Abstract
Purpose
Percutaneous radiofrequency ablation (RFA) and microwave ablation (MWA) are well-validated interventions for hepatocellular carcinoma (HCC). The purpose of this study was to compare their safety and efficacy through a meta-analysis of randomized controlled trials (RCT).
Methods
MEDLINE, Pubmed, and the Cochrane Library were queried up to September 2020 using the terms “microwave”, “radiofrequency”, “hepatocellular”, and “randomized”. Only RCTs investigating MWA versus RFA for HCC were included. Baseline study characteristics, complete ablation rate, ablation time, overall survival, local recurrence, and complication rates were investigated.
Results
Among the five original studies included, a total of 413 and 431 patients were treated with RFA and MWA, respectively. All studies focused on very early and early-stage HCC only (Barcelona Clinic Liver Cancer Stage 0 and A). No statistical significance was observed in terms of complete ablation rate (96.7 vs 96.9%, p = 0.882), overall survival (6 month: 95.7 vs 100%, p = 0.492; 1 year: 91.9 vs 94.1%, p = 0.264; 3 year: 77.5 vs 78.4%, p = 0.905), recurrence-free survival (6 month: 99.1 vs 99.7%, p = 0.717; 1 year: 94.6 vs 93.9%, p = 0.675; 3 year: 76.8 vs 77.1%, p = 0.935), and complication rates (p > 0.05 in all types). The mean ablation time of MWA was significantly shorter than RFA (26.9 vs 14.1 min, p < 0.001).
Conclusion
For very early and early-stage HCC, RFA and MWA are equally safe and effective, though the former is associated with a longer ablation time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of death among all cancer types world-wide [1]. Percutaneous thermal ablation is universally accepted as a minimally invasive therapy for HCC as either definitive treatment or bridge to transplant [2]. Historically, radiofrequency ablation (RFA) has been the most common modality utilized for percutaneous liver ablation [3]. Microwave ablation (MWA), on the contrary, has recently gained popularity for its efficiency, simplicity, and ability to lessen the risk of “charring” and “heat-sink” effects [4]. Consequently, the use of MWA in the US has risen dramatically over the last decade, with a foreseeable market predominance over RFA by more than sevenfolds within the next 5 years [3].
In terms of clinical outcomes, current evidence comparing the two modalities are mostly retrospective in nature [5]. This data is also conflicted with respect toward treatment measures such as the complete ablation rate, overall survival, recurrence, and risk for complication [6,7,8,9,10]. Furthermore, several published meta-analyses on this topic are similarly comprised of retrospective studies, which are inherently prone to selection and measuring biases [5, 11, 12]. Fortunately, randomized clinical trials (RCT) comparing the two modalities have been published recently [13, 14]. The goal of the current study was to perform an updated meta-analysis using RCTs only, comparing the safety and therapeutic efficacy of percutaneous RFA and MWA in the treatment of localized HCCs (BCLC 0 and A).
Methods
Searching strategy and study screening
MEDLINE, Embase and the Cochrane Library were queried from establishment to September 2020 without language restrictions. RCTs comparing hepatic MWA and RFA were identified with keywords: Pubmed: “microwave” AND “radiofrequency” AND “hepatocellular” AND (“random” OR “randomized”) and MeSH terms ((radiofrequency ablation[MeSH Terms]) AND (microwaves[MeSH Terms])) AND (randomized controlled trial[MeSH Terms]); Embase: microwave AND radiofrequency AND hepatocellular AND hepatocellular AND random OR randomized and Emtree terms “‘radiofrequency ablation’/exp AND ‘microwave ablation device’/exp AND ‘randomized controlled trial’/exp”; Cochrane Library: radiofrequency microwave hepatocellular randomized. The following inclusion criteria were adopted: (a) RCTs comparing percutaneous RFA and MWA of HCC; (b) outcomes were reported: complete ablation rate (CA), overall survival (OS), local tumor recurrence-free survival (RFS), and/or procedure-related complications. Exclusion criteria were: (a) non-RCT (case report, case series, retrospective studies, non-randomized prospective studies); (b) pre-clinical experiments of non-human subjects; (c) review, meta-analysis, editorial, commentary, or letter without original data; d) studies containing patient samples used by more than 1 study; (e) abstract or conference paper without full text.
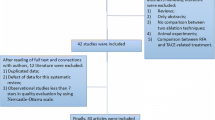
Endnote X8 (Clarivate Analytics, Philadelphia, Pennsylvanian) was implemented to identify duplicates and screen studies. Titles and abstracts were screened initially, followed by reviewing full texts of remaining studies (Fig. 1).
Data extraction and statistical analysis
Baseline information were extracted from each study: author, year of publication, country, patient number, age, nodule number, lesion size, Barcelona Clinic Liver Cancer staging (BCLC), CA, ablation time, OS, RFS, and complication. Quality assessment was performed using the Cochrane Collaboration’s tool for RCTs (S.Table 1). Two researchers screened and extracted the data from the original studies. Any disagreement was discussed and resolved by consensus. Missing outcome data (CA, OS, RFS, and complication rates) were inquired by attempting to contact the authors of the original studies via email.
Per-patient analysis was used for OS, RFS, and complication rate. Per-tumor analysis was used for CA. Per-session analysis was used for ablation time. A lesion was considered completely ablated if CT/MRI at 1-month post procedural follow-up did not show any residual disease within the ablation cavity. RFS was calculated based on the time when local hepatic recurrence occurred post-ablation.
Statistical analysis was performed with Stata 15.1 (STATA Corp., College Station, TX, USA). Meta-analysis was conducted with the -metan function. A fixed-effect model was used if heterogeneity I2 ≤ 30. A random-effect model was used if I2 > 30. CA, OS, and RFS were analyzed with odds ratio (OR) and 95% confidence interval (CI). Ablation time was analyzed with standardized mean difference (SMD). Forest plots were generated. Publication bias was evaluated with funnel plot and Egger’s test. Sensitivity analysis was performed using the -metaninf function (the one-study removal approach).
Results
Baseline characteristics of included studies
The initial search identified 162 unique studies (Fig. 1). After screening based on title, abstract, and full text, 5 RCTs containing 413 RFA and 431 MWA patients were included in the present meta-analysis [13,14,15,16,17,18] (Table 1). Sheta et al. [16] randomized patients to combined therapy of RFA or MWA and transarterial chemoembolization (TACE), and thus was excluded from the quantitative analysis. These studies were conducted in Egypt (n = 2), China (n = 1), Europe (n = 1), and Japan (n = 1). A total of 489 and 519 HCCs with average sizes of 2.4 and 2.5 cm were ablated via RFA and MWA, respectively. All RCTs in the meta-analysis assigned patients to either MWA or RFA only. Despite the slight variations among the inclusion criteria of individuals studies, most studies only enrolled patients who were either Child–Pugh A or B, without evidence of extrahepatic disease, and without portal vein thrombus, which were consistent with BCLC Stage 0 or A (S.Table 2). The pre-procedural baseline lab values and details regarding the ablation systems are recorded in S.Table 2. All procedures were conducted under ultrasound (US)-guidance with the exception of Violi et al. [14], in which a computed tomography (CT) was also implemented in a portion of the enrolled patients.
Complete ablation
The CA was reported by 5 studies [13,14,15, 17, 18] (Fig. 2), none of which implemented combined embolotherapy at the time of treatment. Overall, CA was achieved in 96.7% (range 88.2–98.8%) of the tumor ablated via RFA and 96.9% (89.1% and 99.6%) via MWA. No statistically significant difference was observed between these groups (Fig. 2a, OR: 0.948 [95% CI 0.466–1.927], p = 0.882).
Procedure time
Three studies reported the average ablation time per ablation session [13, 15, 18] (Fig. 3). On average, 26.9 and 14.1 min was required per each RFA and MWA session, respectively. The amount of ablation time required by MWA was statistically shorter than RFA (SMD: 1.696 [1.439–1.954], p < 0.001).
Overall survival
OS was reported by 4 studies [13, 14, 17, 18] (Fig. 4), all of which focused on the treatment of HCC with MWA or RFA only. In the RFA group, the OS at 6 month, 1 year, and 3 year were 95.7%, 91.9%, and 77.5%, respectively; the OS of the MWA were comparable at 6 month (100.0%), 1 year (94.1%), and 3 year (78.4%). The OS was not statistically significant between two groups at 6 month (OR: 0.324 [95 CI 0.013–8.086], p = 0.492), 1 year (OR: 0.705, [95% CI 0.382–1.301], p = 0.264), 3 year (OR: 0.972 [95% CI 0.615–1.538], p = 0.905).
Overall survival between RFA and MWA at 6 month (OR: 0.324 [95 CI 0.013–8.086], p = 0.492), 1 year (OR: 0.705, [95% CI 0.382–1.301], p = 0.264) and 3 year (0.972 [OR: 95% CI 0.615–1.538], p = 0.905). Fixed-effect model. CI: confidence interval. MWA: microwave ablation. RFA: radiofrequency ablation. OR: odds ratio
Recurrence-free survival
Information regarding RFS was investigated in 3 studies [13, 14, 18] (Fig. 5). The pooled RFS at 6 month, 1 year, and 3 year were 99.1%, 94.6%, and 76.8% in RFA group, whereas it was 99.7%, 93.9%, and 77.1% in the MWA group. No statistical significance was observed between two groups at 6 month (OR: 0.69 [95% CI 0.09–5.23], p = 0.717), 1 year (OR: 1.167 [95% CI 0.568–2.396], p = 0.675), and 3 year (OR: 0.981 [95% CI 0.616–1.562], p = 0.935).
Local hepatic recurrence between RFA and MWA at 6-month (OR: 0.69 [95% CI 0.09–5.23], p = 0.717), 1 year (OR: 1.167 [95% CI 0.568–2.396], p = 0.675), and 3 year (OR: 0.981 [95% CI 0.616–1.562], p = 0.935). Fixed-effect model. CI: confidence interval. MWA: microwave ablation. RFA: radiofrequency ablation. OR: odds ratio
Procedure-related complications
Among studies with reported incidences (Table 2), no statistical significance was observed between RFA and MWA regarding subcapsular hematoma [14, 15, 17] (4.9% vs 2.0%, p = 0.159), major bleeding requiring embolization [13, 14] (0.0% vs 2.4%, p = 0.198), segmental necrosis [14, 15] (1.4% vs 0.0%, p = 0.352), skin burn [15, 17] (1.2% vs 2.0%, p = 0.756), local pain [13, 14] (11.4% vs 10.3%, p = 0.654). Major complications requiring medical interventions include gastrointestinal bleeding, needle seeding, bulk pleural effusion, severe hepatic decompensation, and liver abscess [13,14,15, 18]; there was not statistical difference between two groups (1.9% vs 3.8%, p = 0.138). Iatrogenic injuries to the neighboring abdominal viscera were not reported.
Subgroup analysis
Due to the limited number of included studies and reported outcomes, subgroup analyses were performed selectively. In terms of technical efficacy (complete ablation rate), subgroup analyses of tumor size > 3 cm and treatment technique (i.e., ultrasound versus CT-guided ablation) did not reveal statistical differences between the two treatment groups (S.Table 3).
Publication bias and sensitivity analysis
No asymmetry was observed on funnel plots regarding complete ablation rates, ablation time, 1-year OS, and 1-year RFS (S. Figure 1 p = 0.305), and the Egger’s tests were p = 0.305, 0.115, 0.391, and 0.426, respectively. Results of the sensitivity analyses indicated that the elimination of studies did not affect the results (S. Figure 2).
Discussion
RFA and MWA are heat-based thermal technologies with differing mechanisms of action. RFA causes coagulation necrosis through frictional heat generated from an alternating current; by contrast, MWA induces rigorous movement of polar molecules (i.e., water) in tissue through electromagnetic energy, causing tumor necrosis and cell death [4, 19, 20]. As a result, MWA is less influenced by tissue type, conductance, or location. Unlike RFA, MWA is theoretically less susceptible to charring or heat-sink and performs equally for perivascular and non-perivascular tumors [6]. Further, MWA has been shown in pre-clinical and clinical models to produce persistently high temperature throughout the ablation cavity, effectively creating larger and more predictable ablation zones [4, 21, 22]. Several retrospective studies have reported that MWA can provide a durable tumor-free response for HCC within Milan criteria. [23, 24] MWA also does not require creation of a closed circuit, which simplifies the operating system [25]. In the present meta-analysis of RCTs, the ablation time of RFA was significantly longer than MWA (26.9 vs 14.1 min, p < 0.001), while both achieved similarly high CA (89.1% and 99.6%, p = 0.882).
Based on a consensus societal guidelines [26, 27], curative percutaneous thermal ablation is most effective in patients with ≤ 3 nodules that are ≤ 3 cm or a single lesion ≤ 5 cm (i.e., the Milan criteria). The average tumor diameters of the included studies were 2.6 cm in both RFA and MWA groups, respectively. For this reason, the advantage of MWA in energy penetration might be masked by the relatively small lesions among patients included in the present meta-analysis, resulting in comparable cumulative CR rates. In terms of clinical outcomes, both OS and RFS were analyzed in a time-dependent manner, demonstrating no significant differences between MWA and RFA at 6-month, 1-year, and 3-year follow-up (Figs. 4, 5). These findings suggested that MWA and RFA are equally effective in the management of early and very early-stage HCC.
In percutaneous thermal ablation, the tumoricidal effect along the ablation cavity’s margin declines as the tumor size increases. With limited evidence available, subgroup analyses using a 3 cm cutoff did not demonstrate a significant advantage for MWA over RFA in achieving CA of these larger lesions (S.Table 3). As for lesions > 5 cm, additional therapies such as TACE or radioembolization (TARE) can be performed to enhance therapeutic efficacy [28]. Previous literature indicated that MWA + TACE was equally efficacious as RFA + TACE at inducing tumor necrosis, CA, and survival outcomes [8, 9]. Only one RCT enrolled patients with solitary tumors ≥ 5 cm who also received combinational treatment with TACE [16]. No statistical difference was observed between RFA and MWA groups in terms of local recurrence at 1-, 3-, and 6-month, though ablative cavity coverage, overall survival, and long-term outcomes beyond 6 months were not reported.
In practice, the most concerning procedure-related complications associated with percutaneous thermal ablation is iatrogenic injuries to the major vessels or neighboring structures. No statistical significance was noted between MWA and RFA in terms of subcapsular hematoma or segmental necrosis/infarction (Table 2). Injury of the bowel was not observed. It was speculated that a higher skin burn rate could be associated with RFA, because an additional ground pad had to be applied on the surface of patient’s skin to establish a closed circuit [29]. This type of complication was, however, not statistically significant between the two groups (Table 2).
The present meta-analysis should be interpreted with several caveats. First, the treatment efficacy can be influenced by the type of image guidance used, such as US, CT, or even MRI [30, 31]. The majority of included original studies were US-guided, while CT guidance could be more operator-friendly in deeper lesions that were poorly visualized on US [32]. Subgroup analysis of the CT-guided ablation only was not possible. Second, the average size of the included nodules was rather small. Differences for efficacy between MWA and RFA may be more evident in larger tumors. As such, the results of the present meta-analysis mostly apply to patients with early and very early-stage HCCs. Finally, most of the included studies did not enroll patients with tumors located near major vessels, abdominal viscera, gallbladder, etc. In practice, the “heat-sink” effects could be more evident in RFA than MWA under these scenarios.
In conclusion, RFA and MWA are equally safe and effective in the treatment of early-stage and very early-stage HCCs. The CA, OS, RFS, and procedural-related complication rates are comparable between two treatment methods, though RFA requires longer ablation time. Future RCTs with longer follow-up can be designed to compare MWA and RFA of larger sizes (with or without combination therapies such as embolization) for the treatment of HCC beyond Milan Criteria.
Availability of data and material
The present study is a meta-analysis of publish articles.
Abbreviations
- CA:
-
Complete ablation rate
- HCC:
-
Hepatocellular carcinoma
- MWA:
-
Microwave ablation
- OS:
-
Overall survival
- RCT:
-
Randomized controlled trial
- RFA:
-
Radiofrequency ablation
- RFS:
-
Recurrence-free survival
References
J.D. Yang, L.R. Roberts, Epidemiology and management of hepatocellular carcinoma, Infectious Disease Clinics, 24 (2010) 899-919.
S. Shiina, Image-guided percutaneous ablation therapies for hepatocellular carcinoma, Journal of gastroenterology, 44 (2009) 122-131.
V. Patel, C.A. Ritchie, C. Padula, J.M. McKinney, Radiofrequency Ablation, Where It Stands in Interventional Radiology Today, Seminars in Interventional Radiology, Thieme Medical Publishers, 2019, pp. 398-404.
L.S. Poulou, E. Botsa, I. Thanou, P.D. Ziakas, L. Thanos, Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma, World journal of hepatology, 7 (2015) 1054.
M.B. Glassberg, S. Ghosh, J.W. Clymer, R.A. Qadeer, N.C. Ferko, B. Sadeghirad, G.W. Wright, J.F. Amaral, Microwave ablation compared with radiofrequency ablation for treatment of hepatocellular carcinoma and liver metastases: a systematic review and meta-analysis, OncoTargets and therapy, 12 (2019) 6407.
A.A. van Tilborg, H.J. Scheffer, M.C. de Jong, L.G. Vroomen, K. Nielsen, C. van Kuijk, P.M. van den Tol, M.R. Meijerink, MWA versus RFA for perivascular and peribiliary CRLM: a retrospective patient-and lesion-based analysis of two historical cohorts, Cardiovascular and interventional radiology, 39 (2016) 1438-1446.
W. Shady, E.N. Petre, K.G. Do, M. Gonen, H. Yarmohammadi, K.T. Brown, N.E. Kemeny, M. D’Angelica, P.T. Kingham, S.B. Solomon, Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control, Journal of Vascular and Interventional Radiology, 29 (2018) 268-275. e261.
R. Vasnani, M. Ginsburg, O. Ahmed, T. Doshi, J. Hart, H. Te, T.G. Van Ha, Radiofrequency and microwave ablation in combination with transarterial chemoembolization induce equivalent histopathologic coagulation necrosis in hepatocellular carcinoma patients bridged to liver transplantation, Hepatobiliary surgery and nutrition, 5 (2016) 225.
M. Ginsburg, S.P. Zivin, K. Wroblewski, T. Doshi, R.J. Vasnani, T.G. Van Ha, Comparison of combination therapies in the management of hepatocellular carcinoma: transarterial chemoembolization with radiofrequency ablation versus microwave ablation, Journal of vascular and interventional radiology, 26 (2015) 330-341.
K. Ohmoto, N. Yoshioka, Y. Tomiyama, N. Shibata, T. Kawase, K. Yoshida, M. Kuboki, S. Yamamoto, Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas, Journal of gastroenterology and hepatology, 24 (2009) 223-227.
Y.R. Huo, G.D. Eslick, Microwave ablation compared to radiofrequency ablation for hepatic lesions: a meta-analysis, Journal of Vascular and Interventional Radiology, 26 (2015) 1139-1146. e1132.
W. Tan, Q. Deng, S. Lin, Y. Wang, G. Xu, Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis, International Journal of Hyperthermia, 36 (2019) 264-272.
A. Kamal, A.A.A. Elmoety, Y.A.M. Rostom, M.S. Shater, S.A. Lashen, Percutaneous radiofrequency versus microwave ablation for management of hepatocellular carcinoma: a randomized controlled trial, Journal of gastrointestinal oncology, 10 (2019) 562-571.
N. Vietti Violi, R. Duran, B. Guiu, J.P. Cercueil, C. Aube, A. Digklia, I. Pache, P. Deltenre, J.F. Knebel, A. Denys, Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial, The lancet. Gastroenterology & hepatology, 3 (2018) 317-325.
T. Shibata, Y. Iimuro, Y. Yamamoto, Y. Maetani, F. Ametani, K. Itoh, J. Konishi, Small hepatocellular carcinoma: Comparison of radio-frequency ablation and percutaneous microwave coagulation therapy, Radiology, 223 (2002) 331-337.
E. Sheta, F. El-Kalla, M. El-Gharib, A. Kobtan, M. Elhendawy, S. Abd-Elsalam, L. Mansour, I. Amer, Comparison of single-session transarterial chemoembolization combined with microwave ablation or radiofrequency ablation in the treatment of hepatocellular carcinoma: a randomized-controlled study, European journal of gastroenterology & hepatology, 28 (2016) 1198-1203.
A. Abdelaziz, T. Elbaz, H.I. Shousha, S. Mahmoud, M. Ibrahim, A. Abdelmaksoud, M. Nabeel, Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an Egyptian multidisciplinary clinic experience, Surgical endoscopy, 28 (2014) 3429-3434.
J. Yu, X.L. Yu, Z.Y. Han, Z.G. Cheng, F.Y. Liu, H.Y. Zhai, M.J. Mu, Y.M. Liu, P. Liang, Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial, Gut, 66 (2017) 1172-1173.
F. Izzo, V. Granata, R. Grassi, R. Fusco, R. Palaia, P. Delrio, G. Carrafiello, D. Azoulay, A. Petrillo, S.A. Curley, Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update, Oncologist, 24 (2019).
A. Facciorusso, G. Serviddio, N. Muscatiello, Local ablative treatments for hepatocellular carcinoma: an updated review, World journal of gastrointestinal pharmacology and therapeutics, 7 (2016) 477.
F. Primavesi, S. Swierczynski, E. Klieser, T. Kiesslich, T. Jäger, R. Urbas, J. Hutter, D. Neureiter, D. Öfner, S. Stättner, Thermographic real-time-monitoring of surgical radiofrequency and microwave ablation in a perfused porcine liver model, Oncology letters, 15 (2018) 2913-2920.
G.-J. Qian, N. Wang, Q. Shen, Y.H. Sheng, J.-Q. Zhao, M. Kuang, G.-J. Liu, M.-C. Wu, Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: experimental and clinical studies, European radiology, 22 (2012) 1983-1990.
T.A. Potretzke, T.J. Ziemlewicz, J.L. Hinshaw, M.G. Lubner, S.A. Wells, C.L. Brace, P. Agarwal, F.T. Lee Jr, Microwave versus radiofrequency ablation treatment for hepatocellular carcinoma: a comparison of efficacy at a single center, Journal of Vascular and Interventional Radiology, 27 (2016) 631-638.
W. Liu, Y. Zheng, W. He, R. Zou, J. Qiu, J. Shen, Z. Yang, Y. Zhang, C. Wang, Y. Wang, Microwave vs radiofrequency ablation for hepatocellular carcinoma within the Milan criteria: a propensity score analysis, Alimentary pharmacology & therapeutics, 48 (2018) 671-681.
D.M. Lloyd, K.N. Lau, F. Welsh, K.-F. Lee, D.J. Sherlock, M.A. Choti, J.B. Martinie, D.A. Iannitti, International multicentre prospective study on microwave ablation of liver tumours: preliminary results, HPB, 13 (2011) 579-585.
J.K. Heimbach, L.M. Kulik, R.S. Finn, C.B. Sirlin, M.M. Abecassis, L.R. Roberts, A.X. Zhu, M.H. Murad, J.A. Marrero, AASLD guidelines for the treatment of hepatocellular carcinoma, Hepatology (Baltimore, Md.), 67 (2018) 358-380.
E.A.F.T.S.O.T. Liver, EASL clinical practice guidelines: management of hepatocellular carcinoma, Journal of hepatology, 69 (2018) 182-236.
S. Young, J. Golzarain, Locoregional Therapies in the Treatment of 3-to 5-cm Hepatocellular Carcinoma: Critical Review of the Literature, American Journal of Roentgenology, (2020) 1-12.
D. Haemmerich, D.J. Schutt, Sequential activation of multiple grounding pads reduces skin heating during radiofrequency tumor ablation, International Journal of Hyperthermia, 23 (2007) 555-566.
S. Clasen, H. Rempp, R. Hoffmann, H. Graf, P.L. Pereira, C.D. Claussen, Image-guided radiofrequency ablation of hepatocellular carcinoma (HCC): is MR guidance more effective than CT guidance?, European Journal of Radiology, 83 (2014) 111-116.
Y. Minami, N. Nishida, M. Kudo, Therapeutic response assessment of RFA for HCC: contrast-enhanced US, CT and MRI, World Journal of Gastroenterology: WJG, 20 (2014) 4160.
A. Kambadakone, V. Baliyan, H. Kordbacheh, R.N. Uppot, A. Thabet, D.A. Gervais, R.S. Arellano, Imaging guided percutaneous interventions in hepatic dome lesions: Tips and tricks, World journal of hepatology, 9 (2017) 840.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, Q., Liu, C., Navuluri, R. et al. Percutaneous microwave ablation versus radiofrequency ablation of hepatocellular carcinoma: a meta-analysis of randomized controlled trials. Abdom Radiol 46, 4467–4475 (2021). https://doi.org/10.1007/s00261-021-03080-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-03080-1