Abstract
Purpose
The percutaneous ablation of subcapsular hepatocellular carcinoma (S-HCC) may involve a risk of complications such as hemorrhage and tumor seeding, mainly linked to the direct tumor puncture often inevitable with mono-applicator ablation devices. The purpose of this study was to assess the efficacy and safety of no-touch multi-bipolar radiofrequency ablation (NTMBP-RFA) for the treatment of S-HCC ≤ 5 cm not puncturable via the non-tumorous liver parenchyma.
Materials and methods
Between September 2007 and December 2014, 58 consecutive patients (median age: 63 years [46–86], nine females) with 59 S-HCC ≤ 5 cm (median diameter: 25 mm [10–50 mm]), not puncturable via the non-tumorous liver parenchyma, were treated with NTMBP-RFA. Response and follow-up were assessed by CT or MRI. Complications were graded using the Cardiovascular and Interventional Radiological Society of Europe classification. Overall local tumor progression (OLTP)-free survival was assessed using the Kaplan–Meier method. A Cox proportional model evaluated the factors associated with OLTP. Signs of peritoneal or parietal tumor seeding were noted during follow-up imaging studies.
Results
A complete ablation was achieved in 57/58 patients (98.3%) after one (n = 51) or two (n = 6) procedures. Three patients (5.2%) experienced complications (sepsis, cirrhosis decompensation; CIRSE grade 2 or 3). After a median follow-up period of 30.5 months [1–97], no patients had tumor seeding. The 1, 2 and 3-year OLTP-free survival rates were 98%, 94% and 91%, respectively. No factors were associated with OLTP.
Conclusion
NTMBP-RFA is a safe and effective treatment for S-HCC not puncturable via the non-tumorous liver parenchyma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radiofrequency ablation (RFA) is an effective local treatment for small-sized hepatocellular carcinoma (HCC) in patients with cirrhosis [1]. The safety of RFA has been widely demonstrated in this setting [2,3,4,5], but for subcapsular HCC (S-HCC), the percutaneous approach remains controversial because of the higher risk of tumor seeding, hemorrhage and collateral damage [2, 6]. In addition, some reports have suggested that the RFA of subcapsular HCC can lead to a local tumor progression rate as high as 20% [7, 8].
Llovet et al. [9] reported up to 12% of tumor seeding along the needle track after RFA for HCC. A subcapsular location and poor degree of differentiation were found to be independent risk factors for seeding. Several subsequent studies reported much lower rates of tumor seeding after RFA, even in a subcapsular location and after a biopsy performed prior to ablation [7, 8, 10,11,12,13,14].
Under the assumption that most major post-procedural complications in the case of S-HCC (such as hemorrhage and tumor seeding) are mainly caused by tumor rupture into the peritoneal space, it is strongly recommended to introduce the needle via the non-tumorous parenchyma before entering the targeted tumor [8].
However, because many exophytic and/or anterior or lateral S-HCC cannot be punctured via the non-tumorous parenchyma, they are still considered by most practitioners to be contraindicated for standard intra-tumorous mono-applicator techniques such as monopolar RFA or microwave ablation (MWA) [15, 16].
No-touch multi-bipolar RFA (NTMBP-RFA), consisting in inserting several probes around the tumor, can overcome this common limitation of intra-tumorous ablative techniques for S-HCC [17, 18]. In addition, through the use of appropriate needle insertion strategies, this technique can ablate S-HCC that are even larger than 3 cm and can, therefore, improve the global efficacy of RFA for superficially located tumors [19,20,21].
The purpose of this study was, therefore, to assess the efficacy and safety of NTMBP-RFA in the treatment of S-HCC ≤ 5 cm not puncturable via the non-tumorous liver parenchyma.
Materials and Methods
Patient Selection and Tumor Status
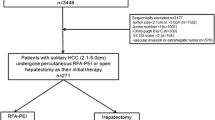
This retrospective study was approved by our institutional review board, and informed written consent from patients was waived. Between September 2007 and December 2014, 439 patients underwent a first NTMBP-RFA procedure in our center for the treatment of HCC. Patients who had previously been treated or were simultaneously being treated with ablation techniques other than NTMBP-RFA, those for whom pre-therapeutic imaging was not available, and patients treated for a recurrence after liver transplantation (but not after liver resection), were all excluded from the study (Fig. 1). Pre-therapeutic multiphasic contrast-enhanced CT scan or MRI of the remaining 375 patients were analyzed in order to locate their tumors.
Among these 375 patients, 97 (25.9%) had one or up to three S-HCC, defined as an HCC nodule abutting the superficial liver capsule. Three patients with infiltrative HCC and five patients with a subcapsular tumor larger than 5 cm were excluded. The imaging studies of the remaining 89 patients with S-HCC were then reviewed by an interventional radiologist (OSe) with more than 15 years of experience in the percutaneous ablation of liver tumors, in order to select those with S-HCC not puncturable via the non-tumorous liver parenchyma (Fig. 2). Finally, 59 S-HCCs (located in Couinaud segments 3, 4, 5, 6 and 8) in 58/89 patients (65.2%) were considered not directly puncturable via the non-tumorous parenchyma and were included in the analysis. The diagnosis of HCC was based on the typical imaging pattern defined by EASL guidelines [22]. All treatment decisions were taken by a multidisciplinary tumor board gathering hepatologists, oncologists, radiologists, pathologists and liver surgeons.
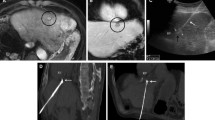
Representative cases of subcapsular HCCs for which no indirect puncture path was feasible because of the location or exophytic growth of the tumor. A CT images of a lateral subcapsular HCC with no possibility of an indirect puncture path. B T2 weighted-MR images showing an anterior and exophytic left lobe subcapsular HCC with no possibility of an indirect puncture path
Tumors were considered to be exophytic if at least 50% of their volume was developed outside the liver margin. Contact with a significant vessel was defined as a tumor abutting a vessel larger than 3 mm. The biological parameters of all patients were retrieved from the blood tests performed on the day of the pre-treatment visit. All patients had undergone an upper gastrointestinal endoscopy during the year prior to the ablation procedure in order to screen or monitor gastric/esophageal varices (Table 1).
NTMBP-RFA Procedures
All NTMBP-RFA procedures were performed under general anesthesia, by the same interventional radiologist (OSe). The probes were inserted using ultrasound guidance alone or fused with CT, MRI or cone beam computed tomography when the target was insufficiently conspicuous on ultrasound alone.
Radiofrequency energy was supplied by a 3 × 2-channel 250-W, 470-kHz radiofrequency generator (CelonLabPower®; Olympus-Celon, Teltow, Germany). Two to six internally cooled 30–40-mm active 15-G electrodes were used, depending on the size and location of the tumors. In this setting, 2 to 4 applicators were usually used to treat tumors < 3 cm and 4 to 6 probes for HCCs between 3 and 5 cm. As previously detailed, the electrodes were inserted using free-hand technique, in compliance with the no-touch concept, with an objective of at least 5-mm margins around the tumor [17]. If necessary, the number of applicators was adjusted according to per-procedural requirements regarding proximity of large vessels or neighboring critical structures. Three electrode insertion strategies were employed to achieve no-touch tumor ablation (Fig. 3) [21]:
Illustrations of electrode implantation strategies. A Standard no-touch strategy. a CT images of a lateral subcapsular HCC treated with the implantation of four electrodes around the tumor in a parallel course. Continuous lines represent electrodes in front of the plan whereas discontinuous lines represent electrodes behind the plan. b. CT images at 1 month showing complete ablation. B Retro-nodular converging no-touch strategy. a CT images of an anterior subcapsular HCC treated with the implantation of four electrodes around the tumor in a retro-nodular converging course. Continuous lines represent electrodes in front of the plan whereas discontinuous lines represent electrodes behind the plan b CT images at 1 month showing complete ablation. Note that the preexisting small amount peri-hepatic ascites slightly increased at 1 month. B. No-touch cutting strategy. a Pre-therapeutic contrast-enhanced T1 weighted-MR images showing an anterior left lobe subcapsular HCC. White lines represent the electrodes. b Three-dimensional volume rendering reconstruction of post-procedural CT showing the ablation zone with a representation of the targeted tumor (circle) treated with the implantation of three electrodes according to a cutting strategy. c Axial and coronal CT images at 1 month showing complete ablation
a) the standard no-touch strategy, consisting in the parallel insertion of electrodes into the non-tumorous liver parenchyma around the tumor, with a maximum distance between adjacent probes of approximately 3 cm; b) the retro-nodular converging no-touch strategy, consisting in the convergent insertion of electrodes into the non-tumorous liver parenchyma, but around the tumor toward its hilar side (mainly used for exophytic tumors and those > 3 cm); c) the no-touch cutting strategy, used for peripheral nodules attached to the right or left lobe by a thin band of non-tumorous liver parenchyma. In this parenchyma band, the electrodes were here inserted in parallel (with a maximum distance of 3 cm between adjacent probes) to achieve complete ablation and wide ablative margins by means of the total upstream vascular deprivation of the tumor.
The electrode implantation strategies, number of electrodes used, amount of energy delivered and time of energy deposition were all recorded. Track ablations were usually not performed because in most case, the ablation zones encompassed the entire path of intra-hepatic needles up to the liver capsule, including that of the per-procedure biopsy if it was performed (using a coaxial needle which was left in the target until the ablation endpoint was reached). If the targeted tumor was located in contact with the gastrointestinal tract or diaphragm, artificial ascites (obtained by filling the peritoneal cavity with 1 to 2 L of isotonic saline solution via a 6-F pigtail catheter inserted under US guidance) was then created.
Technique Efficacy
The efficacy of NTMBP-RFA was assessed at 1 month using a multiphasic CT scan or MRI examination. If the treatment was considered to be complete, the patient underwent a follow-up multiphasic CT scan or MRI every 3 months for 2 years and every 6 months thereafter. Incomplete treatment at 1 month was defined by the persistence of a residual, non-ablated tumor part with nodular or irregular enhancement. In that case, a further NTMBP-RFA procedure was scheduled, followed by early imaging evaluation at 1 month and the same follow-up scheduled as described above. Primary efficacy was defined as achieving complete treatment after one NTMBP-RFA procedure and secondary efficacy after one or two NTMBP-RFA procedures. A primary treatment failure was defined as incomplete ablation after NTMBP-RFA, including, if performed, additional procedures.
The volumes of initial tumors and ablated areas were measured for each patient using a semi-automated segmentation method integrated into the workstation (LiveWire® software, Carestream Health Inc, Rochester, USA). In addition, ablative margins were evaluated on transversal and coronal-reformatted images by comparison with the pre-RFA images [23].
Tumor Progression and Tumor Seeding
Local tumor progression (LTP) was defined by the appearance of a new nodular or irregular tumor enhancement abutting the ablated area after an initially complete treatment. By adding LTP to primary NTMBP-RFA failures, the overall LTP (OLTP) was computed. Intra-hepatic distant tumor progression (DTP) was defined by the appearance of a nodule with a typical imaging pattern of HCC separated from the ablation zone by non-tumorous parenchyma. Adding DTP to OLTP defined overall intra-hepatic tumor progression (OTP). Tumor seeding was defined by the appearance of a parietal or peritoneal nodule close to the ablation zone and was the subject of an additional systematic retrospective review of all follow-up imaging examinations. If applicable, tumor seeding was considered as an LTP for analysis. Time to progression was defined as the time between the last RFA procedure and images revealing tumor progression. All data were collected until March 2016 and, where applicable, were censored at the date of the patient’s last follow-up or liver transplantation.
Complications
The presence of early complications was determined from clinical post-procedure features and imaging evaluations performed during the month after the procedure. These complications were then assessed using the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) classification system for complications [24].
Statistical Analysis
Qualitative variables were expressed as raw numbers and proportions; quantitative variables were expressed as medians with ranges. OLTP-free survival (OLTPFS), OTP-free survival (OTPFS) and overall survival (OS) were measured from the last RFA procedure to the event date (OLTP, OTP or death, respectively) and, where applicable, were censored at the date of the most recent follow-up visit (prior to March 2016) or death or liver transplantation. Rates of OLTPFS, OTPFS and OS were computed using the Kaplan–Meier method. Factors associated with tumor progression and overall survival were determined using univariate Cox model analysis. Those with a P value < 0.10 were entered into a multivariate Cox model. P < 0.05 was considered indicative of a statistically significant difference. Statistical analyses were performed with Stata® (version 13; Stata Corp, College Station, USA).
Results
Patient Characteristics
The mean age of the 58 patients was 63 years (range 46–86). All patients had histologically proven cirrhosis, mainly related to alcohol, alone (n = 19, 32.8%) or in association with hepatitis C virus (n = 11, 19%) or nonalcoholic steatohepatitis (n = 6, 10.3%). 48 patients (82.8%) were Child–Pugh class A, 8 (13.8%) were Child–Pugh class B and 2 (3.4%) had Child–Pugh score C10. Median tumor diameter was 25 mm (range 10–50). 17 patients (29.3%) had a tumor > 3 cm and 12 tumors (20.3%) were exophytic (Table 1).
NTMBP-RFA Procedures
Forty-seven tumors (79.7%) were treated using a standard no-touch electrode implantation strategy, seven (11.8%) according to a no-touch retro-nodular converging approach and five (8.5%) with a cutting no-touch ablation technique. The median number of electrodes used was 3 [range 2–6], the median energy delivered was 91.5 kJ [range 22–276] and median radiofrequency application time was 24 min [range 5–60].
Biopsy was performed for 36 tumors (61%) at the beginning of the ablation procedure. Three procedures (5%) required the creation of artificial ascites.
Technique Efficacy
Among the 59 nodules, complete treatment was achieved in 52 after the first NTMBP-RFA procedure (primary efficacy rate of 88.1%). Six of the remaining seven nodules were completely treated after a second NTMBP-RFA procedure (secondary efficacy rate of 98.3%). One patient with a nodule that was incompletely treated after the first NTMBP-RFA underwent liver transplantation and was considered as a primary NTMBP-RFA failure (1.7%) (Table 2).
Tumor Progression and Seeding
The median follow-up period was 30.5 months [range 1–97]. LTP was detected in 5/59 tumors (8.5%) with a median time to LTP of 15 months [range 11–42]. By adding one case of NTMBP-RFA primary failure (1.7%), the OLTP rate was 10.2% (6/59 tumors). The 1, 2 and 3-year OLTPFS rates were 98%, 94% and 91%, respectively (Fig. 4). After univariate analysis, no specific factor was associated with OLTP (Table 3). No cases of parietal or peritoneal tumor seeding were observed during follow-up.
DTP occurred in 31/58 patients (53.4%) during follow-up, with a median time to DTP of 15 months [range 1–65]. By adding cases of LTP and DTP, OTPFS rates, therefore, reached 77%, 58% and 44% at 1, 2 and 3 years, respectively (Online resource 1). Univariate analysis did not reveal any factors as being linked with OTP (Online resource 2).
During follow-up, 28/58 patients died (48.2%), two patients were transplanted (3.4%) and two patients (3.4%) were lost to follow-up after 1 and 19 months. The 1, 2 and 3-year OS rates determined using the Kaplan–Meier method were 86%, 73% and 62%, respectively (Online resource 3). After univariate analysis, the exophytic growth of the tumor was the only factor significantly associated with the overall survival (HR 2.96 [1.308–6.709]; P < 0.01) (Online resource 4).
Complications
One patient (1.7%) experienced a grade 3 complication: sepsis and liver failure and required transfer to the Intensive Care Unit with favorable outcome after specific therapy. Two additional patients (3.4%) experienced transient cirrhosis decompensation within one month after NTMBP-RFA with an increase in Child–Pugh score > 1 point (due to clinical ascites and/or jaundice and/or encephalopathy; CIRSE grade 2). No cases of death related to NTMBP-RFA were recorded.
At 1 month, 18 patients (31%) exhibited at least one adverse imaging finding but without any clinical significance or need for specific therapy. The most frequent findings were pleural effusion and onset or exacerbation of radiological ascites, seen in 10 (17.2%) and 8 (13.8%) patients, respectively. Among eight patients with onset or exacerbation of radiological ascites, there were three cirrhosis decompensations (see above) and one patient with an isolated thin fluid effusion next to the ablation zone. The remaining four patients had an exacerbation of preexisting radiological ascites without cirrhosis decompensation that may be unrelated to the procedure. In one patient (1.7%), the one-month CT scan revealed a hepatic subcapsular hematoma, without clinical impact or need for specific therapy. The other imaging anomalies found were segmental portal vein thrombosis in two patients (3.4%), biliary modifications (biliary duct dilatation and/or bilioma) limited to one hepatic segment in two patients (3.4%) and parietal burns (including mild necrosis of the peri-hepatic fat or muscles but not skin) in three patients (5.2%).
Discussion
The percutaneous thermal ablation of subcapsular hepatic tumors may involve a risk of major complications such as collateral damage to the diaphragm or gastrointestinal tract, hemorrhage or delayed tumor seeding [2]. Therefore, given the relatively high frequency of S-HCC (23.7% of the entire population in our study), a considerable proportion of patients with non-resectable S-HCC and whose tumors were frequently smaller than 3 cm, sometimes remain contraindicated for ablation by several teams or may experience treatment failures because of technical difficulties. In such a setting, effective solutions to overcome these specific technical limitations of percutaneous ablation are a high priority.
The subcapsular location of tumors is a widely acknowledged risk factor for delayed tumor seeding [6, 9, 14]. Tumor seeding along the needle path is especially feared when using standard mono-applicator ablative technologies (such as monopolar RFA or MWA); direct punctures through the liver capsule without the interposition of non-tumorous parenchyma are performed to reach such superficial tumors [6, 9]. By following precautionary principles such as avoiding a direct tumor puncture and thermocoagulating the puncture path during the needle withdrawal, other groups have reported much lower rates (between 0 and 3.8%) of tumor track seeding in patients treated with mono-applicator intra-tumorous techniques for HCC in a subcapsular location [8, 12, 25, 26]. However, the LTP rates reported after standard intra-tumorous single applicator ablation of S-HCC remain at around 20% [7, 8, 11, 25, 26]. Furthermore, for anterior or lateral subcapsular locations, a puncture path that initially passes through the non-tumorous liver parenchyma is often not feasible when using percutaneous approaches. These particularly risky subcapsular HCCs, therefore, continue to be seen as a contraindication to percutaneous ablation in many centers and thus have to be redirected to palliative treatments such as chemoembolization or stereotactic body radiotherapy, with lower expected local control rates [27, 28]. In our study, NTMBP-RFA of anterior and lateral subcapsular HCCs not puncturable via the non-tumorous liver parenchyma achieved 98.3% of secondary efficacy and an OLTPFS rate at 3 years of 91%, without any cases of needle tract seeding. Moreover, bleeding complications rates were very low (one subcapsular hematoma requiring no specific management). The only significant complication that we observed was a case of sepsis with liver failure which was deemed to be independent of the tumor location. These findings highlight the safety of the no-touch concept for subcapsular HCCs that are not puncturable via the non-tumorous liver parenchyma.
No risk factors for OLTP could be identified in our study probably due to the low number of events (only 6 cases of OLTP for 59 tumors). The exophytic character of tumors appeared to be negatively associated with OS, but this result should be taken with caution because only 12 patients had an exophytic nodule.
Nevertheless, Park et al. [29] recently reported the excellent local control of superficial liver tumors in 15 patients (7 with anterior locations) without any major complications when using monopolar RFA with a single manually angled electrode. It should be noted that the maximum size of the nodules ablated was 26 mm. When compared with such intra-tumorous mono-applicator technique, the need to insert multiple probes in order to perform NTMBP-RFA is clearly counterbalanced by the latter’s ability to induce a large and homogeneous single-block ablation that includes the index tumor to up to 5 cm with safety margins [30, 31]. This point is critical because it has been reported that the use of intra-tumorous mono-applicator ablative techniques involves an increased risk of tumor seeding and hemorrhage, particularly when treating large subcapsular tumors, that frequently require multiple overlapped ablations and/or multiple additional procedures [6, 10, 25, 32]. Interestingly, the no-touch ablation concept has been applied in few studies using multi-applicator monopolar RFA or MWA for the treatment of subcapsular tumors [33,34,35]. Indeed, no-touch ablation can be performed using such centrifugal ablative techniques, but when compared to the multi-bipolar RFA mode, the predictability of the extent and shape of ablation zones and ultimately the safety of the procedure are questionable because of the numerous types of repositioning required in order to fully comply with the no-touch concept [33, 36, 37].
The risk of thermal injury to critical extra-hepatic structures is also a major safety issue when ablating subcapsular liver tumors. In this setting, the laparoscopic ablation has been supported as being the safest approach for subscapular nodules [12, 38]. However, hydro-dissection with artificial ascites enables a safe RFA for superficial tumors because it both separates the targeted tumor from critical structures and increases its visibility under ultrasound [25]. In our study, the no-touch technique combined with artificial ascites (when required) appeared to be notably safe regarding this specific risk. The no-touch cutting strategy was particularly appropriate for subcapsular tumors that were attached to the liver by only a thin band of parenchyma. The superficial tumorous component located closest to critical extra-hepatic structures could be ablated without direct energy deposition; thanks to vascular deprivation. Interestingly, when comparing superficial no-touch RFA using the bipolar versus the monopolar mode in an experimental liver model, Chang et al. [33] showed that the extent of capsular burn outside the electrode field was significantly more limited in the bipolar mode. These results were in line with those reported in an animal study by Kawamura et al. [39] who used the same multi-bipolar RFA device and achieved highly predictable wedge ablations of subcapsular liver tumors without inducing thermal damage to neighboring extra-hepatic structures.
Our study has several limitations, mostly relative to its retrospective and non-comparative design. Furthermore, the number of cases was smaller than those reported in the previous studies focused on the same topic. However, this was due to the fact that we deliberately restricted our analysis to subcapsular HCCs not puncturable via the non-tumorous liver parenchyma.
Conclusion
NTMBP-RFA can broaden the spectrum of subcapsular hepatic tumors that are amenable to ablation by avoiding the direct tumor punctures that are often inevitable with mono-applicator ablative technologies, and particularly when treating tumors in anterior and lateral locations. In addition, thanks to the centripetal energy deposition between dipolar combinations of electrodes; NTMBP-RFA can reduce the risk of thermal injuries to extra-hepatic structures located near the ablation zone. Further studies are still needed to confirm the good efficacy and safety profile of NTMBP-RFA applied in such difficult cases.
References
Liver EA for the S of the, Cancer EO for R and T of. EASL–EORTC Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43.
Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–51.
Bertot LC, Sato M, Tateishi R, Yoshida H, Koike K. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: a systematic review. Eur Radiol. 2011;21:2584–96.
Giorgio A, Merola MG, Montesarchio L, Merola F, Gatti P, Coppola C, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma in cirrhosis: analysis of complications in a single centre over 20 years. Br J Radiol. 2017;90:20160804.
Rhim H, Yoon K-H, Lee JM, Cho Y, Cho J-S, Kim SH, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiogr Rev Publ Radiol Soc N Am Inc. 2003;23:123–34 discussion 134–6.
Jaskolka JD, Asch MR, Kachura JR, Ho CS, Ossip M, Wong F, et al. Needle tract seeding after radiofrequency ablation of hepatic tumors. J Vasc Interv Radiol. 2005;16:485–91.
Kang TW, Lim HK, Lee MW, Kim Y-S, Rhim H, Lee WJ, et al. Long-term therapeutic outcomes of radiofrequency ablation for subcapsular versus nonsubcapsular hepatocellular carcinoma: a propensity score matched study. Radiology. 2016;280:300–12.
Kim YJ, Raman SS, Yu NC, Busuttil RW, Tong M, Lu DSK. Radiofrequency ablation of hepatocellular carcinoma: can subcapsular tumors be safely ablated? AJR Am J Roentgenol. 2008;190:1029–34.
Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatol Baltim Md. 2001;33:1124–9.
Livraghi T, Lazzaroni S, Meloni F, Solbiati L. Risk of tumour seeding after percutaneous radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 2005;92:856–8.
Sartori S, Tombesi P, Macario F, Nielsen I, Tassinari D, Catellani M, et al. Subcapsular liver tumors treated with percutaneous radiofrequency ablation: a prospective comparison with nonsubcapsular liver tumors for safety and effectiveness. Radiology. 2008;248:670–9.
Poon RT-P, Ng KK-C, Lam C-M, Ai V, Yuen J, Fan S-T. Radiofrequency ablation for subcapsular hepatocellular carcinoma. Ann Surg Oncol. 2004;11:281–9.
Francica G, Meloni MF, de Sio I, Smolock AR, Brace CL, Iadevaia MD, et al. Radiofrequency and microwave ablation of subcapsular hepatocellular carcinoma accessed by direct puncture: safety and efficacy. Eur J Radiol. 2016;85:739–43.
Zhong-yi Z, Wei Y, Kun Y, Ying D, Wei W, Jung-chieh L, et al. Needle track seeding after percutaneous radiofrequency ablation of hepatocellular carcinoma: 14-year experience at a single centre. Int J Hyperth. 2017;33:454–8.
Bonny C, Abergel A, Gayard P, Chouzet S, Ughetto S, Slim K, et al. Radiofrequency ablation of hepatocellular carcinoma in patients with cirrhosis. Gastroentérol Clin Biol. 2002;1220:665.
Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Interv Radiol. 2010;33:11–7.
Seror O, N’Kontchou G, Nault J-C, Rabahi Y, Nahon P, Ganne-Carrié N, et al. Hepatocellular carcinoma within Milan criteria: no-touch multibipolar radiofrequency ablation for treatment-long-term results. Radiology. 2016;280:981.
Seror O. Percutaneous hepatic ablation: what needs to be known in 2014. Diagn Interv Imaging. 2014;95:665–75.
Seror O, N’Kontchou G, Ibraheem M, Ajavon Y, Barrucand C, Ganne N, et al. Large (>or=5.0-cm) HCCs: multipolar RF ablation with three internally cooled bipolar electrodes–initial experience in 26 patients. Radiology. 2008;248:288–96.
Mohkam K, Dumont P-N, Manichon A-F, Jouvet J-C, Boussel L, Merle P, et al. No-touch multibipolar radiofrequency ablation vs. surgical resection for solitary hepatocellular carcinoma ranging from 2 to 5 cm. J Hepatol. 2018;68:1172–80.
N’Kontchou G, Nault J-C, Sutter O, Bourcier V, Coderc E, Grando V, et al. Multibipolar radiofrequency ablation for the treatment of mass-forming and infiltrative hepatocellular carcinomas > 5 cm: long-term results. Liver Cancer. 2018;1–14.
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2.
Motoyama T, Ogasawara S, Chiba T, Higashide T, Yokota H, Kanogawa N, et al. Coronal reformatted CT images contribute to the precise evaluation of the radiofrequency ablative margin for hepatocellular carcinoma. Abdom Imaging. 2014;39:262–8.
Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc Interv Radiol. 2017;40:1141–6.
Kang TW, Lim HK, Lee MW, Kim Y-S, Choi D, Rhim H. First-line radiofrequency ablation with or without artificial ascites for hepatocellular carcinomas in a subcapsular location: local control rate and risk of peritoneal seeding at long-term follow-up. Clin Radiol. 2013;68:e641–51.
Patidar Y, Singhal P, Gupta S, Mukund A, Sarin SK. Radiofrequency ablation of surface v/s intraparenchymal hepatocellular carcinoma in cirrhotic patients. Indian J Radiol Imaging. 2017;27:496.
Hocquelet A, Seror O, Blanc J-F, Frulio N, Salut C, Nault J-C, et al. Transarterial chemoembolization for early stage hepatocellular carcinoma decrease local tumor control and overall survival compared to radiofrequency ablation. Oncotarget. 2017;8:32190.
Mohamed M, Katz AW, Tejani MA, Sharma AK, Kashyap R, Noel MS, et al. Comparison of outcomes between SBRT, yttrium-90 radioembolization, transarterial chemoembolization, and radiofrequency ablation as bridge to transplant for hepatocellular carcinoma. Adv Radiat Oncol. 2016;1:35–42.
Park SI, Kim IJ, Lee SJ, Shin MW, Shin W, Chung YE, et al. Angled cool-tip electrode for radiofrequency ablation of small superficial subcapsular tumors in the liver: a feasibility study. Korean J Radiol. 2016;17:742.
Hocquelet A, Aubé C, Rode A, Cartier V, Sutter O, Manichon AF, et al. Comparison of no-touch multi-bipolar vs. monopolar radiofrequency ablation for small HCC. J Hepatol. 2017;66:67–74.
Seror O, N’Kontchou G, Van Nhieu JT, Rabahi Y, Nahon P, Laurent A, et al. Histopathologic comparison of monopolar versus no-touch multipolar radiofrequency ablation to treat hepatocellular carcinoma within Milan criteria. J Vasc Interv Radiol. 2014;25:599–607.
Stigliano R, Marelli L, Yu D, Davies N, Patch D, Burroughs AK. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Cancer Treat Rev. 2007;33:437–47.
Chang W, Lee JM, Lee SM, Han JK. No-touch radiofrequency ablation: a comparison of switching bipolar and switching monopolar ablation in ex vivo bovine liver. Korean J Radiol. 2017;18:279.
Chang W, Lee JM, Yoon JH, Lee DH, Lee SM, Lee KB, et al. No-touch radiofrequency ablation using multiple electrodes: an in vivo comparison study of switching monopolar versus switching bipolar modes in porcine livers. PLoS ONE. 2017;12:e0176350.
Patel PA, Ingram L, Wilson IDC, Breen DJ. No-touch wedge ablation technique of microwave ablation for the treatment of subcapsular tumors in the liver. J Vasc Interv Radiol. 2013;24:1257–62.
Nault J-C, Sutter O, Nahon P, Ganne-Carrié N, Séror O. Percutaneous treatment of hepatocellular carcinoma: state of the art and innovations. J Hepatol. 2018;68:783–97.
Ziegle J, Audigier C, Krug J, Ali G, Kim Y, Boctor EM, et al. RF-ablation pattern shaping employing switching channels of dual bipolar needle electrodes: ex vivo results. Int J Comput Assist Radiol Surg. 2018;13:905–16.
de la Serna S, Vilana R, Sánchez-Cabús S, Calatayud D, Ferrer J, Molina V, et al. Results of laparoscopic radiofrequency ablation for HCC. Could the location of the tumour influence a complete response to treatment? A single European centre experience. HPB. 2015;17:387–93.
Kawamura Y, Ikeda K, Fukushima T, Hara T, Hosaka T, Kobayashi M, et al. Potential of a no-touch pincer ablation procedure for small hepatocellular carcinoma that uses a multipolar radiofrequency ablation system: an experimental animal study: no-touch ablation procedure for hepatic tumors. Hepatol Res. 2014;44:1234–40.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Thisstudy has obtained approval from IRB and the need for informed consent was waived.
Consent for Publication
For this type of study, consent for publication is not required
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Petit, A., Hocquelet, A., N’kontchou, G. et al. No-Touch Multi-bipolar Radiofrequency Ablation for the Treatment of Subcapsular Hepatocellular Carcinoma ≤ 5 cm Not Puncturable via the Non-tumorous Liver Parenchyma. Cardiovasc Intervent Radiol 43, 273–283 (2020). https://doi.org/10.1007/s00270-019-02357-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-019-02357-9