Abstract
Objectives
To assess the prognostic value of Alberta Stroke Program Early Computed Tomography Score (ASPECTS) on post-treatment diffusion-weighted imaging (DWI) for acute ischemic stroke (AIS) patients after endovascular thrombectomy (EVT) and compare it with that of infarction volume.
Methods
Ninety-eight consecutive AIS patients who underwent EVT and post-treatment DWI were retrospectively enrolled. ASPECTS and infarction volume were evaluated based on post-treatment DWI, respectively. Good clinical outcome was defined as modified Rankin Scale score of 0–2 at 90 days. Predictors of good clinical outcome were evaluated using univariate and multivariate logistic regression analysis. Prognostic value of post-treatment DWI ASPECTS and infarction volume were assessed and compared using receiver-operating-characteristic curves and the DeLong method.
Results
Favorable outcome was achieved in 62 (63.3%) patients. A strong correlation was found between post-treatment DWI ASPECTS and infarction volume (ρ = −0.847). Due to strong correlation and potential collinearity, two multivariate logistic regression models were respectively developed which included post-treatment DWI ASPECTS or infarction volume. As a result, post-treatment DWI ASPECTS (OR, 2.401; 95%CI, 1.567–3.678; p < 0.001) and infarction volume (OR, 0.982; 95%CI, 0.846–0.998; p = 0.002) were both independent predictors of good clinical outcome. Setting post-treatment DWI ASPECTS ≥ 6 as a cut-off value, optimal performance (AUC = 0.836; sensitivity, 87.1%; specificity, 66.7%) could be obtained in predicting good clinical outcome, which was comparable with that of infarction volume (cut-off volume, ≤ 94.87 ml; AUC = 0.821; sensitivity, 90.3%; specificity, 55.6%).
Conclusions
Post-treatment DWI ASPECTS might be a potential surrogate of infarction volume and be effective in predicting the clinical outcome of AIS patients after EVT.
Key Points
• Post-treatment DWI ASPECTS correlated significantly with infarction volume.
• A post-treatment DWI ASPECTS ≥ 6 best predicts good outcomes for AIS patients after EVT.
• Post-treatment DWI ASPECTS has the potential in substituting infarction volume in predicting the clinical outcome of AIS patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endovascular thrombectomy (EVT) has been proven to benefit acute ischemic stroke (AIS) patients with large vessel occlusion in anterior circulation, as long as 24 h after stroke onset [1,2,3,4,5]. However, although the safety and effectiveness of EVT have been fully verified, we must be aware that the rate of functional independence after EVT was only about 50% [6]. Previous studies indicated that infarction volume on follow-up imaging was closely associated with the clinical outcome of AIS patients after EVT [7,8,9,10]. However, the delineation of the infarction area was usually performed manually, which was time-consuming. Recently, some studies indicated that infarction volume could be calculated using automated software; however, it did not always perform well, especially when diffuse infarction lesions occurred or cerebral hemorrhage coexisted [7,8,9,10,11]. Therefore, establishing a convenient and accurate method to assess the infarct extent and to predict the clinical outcome of AIS patients after EVT is urgently needed in clinical practice.
Alberta Stroke Program Early Computed Tomography Score (ASPECTS) was a semi-quantitative grading system for assessing the extent and distribution of early ischemic changes in anterior circulation [12, 13]. Initially, ASPECTS was evaluated based on pre-treatment non-contrast computed tomography (NCCT) images, and then it was also applied in diffusion-weighted imaging (DWI) [14, 15]. Previously, Yoshimoto et al demonstrated that pre-treatment DWI ASPECTS significantly correlated with pre-treatment DWI infarction volume and could help to predict an unfavorable outcome [15]. Recently, Leker RR et al reported that ASPECTS evaluated on post-treatment NCCT was useful for predicting the functional outcome in AIS patients after EVT [16]. However, the post-treatment NCCT was mostly scanned at 24–72 h after EVT in their study. The infarction area shown on the NCCT scanned at a short interval after EVT might not precisely represent the final infarct [16]. Besides that, the prognostic value of ASPECTS on post-treatment NCCT was not compared with that of infarction volume in their study. Considering the superiority of DWI to NCCT in delineating the infarction area and evaluating the final infarction extent [17, 18], we evaluated the ASPECTS and infarction volume on post-treatment DWI, and we hypothesized that ASPECTS evaluated on post-treatment DWI could help to predict the prognosis of AIS patients after EVT, and its prognostic value was comparable with that of infarction volume.
Therefore, the aims of the present study were to (1) determine whether ASPECTS on post-treatment DWI was associated with the clinical outcome of AIS patients after EVT; (2) compare the prognostic value between ASPECTS and infarction volume derived from post-treatment DWI, for clarifying whether post-treatment DWI ASPECTS could serve as a surrogate of infarction volume for predicting the clinical outcome of AIS patients after EVT.
Materials and methods
Patients
This study protocol was approved by the human ethics committee of our center. Written informed consent was waived due to the nature of a retrospective study. This retrospective study was based on the consecutive AIS patients who underwent EVT from July 2017 to April 2021 in our stroke center. We included the patients who (1) had the occlusion of the middle cerebral artery (MCA) segment 1 or 2 (M1 or M2) and/or the internal carotid artery (ICA); (2) had a baseline modified Rankin Scale (mRS) score less than 2; (3) underwent post-treatment MRI assessment including DWI and susceptibility-weighted imaging (SWI) sequences within 7 days after EVT. We excluded patients who (1) had a previous history of intracranial hemorrhage or brain surgery; (2) failed to undergo MRI examination because of poor state of consciousness and symptomatic hemorrhage transformation; (3) had poor image quality of post-treatment DWI due to motion artifacts.
Clinical information
We collected the clinical information from our stroke database, including (1) demographic features: gender, age; (2) stroke-related risk factors: hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, and smoking history; (3) stroke- and treatment- related characteristics: stroke onset time, occlusion sites of artery, door-to-puncture time, National Institute of Health Stroke Scale (NIHSS) scores at admission and 24 h after EVT, whether intravenous thrombolysis (IVT) was used before EVT or not, recanalization status, and 90-day mRS scores. Recanalization status was evaluated based on the final angiographic results by the operator, according to the modified Thrombolysis in Cerebral Infarction (mTICI) scale. Successful recanalization was defined as mTICI 2b-3 [19]. Patients were dichotomized into groups with good (mRS scores ≤ 0–2) and poor (mRS scores > 2) clinical outcomes at 90 days.
Imaging protocol
Post-treatment MRI assessment was performed on a 3.0-T scanner (Magnetom Skyra, Siemens Healthcare) equipped with a 20-channel head-neck coil. DWI was scanned with b-values of 0 and 1000 mm2/s, repetition time (TR) of 6400 ms, echo time (TE) of 98 ms, field-of-view (FOV) of 220 × 220 mm2, matrix of 192 × 192, slice thickness of 4 mm, slice numbers of 20. SWI was scanned with a TR of 28 ms, TE of 20 ms, FOV of 220 × 220 mm2, matrix of 384 × 307, slice thickness of 2 mm, and slice numbers of 48.
Imaging analysis
ASPECTS and infarction volume were analyzed based on post-treatment DWI. Firstly, two readers (both with more than 10 years of experience in neuroradiology), who were blinded for clinical information (except for the affected hemisphere) and study design, assessed the ASPECTS. MCA territory was divided into 10 regions (caudate nucleus, lentiform nucleus, insula, the posterior limb of the internal capsule, and M1–M6), corresponding to 10 points of the score [12]. The readers visually assigned each ASPECTS region for relative hyperintensity on DWI, compared with the same region in the contralateral hemisphere [14]. In the case of patients with scattered infarction, an ASPECTS region with an infarction encompassing more than 20% of that region was regarded as a positive region [9]. A normal DWI had ASPECTS of 10 points. One point would be subtracted for one positive region. After all 10 regions were assessed, a total ASPECTS could be generated. Once a discrepancy occurred between two readers, a third reader (with more than 20 years of experience in neuroradiology) would make a final decision. Secondly, the same two experienced neuroradiologists manually delineated the infarction area on DWI section-by-section using Carestream Vue PACS (v12.1, Carestream Health). If hemorrhagic transformation which is determined by the hypointensity on SWI imaging was found within the infarction area, the hemorrhage regions were also incorporated into the boundaries of the infarct. Then, infarction volume was calculated by multiplying the sum of lesion areas by the slice thickness.
Statistical analysis
Continuous variables were reported as medians with interquartile ranges (IQRs). Categorical variables were presented as numbers and percentages. Intraclass correlation coefficient (ICC) was used to assess the reproducibility of post-treatment DWI ASPECTS. Correlation between post-treatment DWI ASPECTS and infarction volume was analyzed using the Spearman rank correlation. The values of correlation coefficient and ICC were interpreted as follows: slight correlation or agreement, 0.000–0.200; fair correlation or agreement, 0.201–0.400; moderate correlation or agreement, 0.401–0.600; good correlation or agreement, 0.601–0.800; excellent correlation or agreement, 0.801–1.000 [20]. Univariate analyses including the Mann-Whitney U test or chi-square test were used to investigate the association between clinical variables and clinical outcomes at 90 days. Variables with p value less than 0.05 in univariate analyses were included in the multivariate logistic regression using a stepwise backward method. Receiver operating characteristic (ROC) curves were used to assess the ability of post-treatment DWI ASPECTS and infarction volume in predicting a good clinical outcome. The area under the ROC curve (AUC), sensitivity, and specificity were calculated. The optimal threshold was determined as the value that would maximize the Youden index. A dichotomized analysis was also performed based on the optimal cut-off point of post-treatment DWI ASPECTS using a Mann-Whitney U test or chi-square test to investigate the differences in clinical characteristics between these patients. All statistical analyses were performed using SPSS package (version 23.0) or MedCalc (version 12.3.0). A two-sided p value less than 0.05 indicated a significant difference.
Results
Patient characteristics
A total of 98 patients who fulfilled our inclusion criteria were finally enrolled. The median age of included patients was 69 years (IQR 61–74), and 69 (70.4%) patients were male. The median time interval between stroke onset and pre-treatment CT imaging evaluation was 269 min (IQR 181–439), and the median door-to-puncture time was 85 min (IQR 68–110). Median admission NIHSS score and NIHSS score at 24 h after EVT were 12 (IQR 9–16) and 8 (3–13), respectively. Good clinical outcomes were achieved in 66 (67.3%) patients and 46 (46.9%) patients had hemorrhagic transformation on post-treatment imaging. There were 35 (35.7%) patients who received IVT before EVT. The median time interval between EVT and follow-up MRI examination was 4 (IQR 3–5) days. Detailed clinical information of our patient cohort was shown in Table 1. Excellent inter-reader reproducibility was obtained in the assessment of post-treatment DWI ASPECTS (ICC, 0.866; 95% confidence interval [CI], 0.807–0.908).
Relationship between clinical and imaging characteristics and clinical outcome
Significant differences were found in both infarction volume and post-treatment DWI ASPECTS, which were 19.09 ml (IQR 10.61–48.15) and 7 (6–8) for patients with good outcomes and 100.53 ml (IQR 41.63–142.95) and 5 (3–6) for patients with poor outcomes, respectively (both p < 0.001). Besides that, patients with good outcomes had significantly lower admission NIHSS score (11 vs 14, p = 0.017) and post-treatment NIHSS score at 24 h (5 vs 12, p < 0.001) than patients with poor outcomes. Successful recanalization was more frequently achieved in patients with good outcomes than those without (95.2% vs 58.3%, p < 0.001) (Table 2).
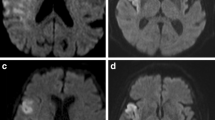
In multivariate logistic regression analysis, two models were established due to the strong correlation between post-treatment DWI ASPECTS and infarction volume (ρ = −0.847), which might influence the result of the logistic analysis on account of the collinearity. Post-treatment DWI ASPECTS and infarction volume were included in further multivariate logistic regression models, respectively. As a result, together with post-treatment NIHSS score at 24 h and successful recanalization, post-treatment DWI ASPECTS (OR, 2.401; 95%CI, 1.567–3.678; p < 0.001) and infarction volume (OR, 0.982; 95%CI, 0.846–0.998; p = 0.002) were both independent predictors of good clinical outcome (Table 3). Two representative cases were shown in Figure 1 and Figure 2.
A 71-year-old male patient with acute ischemic stroke due to occlusion of the left M1 segment of the middle cerebral artery (a). Post-treatment diffusion-weighted imaging showed a small volume infarction of 56.75 ml, and a high DWI ASPECTS of 6 (caudate, lentiform, insula, and M5 region) (b, c), though hemorrhagic transformation occurred in the basal ganglia region. Successful recanalization (mTICI 3) was achieved after endovascular treatment (d, e). The modified Rankin Scale (mRS) score of this patient was 2 at 90 days
A 66-year-old male patient with acute ischemic stroke due to occlusion of the left M1 segment of the middle cerebral artery (a). Post-treatment diffusion-weighted imaging showed a large volume infarction of 112.19 ml, and a low DWI ASPECTS of 4 (lentiform, insula, M2, M4, M5, and M6 regions) (b, c). A poor state of recanalization was achieved (mTICI 2a) after endovascular treatment (d, e). The modified Rankin Scale (mRS) score of this patient was 5 at 90 days
Prognostic value of post-treatment DWI ASPECTS and infarction volume
Table 4 summarized the performance of post-treatment DWI ASPECTS in predicting a good outcome. Setting post-treatment DWI ASPECTS ≥ 6 as the cut-off value, optimal predicting performance (sensitivity, 87.1%; specificity, 66.7%; AUC, 0.836) could be obtained in predicting good outcomes at 90 days. In addition, if setting infarction volume ≤ 94.87 ml as a threshold value, optimal predicting performance (sensitivity, 90.3%; specificity, 55.6%; AUC, 0.821) could be achieved. There was no significant difference in AUC between post-treatment DWI ASPECTS and infarction volume in predicting good outcomes (p = 0.686) (Figure 3).
Diagnostic performance of post-treatment DWI ASPECTS and infarction volume in predicting good clinical outcomes (mRS 0–2) for acute ischemic stroke (AIS) patients underwent endovascular treatment. The area under the curve (AUC) of post-treatment DWI ASPECTS is 0.836, with no significant difference compared to that of infarction volume (AUC = 0.821) (p = 0.686)
Dichotomized analysis based on post-treatment DWI ASPECTS
Compared with the patients with post-treatment DWI ASPECTS < 6, patients with post-treatment DWI ASPECTS ≥ 6 had lower NIHSS scores at admission (12 vs 15; p = 0.026) and NIHSS scores 24 h after EVT (6 vs 11; p = 0.007). Patients with post-treatment DWI ASPECTS ≥ 6 had significantly smaller infarction volume than those with post-treatment DWI ASPECTS < 6 (19.1 vs 119.2 ml; p < 0.001). In the group with post-treatment DWI ASPECT ≥ 6, the median infarction volume was 19.1 ml with an intermediate dispersion degree (IQR 10.6–35.9 ml). While in the group with post-treatment DWI ASPECTS < 6, the median infarction volume was 119.2 ml but the dispersion was large (IQR 95.1–174.0 ml). No significant difference in the occurrence of hemorrhagic transformation after EVT was found between the two groups (40.9% vs 59.4%; p = 0.130). Patients with a post-treatment DWI ASPECTS ≥ 6 showed a higher proportion of successful recanalization (87.9% vs 68.7%; p = 0.028) and good clinical outcomes (81.8% vs 25.0%; p < 0.001) than those with post-treatment DWI ASPECTS < 6. Dichotomized analysis of study patients based on post-treatment DWI ASPECTS was summarized in Table 5 and Figure 4.
Dichotomized analysis of study patients based on post-treatment DWI ASPECTS. There were significant differences between post-treatment DWI ASPECTS ≥ 6 group and post-treatment DWI ASPECTS < 6 group in (a) infarction volume (19.1 vs 119.2ml; p < 0.001), (b) NIHSS scores at admission (12 vs 15; p = 0.026), (c) NIHSS scores at 24 h after EVT (6 vs 11; p = 0.007), (d) successful recanalization rate (87.9% vs 68.7%; p = 0.028), and the (f) proportion of patients with good clinical outcomes (81.8% vs 25.0%; p < 0.001). No significant difference was found in the proportion of patients with the occurrence of hemorrhagic transformation between these two groups (40.9% vs 59.4%; p = 0.130). ASPECTS, Alberta Stroke Program Early Computed Tomography Score; DWI, diffusion-weighted imaging; NIHSSpre, admission National Institutes of Health Stroke Scale; NIHSS24h, NIHSS at 24 h after EVT; mRS, modified Rankin Scale scores
Discussion
Our study found that, together with a successful recanalization and a lower NIHSS scores at 24 h, both a higher post-treatment DWI ASPECTS and a smaller infarction volume were independently associated with a good clinical functional outcome at 90 days for stroke patients after EVT. A post-treatment DWI ASPECTS ≥ 6 or an infarction volume of ≤ 94.87 ml was identified as the optimal cut-off value for predicting a good clinical outcome at 90 days. Comparable performance was found for post-treatment DWI ASPECTS and infarction volume to predict a good outcome.
Previously, many studies had reported the close relationship between pre-treatment infarction volume and the prognosis of stroke patients after EVT [7,8,9,10]. However, Goyal M et al recently pointed out that the commonly used CT or MRI technique might not be able to precisely determine whether and to what extent brain tissue would be infarcted, because of the variability in tissue vulnerability, the phenomenon of selective neuronal loss, and lack of a reliable gold standard [21]. Meanwhile, the estimated infarct core on baseline imaging might be influenced by the time to reperfusion and the state of recanalization in the EVT procedures [21]. By contrast, the evaluation of infarction burden on post-treatment follow-up imaging might be closer to the extent of final infarction and provide a more accurate prediction for the functional outcomes.
Several trials had indicated that post-treatment infarct volume could effectively predict the functional outcomes after EVT [7,8,9,10], which was also confirmed in the present study. Setting an infarction volume of 94.87 ml as a threshold value, optimal predicting performance could be achieved in predicting the clinical outcome. However, in the present study and some previous studies, the calculation of infarction volume on follow-up imaging was conducted using a manual delineation, which was complicated and time-consuming [7,8,9,10]. Recently, some artificial intelligence–based software could help us to determine the infarction volume automatically; however, it did not always provide an accurate evaluation, especially when diffuse infarct lesions or concomitant hemorrhagic conversion occurred [11]. In addition, suchlike software was not always available, especially in primary stroke center. Thus, a simple and accurate surrogate of infarction volume was needed in clinical practice to predict the clinical outcome of stroke patients after EVT.
ASPECTS was a semi-quantitative grading tool to assess the extent of ischemic changes [12, 13]. After initially applied on NCCT, it was extended to be applied on the perfusion colored maps and DWI [14, 15, 22]. Several studies had reported the excellent correlation between ASPECTS and infarction volume, and the reliability of using pre-treatment DWI ASPECTS as a surrogate of infarction core volume for EVT patient selection [14, 15]. However, only few studies reported the relationship between stroke prognosis and post-treatment ASPECTS [16]. Leker RR et al reported that a post-treatment ASPECTS ≥ 7 on follow-up NCCT correlated with a good outcome after thrombectomy [16]. In our study, we found that a post-treatment DWI ASPECTS ≥ 6 was optimal for predicting a favorable outcome. In our opinion, our study holds the following two advantages: firstly, post-treatment ASPECTS was evaluated based on follow-up DWI. In our study cohort, follow-up MRIs were all scanned within 7 days after EVT; therefore, the infarct area would show as remarkable hyper-intensity on post-treatment DWI. Post-treatment DWI would be more sensitive for the detection and delineation of infarct lesion and also for assessing the ASPECTS and infarction volume [17, 18]. Secondly, the median time interval between EVT and follow-up MRI examination was 4 days, which was longer than the time interval (1–3 days) reported in the study of Leker RR et al Ischemic lesions scanned at a longer interval might be more rational to be considered the final infarct. Overall, the excellent correlation and comparable predictive ability indicated that post-treatment ASPECTS, especially on DWI, might be an eligible surrogate of infarction volume for predicting the clinical outcomes of stroke patients.
Interestingly, in the 62 patients with post-treatment DWI ASPECTS ≥ 6, there were only 2 patients with infarction volume > 94.87 ml and a relatively small dispersion degree (IQR 10.6–35.9 ml) was observed. By contrast, in the 36 patients with post-treatment DWI ASPECTS < 6, there was an obvious dispersion of infarction volume (IQR 95.1–174.0 ml), and there were 6 patients with infarction volume ≤ 94.87 ml. In our opinion, the different dispersions stratified by post-treatment DWI ASPECTS were derived from the limitation of the ASPECTS methodology. As a semi-quantitative grading system, ASPECTS assigned each of its 10 regions with the same value, while several ASPECTS regions (lentiform, insular, caudate, and internal capsule) represented small-volume brain tissues. This methodology could result in low ASPECTS, even though a small-volume ischemic lesion actually occurred. This result indicated that post-treatment DWI ASPECTS might be more suitable to substitute infarction volume in the patients with higher ASPECTS and smaller infarction volume. However, a discrepancy might occur when it turned to patients with lower ASPECTS and larger infarction volume.
Our study had some limitations. Firstly, besides the infarction volume, the infarct location was also focused on the prediction of functional outcomes [23, 24]. The total ASPECTS used in our study could not reveal the information on infarct distribution. This might be reflected that 12 patients had a post-treatment DWI ASPECTS ≥ 6; however, they suffered a poor clinical outcome at 90 days. We assumed that the infarct location might contribute to their poor outcomes. Further studies were suggested to study the association of each ASPECTS region on follow-up imaging with the clinical outcome. Secondly, follow-up MRIs were scanned at varied time intervals with a median of 4 days after EVT; therefore, the median time interval from stroke onset to follow-up MRI scan was about 4–5 days. A previous study indicated that cerebral edema after stroke commonly had a maximum peak on the fourth day, which might influence the measurement of FIV [25]. By contrast, a 7-day follow-up MRI after EVT was considered a more appropriate evaluation for FIV [18]. Thirdly, patients with a poor state of consciousness and symptomatic hemorrhage transformation were excluded from the MRI evaluation. Therefore, a selection bias might exist. Lastly, this study was a retrospective study conducted in a single center and the sample was relatively small. Further multiple-center study with a large sample size is warranted to confirm our results.
In conclusion, our study indicated that both post-treatment DWI ASPECTS and infarction volume were independently associated with the functional outcome of AIS patients after EVT. They showed comparable performance in predicting the good outcome. Post-treatment DWI ASPECTS might be a potential surrogate of infarction volume in terms of providing the prognostic information for AIS patients after EVT.
Abbreviations
- AIS:
-
Acute ischemic stroke
- ASPECTS:
-
Alberta Stroke Program Early Computed Tomography Score
- AUC:
-
Area under the ROC curve
- DWI:
-
Diffusion-weighted imaging
- EVT:
-
Endovascular thrombectomy
- FOV:
-
Field-of-view
- ICA:
-
Internal carotid artery
- ICC:
-
Intraclass correlation coefficient
- IQRs:
-
Interquartile ranges
- IVT:
-
Intravenous thrombolysis
- MCA:
-
Middle cerebral artery
- mRS:
-
Modified Rankin Scale
- mTICI:
-
Modified Thrombolysis in Cerebral Infarction
- NCCT:
-
Non-contrast computed tomography
- NIHSS:
-
National Institute of Health Stroke Scale
- ROC:
-
Receiver operating characteristic
- SWI:
-
Susceptibility-weighted imaging
- TE:
-
Echo time
- TR:
-
Repetition time
References
Albers GW, Marks MP, Kemp S et al (2018) Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 378:708–718
Nogueira RG, Jadhav AP, Haussen DC et al (2018) Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 378:11–21
Berkhemer OA, Fransen PS, Beumer D et al (2015) A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372:11–20
Jovin TG, Chamorro A, Cobo E et al (2015) Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 372:2296–2306
Saver JL, Goyal M, Bonafe A et al (2015) Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 372:2285–2295
Goyal M, Menon BK, van Zwam WH et al (2016) Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387:1723–1731
Yoo AJ, Chaudhry ZA, Nogueira RG et al (2012) Infarct volume is a pivotal biomarker after intra-arterial stroke therapy. Stroke 43:1323–1330
Albers GW, Goyal M, Jahan R et al (2015) Relationships between imaging assessments and outcomes in solitaire with the intention for thrombectomy as primary endovascular treatment for acute ischemic stroke. Stroke 46:2786–2794
Boers AMM, Jansen IGH, Brown S et al (2019) Mediation of the relationship between endovascular therapy and functional outcome by follow-up infarct volume in patients with acute ischemic stroke. JAMA Neurol 76:194–202
Regenhardt RW, Etherton MR, Das AS et al (2021) Infarct growth despite endovascular thrombectomy recanalization in large vessel occlusive stroke. J Neuroimaging 31:155–164
Boers AM, Marquering HA, Jochem JJ et al (2013) Automated cerebral infarct volume measurement in follow-up noncontrast CT scans of patients with acute ischemic stroke. AJNR Am J Neuroradiol 34:1522–1527
Barber PA, Demchuk AM, Zhang J et al (2000) Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 355:1670–1674
Pexman JH, Barber PA, Hill MD et al (2001) Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. Am J Neuroradiol 22:1534–1542
Mitomi M, Kimura K, Aoki J et al (2014) Comparison of CT and DWI findings in ischemic stroke patients within 3 hours of onset. J Stroke Cerebrovasc Dis 23:37–42
Yoshimoto T, Inoue M, Yamagami H et al (2019) Use of diffusion-weighted imaging-Alberta Stroke Program Early Computed Tomography Score (DWI-ASPECTS) and ischemic core volume to determine the malignant profile in acute stroke. J Am Heart Assoc 8:e012558
Leker RR, Honig A, Filioglo A et al (2021) Post-stroke ASPECTS predicts outcome after thrombectomy. Neuroradiology 63:769–775
Chalela JA, Kidwell CS, Nentwich LM et al (2007) Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet 369:293–298
Krongold M, Almekhlafi MA, Demchuk AM et al (2015) Final infarct volume estimation on 1-week follow-up MR imaging is feasible and is dependent on recanalization status. Neuroimage Clin 7:1–6
Zaidat OO, Yoo AJ, Khatri P et al (2013) Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 44:2650–2663
Shrout PE, Fleiss JL (1979) Intra-class correlations: uses in assessing rater reliability. Psychol Bull 86:420–428
Goyal M, Ospel JM, Menon B et al (2020) Challenging the ischemic core concept in acute ischemic stroke imaging. Stroke 51:3147–3155
Naylor J, Churilov L, Chen Z et al (2017) Reliability, reproducibility and prognostic accuracy of the Alberta Stroke Program Early CT score on CT perfusion and non-contrast CT in hyperacute stroke. Cerebrovasc Dis 44:195–202
Phan TG, Demchuk A, Srikanth V et al (2013) Proof of concept study: relating infarct location to stroke disability in the NINDS rt-PA trial. Cerebraovasc Dis 35:560–565
Sheth SA, Malhotra K, Liebeskind DS et al (2018) Regional contributions to poststroke disability in endovascular therapy. Interv Neurol 7:533–543
Austein F, Riedel C, Kerby T et al (2016) Comparison of perfusion CT software to predict the final infarct volume after thrombectomy. Stroke 47:2311–2317
Funding
This study has received funding from the National Natural Science Foundation of China (grant number: 82171907 to Shan-shan Lu; 81971613 to Hai-Bin Shi).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Fei-Yun Wu.
E-mail address: wfy_njmu@163.com
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Case-control study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, XQ., Chu, Y., Shen, GC. et al. Prognostic value of ASPECTS on post-treatment diffusion-weighted imaging for acute ischemic stroke patients after endovascular thrombectomy: comparison with infarction volume. Eur Radiol 32, 8079–8088 (2022). https://doi.org/10.1007/s00330-022-08888-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08888-z