrequency ablation for hepatocellular carcinoma before liver transplantation: a prospective study with histopathologic comparison. AJR Am J Roentgenol 186:S296–S305. https://doi.org/10.2214/ajr.04.1927
Lee S, Kang TW, Song KD et al (2021) Effect of microvascular invasion risk on early recurrence of hepatocellular carcinoma after surgery and radiofrequency ablation. Ann Surg 273:564–571. https://doi.org/10.1097/sla.0000000000003268
Teng W, Liu KW, Lin CC et al (2015) Insufficient ablative margin determined by early computed tomography may predict the recurrence of hepatocellular carcinoma after radiofrequency ablation. Liver Cancer 4:26–38. https://doi.org/10.1159/000343877
Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN (2003) Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 226:441–451. https://doi.org/10.1148/radiol.2262012198
Angonese C, Baldan A, Cillo U et al (2006) Complications of radiofrequency thermal ablation in hepatocellular carcinoma: what about “explosive” spread? Gut 55:435–436. https://doi.org/10.1136/gut.2005.080515
Lin ZY, Li GL, Chen J, Chen ZW, Chen YP, Lin SZ (2016) Effect of heat sink on the recurrence of small malignant hepatic tumors after radiofrequency ablation. J Cancer Res Ther 12:C153–c158. https://doi.org/10.4103/jcrt.JCRT_959_16
Francica G, Saviano A, De Sio I et al (2013) Long-term effectiveness of radiofrequency ablation for solitary small hepatocellular carcinoma: a retrospective analysis of 363 patients. Dig Liver Dis 45:336–341. https://doi.org/10.1016/j.dld.2012.10.022
Kim TH, Choi HI, Kim BR et al (2018) No-touch radiofrequency ablation of VX2 hepatic tumors in vivo in rabbits: a proof of concept study. Korean J Radiol 19:1099–1109. https://doi.org/10.3348/kjr.2018.19.6.1099
Seror O, N'Kontchou G, Van Nhieu JT et al (2014) Histopathologic comparison of monopolar versus no-touch multipolar radiofrequency ablation to treat hepatocellular carcinoma within Milan criteria. J Vasc Interv Radiol 25:599–607. https://doi.org/10.1016/j.jvir.2013.11.025
Petit A, Hocquelet A, N'Kontchou G et al (2020) No-touch multi-bipolar radiofrequency ablation for the treatment of subcapsular hepatocellular carcinoma </= 5 cm not puncturable via the non-tumorous liver parenchyma. Cardiovasc Intervent Radiol 43:273–283. https://doi.org/10.1007/s00270-019-02357-9
Qu C, Li XQ, Li C, Xia F, Feng K, Ma K (2021) The short-term efficacy of novel no-touch combined directional perfusion radiofrequency ablation in the treatment of small hepatocellular carcinoma with cirrhosis. J Invest Surg:1–8. https://doi.org/10.1080/08941939.2021.1931575
Seror O, N'Kontchou G, Nault JC et al (2016) Hepatocellular carcinoma within Milan criteria: no-touch multibipolar radiofrequency ablation for treatment-long-term results. Radiology 280:611–621. https://doi.org/10.1148/radiol.2016150743
Wu L-W, Chen C-Y, Liu C-J et al (2014) Multipolar radiofrequency ablation with non-touch technique for hepatocellular carcinoma ≤ 3 cm: a preliminary report. Adv Digest Med 1:80–85. https://doi.org/10.1016/j.aidm.2013.09.004
Chai Y, Li K, Zhang C, Chen S, Ma K (2019) The short-term efficacy of no-touch radiofrequency ablation in treating cirrhosis-based small hepatocellular carcinoma. BMC Cancer 19:497. https://doi.org/10.1186/s12885-019-5707-0
Hocquelet A, Aube C, Rode A et al (2017) Comparison of no-touch multi-bipolar vs. monopolar radiofrequency ablation for small HCC. J Hepatol 66:67–74. https://doi.org/10.1016/j.jhep.2016.07.010
Kawamura Y, Ikeda K, Fujiyama S et al (2017) Potential of a no-touch pincer ablation procedure that uses a multipolar radiofrequency ablation system to prevent intrasubsegmental recurrence of small and single hepatocellular carcinomas. Hepatol Res 47:1008–1020. https://doi.org/10.1111/hepr.12838
Park SJ, Cho EJ, Lee JH et al (2021) Switching monopolar no-touch radiofrequency ablation using octopus electrodes for small hepatocellular carcinoma: a randomized clinical trial. Liver Cancer 10:72–81. https://doi.org/10.1159/000512338
Suh YS, Choi JW, Yoon JH et al (2021) No-Touch vs. Conventional Radiofrequency Ablation Using Twin Internally Cooled Wet Electrodes for Small Hepatocellular Carcinomas: A Randomized Prospective Comparative Study. Korean J Radiol. 22(12):1974–1984. https://doi.org/10.3348/kjr.2021.0319
Hirooka M, Hiraoka A, Ochi H et al (2019) Prospective cohort trial to confirm the efficacy of no-touch radio frequency ablation. J Gastroenterol Hepatol 34:567–574. https://doi.org/10.1111/jgh.14476
Lee DH, Lee MW (2021) Outcome of no-touch radiofrequency ablation for small hepatocellular carcinoma: a multicenter clinical trial. Radiology 301:229–236. https://doi.org/10.1148/radiol.2021210309
Mohkam K, Dumont PN, Manichon AF et al (2018) No-touch multibipolar radiofrequency ablation vs. surgical resection for solitary hepatocellular carcinoma ranging from 2 to 5cm. J Hepatol 68:1172–1180. https://doi.org/10.1016/j.jhep.2018.01.014
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
McInnes MDF, Moher D, Thombs BD et al (2018) Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA 319:388–396. https://doi.org/10.1001/jama.2017.19163
Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C (2013) Assessing bias in studies of prognostic factors. Ann Intern Med 158:280–286. https://doi.org/10.7326/0003-4819-158-4-201302190-00009
Lee J, Kim KW, Choi SH, Huh J, Park SH (2015) Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part II. Statistical methods of meta-analysis. Korean J Radiol 16:1188–1196. https://doi.org/10.3348/kjr.2015.16.6.1188
Guyot P, Ades AE, Ouwens MJ, Welton NJ (2012) Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 12:9. https://doi.org/10.1186/1471-2288-12-9
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558. https://doi.org/10.1002/sim.1186
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634. https://doi.org/10.1136/bmj.315.7109.629
Doyle A, Gorgen A, Muaddi H et al (2019) Outcomes of radiofrequency ablation as first-line therapy for hepatocellular carcinoma less than 3 cm in potentially transplantable patients. J Hepatol 70:866–873. https://doi.org/10.1016/j.jhep.2018.12.027
Kang TW, Lim HK, Lee MW et al (2015) Aggressive intrasegmental recurrence of hepatocellular carcinoma after radiofrequency ablation: risk factors and clinical significance. Radiology 276:274–285. https://doi.org/10.1148/radiol.15141215
Tung-Ping Poon R, Fan ST, Wong J (2000) Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg 232:10–24. https://doi.org/10.1097/00000658-200007000-00003
Tateishi R, Shiina S, Akahane M et al (2013) Frequency, risk factors and survival associated with an intrasubsegmental recurrence after radiofrequency ablation for hepatocellular carcinoma. PLoS One 8:e59040. https://doi.org/10.1371/journal.pone.0059040
Sun AX, Cheng ZL, Wu PP et al (2015) Clinical outcome of medium-sized hepatocellular carcinoma treated with microwave ablation. World J Gastroenterol 21:2997–3004. https://doi.org/10.3748/wjg.v21.i10.2997
Chang W, Lee JM, Yoon JH et al (2017) No-touch radiofrequency ablation using multiple electrodes: an in vivo comparison study of switching monopolar versus switching bipolar modes in porcine livers. PLoS One 12:e0176350 https://doi.org/10.1371/journal.pone.0176350
Funding
Financial support: This work was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT; the Ministry of Trade, Industry and Energy; the Ministry of Health & Welfare; the Ministry of Food and Drug Safety (KMDF_PR_20200901 0303).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jeong Min Lee.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Tae-Hyung Kim, one of the authors, has significant statistical expertise in meta-analysis.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval is not required because the study design is systematic review and meta-analysis.
Methodology
• retrospective
• not applicable
• multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 45.3 KB)
Rights and permissions
About this article
Cite this article
Kim, TH., Lee, J.M., Lee, D.H. et al. Can “no-touch” radiofrequency ablation for hepatocellular carcinoma improve local tumor control? Systematic review and meta-analysis. Eur Radiol 33, 545–554 (2023). https://doi.org/10.1007/s00330-022-08991-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08991-1