Abstract
Objectives
To explore the value of post-treatment fluid-attenuated inversion recovery vascular hyperintensity (FVH) in predicting clinical outcome in patients with acute ischemic stroke (AIS) after endovascular thrombectomy (EVT).
Methods
This retrospective study reviewed data from consecutive patients with large vessel occlusion of anterior circulation between July 2017 and February 2021. Together with other variables, status of post-treatment FVH was assessed for each patient. Good outcome was defined as a 3-month modified Rankin Scale score of 0–2. Chi-square test, Fisher’s exact test, independent-samples t test, multivariate logistic regression analysis, and receiver operating characteristic analysis were used as appropriate.
Results
Among 84 included patients, 48 (57.1%) patients showed post-treatment FVH. Post-treatment FVH significantly correlated with incomplete recanalization (p < 0.05) and low Alberta Stroke Project Early CT Changes Score on post-treatment diffusion-weighted imaging (p < 0.05). Higher incidence of hemorrhage transformation was observed in patients with post-treatment FVH than those without (27.1% vs. 16.7%); however, the difference did not reach significance (p = 0.259). Successful recanalization (odds ratio [OR], 0.024; 95% confidence interval [CI] 0.003–0.194; p < 0.05), lower National Institutes of Health Stroke Scale scores at admission (NIHSSpre) (OR, 1.196; 95% CI, 1.017–1.406; p < 0.05), and no post-treatment FVH (OR, 74.690; 95% CI, 4.624–1206.421; p < 0.05) were found to be independent predictors of good outcomes. Combined models integrating all three independent predictors (recanalization+NIHSSpre+post-treatment FVH) significantly outperformed the combined model without post-treatment FVH (recanalization+NIHSSpre) in predicting clinical outcome (p = 0.004).
Conclusions
Post-treatment FVH may be an effective prognostic marker associated with clinical outcome in patients with AIS after EVT.
Key Points
• Post-treatment FVH correlates with incomplete recanalization and higher infarct volume.
• Post-treatment FVH is independently associated with an unfavorable outcome.
• Post-treatment FVH may provide prognostic information in patients with AIS after EVT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute ischemic stroke (AIS) is a major global health burden due to the high rate of disability and mortality. Endovascular thrombectomy (EVT) has been strongly recommended for patients with AIS due to large vessel occlusion (LVO) [1,2,3]. However, even if a successful recanalization is achieved, more than 50% of patients cannot obtain a good outcome at 3 months [3]. Therefore, accurately predicting the prognosis of patients with AIS after EVT is crucial for the determination of individual treatment strategy. Previously, some studies have reported that clinical variables such as age, reperfusion status, and pre- and post-treatment National Institutes of Health Stroke Scale (NIHSS) scores might be promising in predicting the prognosis of patients with AIS after EVT [4,5,6,7,8]. In addition to the clinical variables, some imaging metrics, such as collateral circulation, early infarct growth, and final infarct volume, have been reported to be associated with the prognosis of patients with AIS after EVT [9,10,11,12]. However, given that some metrics are highly dependent on the sophisticated post-processing software, they cannot be widely applied in clinical practice. Therefore, a simple, accessible, and reproducible imaging marker with the potential to predict the prognosis of patients with AIS after EVT is urgently needed.
Fluid-attenuated inversion recovery vascular hyperintensity (FVH) has been defined as circular, continuous, or punctuated linear hyperintensity present in the subarachnoid space relative to the hypointensity of cerebrospinal fluid with a typical arterial course [13]. FVH can be found in about 45 to 100% of stroke patients with intracranial arterial occlusion [14, 15]. Previous studies have indicated that it might represent slow retrograde flow in leptomeningeal collaterals rather than thrombus itself [16, 17]. In regard to the relationship between FVH and the prognosis of patients with AIS who underwent EVT, most studies suggest that FVH may represent sufficient collateralization for providing blood supply to penumbral areas, slowing the progress of ischemic lesions and improving prognosis [13, 18,19,20,21]. However, all abovementioned results are based on the pre-treatment FVH; the association between post-treatment FVH and stroke prognosis has only been reported in limited studies [19, 22, 23]. Sakuta et al and Liu et al have reported that FVH would decrease or disappear on follow-up images when antegrade circulation was restored after successful revascularization [22, 23]. Jiang et al believed that the persistence of post-treatment FVH might be associated with continued hemodynamic impairment and poor functional outcome [19]; however, the study cohort from Jiang et al was mostly composed of patients with AIS who accepted only intravenous thrombolysis due to non-LVO. The association between post-treatment FVH and the prognosis of patients with AIS who underwent EVT due to LVO is still not clear until now.
Therefore, we hypothesized that there would be a significant correlations between post-treatment FVH and prognosis of patients with AIS due to anterior circulation LVO after EVT. Our study aimed to assess (1) the relationship between post-treatment FVH and the clinical or imaging variables, such as demographics, risk factors, hemorrhage transformation (HT), and recanalization status, and (2) whether post-treatment FVH can be used as an imaging marker to predict 3-month functional outcomes in patients with AIS after EVT.
Methods
Patient selection
This study was approved by the ethics committee of our center. The requirement of written informed consent was waived due to the retrospective nature. Consecutive adult patients with AIS who underwent EVT from July 2017 to February 2021 were enrolled. Inclusion criteria included (1) occlusion of the internal carotid artery (ICA) and/or M1/M2 segment of middle cerebral artery (MCA); (2) follow-up magnetic resonance imaging (MRI) examinations, including diffusion-weighted imaging (DWI), fluid-attenuated inversion recovery (FLAIR) and susceptibility-weighted imaging (SWI) were performed within 1 week after EVT; (3) baseline modified Rankin Scale (mRS) score < 2. Exclusion criteria included (1) image quality of MRI was not adequate for subsequent analysis; (2) follow-up mRS at 3 months was not available. Finally, 84 patients were enrolled in our study. The detailed flowchart is showed in Fig. 1.
Flowchart of the study cohort. MCA indicates middle cerebral artery; ICA, internal carotid artery; BA, basilar artery; VA, vertebral artery; PCA, posterior cerebral artery; ACA, anterior cerebral artery; MRI, magnetic resonance imaging; DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery; SWI, susceptibility-weighted imaging; mRS, modified Rankin Scale
Clinical information
Clinical information was retrospectively collected from our stroke database. The following information included (1) demographic data: age, gender; (2) medical history: history of hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, and smoking; (3) stroke and EVT-related data: onset-to-door time (ODT), door-to-puncture time (DPT), NIHSS scores at admission (NIHSSpre) and that at 24 h after EVT (NIHSS24h), and functional outcome at 3 months. Patients were dichotomized into good (mRS score ≤ 2) and poor (mRS score > 2) outcome at 3 months after EVT.
Follow-up image acquisition
In our center, a non-contrast CT scan was routinely suggested at 24 h after EVT procedure. If the patient suffered sudden limb weakness or other neurological symptoms, an emergent CT scan would be performed immediately. Follow-up MRI scans would be performed using a 3.0-T MRI scanner (Magnetom Skyra, Siemens Healthcare) within 1 week after EVT, if only the patient’s condition was adequate. Axial DWI, FLAIR and SWI were included in MRI protocol, and scanned using the following parameters: (1) DWI: b value = 0 and 1000 mm2/s; TR = 6400 ms; TE = 98 ms; field of view (FOV) = 220 × 220 mm2; matrix = 192 × 192; slice thickness = 4 mm; number of slices = 20; (2) FLAIR: TR = 8000 ms; TE = 97 ms, inversion time (TI) = 2370 ms; FOV = 230 × 230 mm2; matrix = 256 × 230; slice thickness = 5 mm; number of slices = 20; (3) SWI: TR = 28 ms; TE = 20 ms; FOV = 220 × 220 mm2; matrix = 384 × 307; slice thickness = 2 mm; number of slices = 48.
Imaging analysis
FVH was defined as circular, continuous, or punctuated linear hyperintensity running along the cortical sulci and subarachnoid space relative to the hypointensity of cerebrospinal fluid [17]. Two experienced radiologists (with 4 and 12 years of experience in neuroradiology, respectively) blinded to the clinical information accessed the status of FVH. They would also evaluate the Alberta Stroke Project Early CT Changes Score on post-treatment DWI images (DWIASPECTS). HT was assessed based on follow-up CT images. If uncertain, SWI was referred. During the process of abovementioned evaluations, in case of any disagreement, consensus would be formed after consulting another senior radiologist (with 27 years of experience in neuroradiology). Status of recanalization was assessed based on the post-treatment digital subtraction angiography by another experienced neurointerventionist (with 14 years of experience) who was blinded to the clinical information. Modified Thrombolysis in Cerebral Infarction (mTICI) Scale was used, and a successful recanalization was defined as mTICI 2b-3.
Statistical analysis
The inter-rater agreement for evaluating the status of FVH was assessed using Cohen’s kappa statistics, and reliabilities of more than 0.75 indicated excellent agreement [24]. Continuous variables were reported as mean ± standard deviation, and categorical variables were presented as absolute and relative frequencies. Categorical variables were evaluated using chi-squared test or Fisher’s exact test, and continuous variables were assessed using independent-samples t test. When assessing the prognosis-associated risk factors, the variables with p values less than 0.05 at univariate analysis were included in the multivariate logistic regression analysis. Receiver operating characteristic (ROC) analyses were performed to evaluate the performance of significant variables alone and their combinations for predicting the 90-day functional outcomes after EVT. Area under the ROC curve (AUC), sensitivity, and specificity were calculated with a threshold criterion determined as the value would maximize the Youden index. The AUCs were compared using the method of Delong et al [25]. All statistical analyses were performed using SPSS version 25.0 (IBM) and MedCalc version 12.3.0. A two-sided p value less than 0.05 indicated a statistical difference.
Result
Patients’ characteristics
A total of 84 patients (mean age, 67.5 ± 11.2 years; range 35–87 years; male, 58 patients) were enrolled in this analysis. The patients had occlusion in intracranial ICA (n = 16), M1 segment (n = 47), M2 segment (n = 9), or ICA combined with M1 (n = 12). The mean NIHSSpre score was 12.6 ± 6.1 (range: 1–35), and the mean NIHSS24h score was 7.9 ± 7.0 (ranged: 0–35). Successful recanalization was achieved in 67 (79.8%) of 84 patients. The presence of post-treatment FVH was observed in 48 (57.1%) of 84 patients. Inter-rater agreement for evaluating the status of post-treatment FVH was excellent (κ = 0.873). HT was found in 19 (22.6%) of 84 patients. Good functional outcomes were achieved in 56 (66.7%) of 84 patients. Patients’ characteristics of the present study cohort are summarized in Table 1.
Relationship between post-treatment FVH and clinical characteristics
Successful recanalization was observed in 91.7% (33/36) of patients without post-treatment FVH, and 70.8% (34/48) of patients with post-treatment FVH (p = 0.027). Patients without post-treatment FVH had a significantly higher proportion of DWIASPECTS ≥ 7 (75% vs. 35.4%, p < 0.001). The rate of good functional outcome was also significantly higher in patients who had no post-treatment FVH (94.4% vs. 45.8%, p < 0.001) (Fig. 2). Compared with the patients without post-treatment FVH, those patients with post-treatment FVH had a higher proportion of HT (27.1% vs. 16.7%, p = 0.259) and hypertension (77.1% vs. 58.3%, p = 0.066), although we found no evidence of a difference between groups. Representative cases with and without FVH are shown in Figs. 3 and 4, respectively. Detailed differences of patients’ characteristics stratified by FVH are shown in Table 2.
Comparisons of post-treatment DWIASPECTS and mRS at 3 months between post-treatment FVH positive and post-treatment FVH negative groups. DWI indicates diffusion-weighted imaging; ASPECTS, Alberta Stroke Project Early CT Changes Score; mRS, modified Rankin Scale; FVH, fluid-attenuated inversion recovery vascular hyperintensity
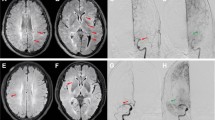
A 74-year-old man presented with stroke syndrome due to right middle cerebral artery occlusion (a). Postprocedural angiography demonstrated successful revascularization of the right middle cerebral artery after endovascular thrombectomy (b). FVH was observed at the right middle cerebral artery territory (c, arrowhead). Post-treatment DWI showed high signal in the right temporal lobe and lateral ventricle (d). Hemorrhage transformation was depicted on SWI (e). The mRS score was 3 at 3 months. FVH indicates fluid-attenuated inversion recovery vascular hyperintensity; DWI, diffusion-weighted imaging; SWI, susceptibility-weighted imaging; mRS, modified Rankin Scale
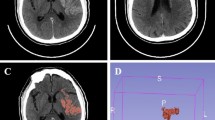
A 77-year-old man presented with stroke syndrome due to left middle cerebral artery occlusion (a). After successful recanalization (b), no FVH was observed on the follow-up images (c). DWI showed a patchy and scattered high signal in the left temporal and occipital lobe (d). No hemorrhage transformation was detected on SWI (e). The mRS score was 0 at 3 months. FVH indicates fluid-attenuated inversion recovery vascular hyperintensity; DWI, diffusion-weighted imaging; SWI, susceptibility-weighted imaging; mRS, modified Rankin Scale
Relationship between post-treatment FVH and clinical outcome
Univariate analysis indicated that patients with a good clinical outcome had a higher proportion of successful recanalization, lower NIHSSpre, lower NIHSS24h, a higher proportion of post-treatment DWIASPECTS ≥ 7, no post-treatment HT, and no post-treatment FVH (all p < 0.05) (Table 3). In the subsequent multivariate analysis, successful recanalization, no post-treatment FVH, and lower NIHSSpre were found to be independently associated with good functional outcome at 3 months after EVT (all p < 0.05) (Table 4).
The performances of independent variables alone in predicting disease prognosis are summarized in Table 4. Post-treatment FVH alone showed the optimal diagnostic performance, with AUC of 0.768, sensitivity of 92.9%, and specificity of 60.7%. Logistic regression was used to integrate independent variables and establish two combined models (model_1: recanalization + NIHSSpre; model_2: recanalization + NIHSSpre + post-treatment FVH). Model_2 showed an excellent performance in predicting clinical outcome (AUC, 0.940; sensitivity, 92.9%; specificity, 82.1%), which outperformed model_1 significantly (p = 0.004) (Fig. 5).
Change of post-treatment FVH according to the interval between EVT and MRI
Four patients underwent follow-up MRI scans within 36 h, while the other 80 patients underwent MRI scan at more than 36 h after EVT. Post-treatment FVH was observed in one (25%) patient who underwent MRI examination within 36 h, in 4 (50%) patients at 36 h to the 2nd day, in 12 (50%) patients at the 3rd day, in 10 (52.6%) patients at the 4th day, 7 (58.3%) patients at the 5th day, in 8 (88.9%) patients at the 6th day, and 6 (75%) patients at the 7th day, respectively (Table 5).
Discussion
In the present study, we found that post-treatment FVH was significantly associated with imcomplete recanalization and low post-treatment DWIASPECTS. Additionally, a higher incidence of HT was observed in patients with post-treatment FVH than those without, but the difference did not reach significance. Moreover, our study indicated that, together with successful recanalization and lower NIHSSpre, no post-treatment FVH was found to be an independent predictor of good outcomes at 90 days. The combined models integrating post-treatment FVH status with the other two variables (recanalization and NIHSSpre) showed optimal performance in predicting the clinical outcome of patients with AIS after EVT.
Due to the association with the severity of neurological deficit, NIHSS score on admission has been suggested to be an important predictor of clinical outcome in patients with AIS [4, 5]. In line with previous results, our study also found that NIHSSpre was associated with the clinical outcome in patients with AIS after EVT. As a previous study indicated, a higher NIHSS usually implies larger infarction core and poorer collateral status [6]. All of these characteristics were negatively associated with the clinical outcomes. Recently, NIHSS score at 24 h after treatment (NIHSS24h) and derived changes ratio of NIHSS score have been considered effective imaging markers for predicting functional outcomes [5, 7]. In our study, a significant difference was also found on NIHSS24h in univariate analysis, but not in multivariable analysis, which might be related to the different study cohort and sample size.
The association between infarction volume on post-treatment images and the clinical outcome has been proven in several studies [26, 27]. Lu et al reported that the infarct volume on post-treatment DWI was an independent predictor of good functional outcomes [26]. Ospel et al further found that infarct volume was linearly associated with decreased chances of achieving good outcome in patients with moderate to large infarcts, but not in those with small infarcts [27]. However, in the abovementioned studies, infarct volume was measured in a manual pattern, which was time consuming. As an alternative approach, some researchers tried to quantify the post-treatment infarct volume using an ASPECTS approach [28, 29]. Leker et al reported that an ASPECT ≥ 7 on post-treatment CT correlated with a favorable outcome at 90 days [8]. In our study, we also quantified the post-treatment infarct volume on DWI using an ASPECTS approach, and found that the proportion of post-treatment DWIASPECTS ≥ 7 differed significantly between two groups in the univariate analysis. Considering the reported relationship between ASPECTS and infarct volume as reported [28], we suggest that post-treatment ASPECTS might be a simple and effective approach for semiquantitatively assessing the infarct volume, and further predicting the prognosis.
Previous studies have indicated that FVH is associated with good collaterals, larger PWI–DWI mismatch [30], milder hypoperfusion [31], smaller infarct growth [32], and better clinical outcome [14, 18, 19]. However, most of the previous reports focused on the admission FVH; now increasing studies paid close attention on the post-treatment FVH. Some studies found that the persistence of FVH on follow-up MRI was associated with persistent vessel occlusion in patients with AIS [33]. Furthermore, Aoki et al reported that stroke recurrence risk was high when patients exhibited persistent FVH [34]. In the present study, we found that post-treatment FVH correlated with incomplete recanalization and higher infarct volume, and it was also independently associated with an unfavorable outcome. As for the potential reason, we deemed that incomplete recanalization could not maintain normal blood flow via antegrade circulation. Thus, the compensatory and slow retrograde flow might open, and be detected as FVH. Besides that, patients with post-treatment FVH might have a higher risk of HT, although the difference did not reach significance in our study. The non-significant difference might be due to the potential selection bias of our study, namely, only patients with relatively mild symptoms could undergo MRI scan, while patients with symptomatic HT or severe symptoms could not accept post-treatment MRI evaluation. This situation would reduce the detection rate of HT in the severe group. As for the potential clinical significance of this finding, we think that FVH is a simple, accessible, and reproducible imaging marker in clinical practice. If FVH is observed on the post-treatment MRI, a reinforced medication or rehabilitation therapy should be suggested or initiated as soon as possible for improving the prognosis.
It was worthy noting and discussing that post-treatment FVH was only assessed at one time point after EVT in the present study. However, FVH might dynamically change within the process of stroke disease. Grosch et al reported a decrease in the presence and extent of FVH on follow-up MRI as compared with baseline, and this effect being much more pronounced in patients who achieved successful recanalization [35]. Therefore, theoretically post-treatment FVH would still exist in the early period after EVT, and then gradually disappear in the subsequent follow-up MRI. However, in our study, we did not retrospectively observe a descending tendency of post-treatment FVH along with the increased time interval between EVT and MRI scan. In my opinion, it might be due to the retrospective nature of our study. In our center, the time interval between EVT and follow-up MRI scan was influenced by the disease severity to a certain extent. If the patients recovered well, follow-up MRI would be performed in an early period. For the patients with more severe symptoms, the intervals between EVT and follow-up MRI would be prolonged, or even follow-up MRI would be canceled. Patients with more severe symptoms usually suffered a larger infarct core volume, heavier hemodynamic damage, and unsuccessful recanalization; therefore, FVH could still be detected on follow-up MRI. Future prospective and longitudinal studies evaluating the time course of post-treatment FVH would be more valuable for exploring the potential mechanism of FVH, and its relationship with clinical outcome.
Our study had some limitations. First, the sample size was limited. When we designed our study, if a power value of 0.8 was set, the power analysis indicated that nearly 143 samples were needed. However, only 84 patients were enrolled according to our inclusion and exclusion criteria, which would affect our results to reach a sound conclusion. Future studies with a larger study population are needed to confirm our results. Second, patients with symptomatic HT or severe symptoms after EVT could not accept post-treatment MRI scan. Only the patients with relatively mild symptoms possessed the chance to undergo the MRI assessment, which might lead to a selection bias. Third, due to that CT techniques were applied to evaluate the patients with AIS in our center, admission FVH and dynamic change of FVH could not be analyzed in the present study. Fourth, only a dichotomized FVH status was studied. Future studies exploring the association between FVH topography (FVH ASPECTS, positional relationship between FVH and DWI lesions) and clinical outcomes after EVT will be valuable.
In conclusion, the present study suggested that post-treatment FVH might be associated with poor clinical outcome. It might provide prognostic information in patients with AIS after EVT. Future larger prospective cohort studies are needed to validate our findings.
Abbreviations
- AIS:
-
Acute ischemic stroke
- AUC:
-
Area under the ROC curve
- DPT:
-
Door-to-puncture time
- DWI:
-
Diffusion-weighted imaging
- EVT:
-
Endovascular thrombectomy
- FLAIR :
-
Fluid-attenuated inversion recovery
- FOV:
-
Field-of-view
- FVH:
-
Fluid-attenuated inversion recovery vascular hyperintensity
- HT:
-
Hemorrhage transformation
- ICA:
-
Internal carotid artery
- LVO:
-
Large vessel occlusion
- MCA:
-
Middle cerebral artery
- MRI :
-
Magnetic resonance imaging
- mRS:
-
Modified Rankin Scale
- mTICI:
-
Modified Thrombolysis in Cerebral Infarction
- NIHSS:
-
National Institutes of Health Stroke Scale
- ODT:
-
Onset-to-door time
- ROC:
-
Receiver operating characteristic
- SWI:
-
Susceptibility-weighted imaging
- TE:
-
Echo time
- TI :
-
Inversion time
- TR:
-
Repetition time
References
Nogueira RG, Jadhav AP, Haussen DC et al (2018) Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 378:11–21
Albers GW, Marks MP, Kemp S et al (2018) Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 378(8):708–718
Goyal M, Menon BK, van Zwam WH et al (2016) Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387:1723–1731
Meyer L, Broocks G, Bechstein M et al (2020) Early clinical surrogates for outcome prediction after stroke thrombectomy in daily clinical practice. J Neurol Neurosurg Psychiatry 91:1055–1059
Wirtz MM, Hendrix P, Goren O et al (2019) Predictor of 90-day functional outcome after mechanical thrombectomy for large vessel occlusion stroke: NIHSS score of 10 or less at 24 hours. J Neurosurg 20:1–7
Du M, Huang X, Li S, Xu L et al (2020) A nomogram model to predict malignant cerebral edema in ischemic stroke patients treated with endovascular thrombectomy: an observational study. Neuropsychiatr Dis Treat 16:2913–2920
Heit JJ, Mlynash M, Kemp SM et al (2019) Rapid neurologic improvement predicts favorable outcome 90 days after thrombectomy in the DEFUSE 3 study. Stroke 50:1172–1177
Leker RR, Honig A, Filioglo A et al (2021) Post-stroke ASPECTS predicts outcome after thrombectomy. Neuroradiology 63(5):769–775
Bang OY, Goyal M, Liebeskind DS et al (2015) Collateral circulation in ischemic stroke: assessment tools and therapeutic strategies. Stroke 46:3302–3309
Albers GW, Goyal M, Jahan R et al (2015) Relationships between imaging assessments and outcomes in solitaire with the intention for thrombectomy as primary endovascular treatment for acute ischemic stroke. Stroke 46(10):2786–2794
Boers AMM, Jansen IGH, Brown S et al (2019) Mediation of the relationship between endovascular therapy and functional outcome by follow-up infarct volume in patients with acute ischemic stroke. JAMA Neurol 76(2):194–202
Al-Ajlan FS, Al Sultan AS, Minhas P et al (2018) Posttreatment infarct volumes when compared with 24-hour and 90-day clinical outcomes: insights from the REVASCAT randomized controlled trial. AJNR Am J Neuroradiol 39(1):107–110
Liu D, Scalzo F, Rao NM et al (2016) Fluid-attenuated inversion recovery vascular hyperintensity topography, novel imaging marker for revascularization in middle cerebral artery occlusion. Stroke 47:2763–2769
Ebinger M, Kufner A, Galinovic I et al (2012) Fluid-attenuated inversion recovery images and stroke outcome after thrombolysis. Stroke 43:539–542
Cheng B, Ebinger M, Kufner A et al (2012) Stroke Imaging Repository (STIR) Investigators. Hyperintense vessels on acute stroke fluid-attenuated inversion recovery imaging: associations with clinical and other MRI findings. Stroke 43:2957-2961
Kufner A, Galinovic I, Ambrosi V et al (2015) Hyperintense vessels on FLAIR: hemodynamic correlates and response to thrombolysis. AJNR Am J Neuroradiol 36:1426–1430
Lee JY, Lee KY, Suh SH (2010) Different meaning of vessel signs in acute cerebral infarction. Neurology 75:668
Derraz I, Ahmed R, Benali A et al (2021) FLAIR vascular hyperintensities and functional outcome in nonagenarians with anterior circulation large-vessel ischemic stroke treated with endovascular thrombectomy. Eur Radiol. 31(10):7406–7416
Jiang L, Chen YC, Zhang H et al (2019) FLAIR vascular hyperintensity in acute stroke is associated with collateralization and functional outcome. Eur Radiol 29(9):4879–4888
Legrand L, Turc G, Edjlali M et al (2019) Benefit from revascularization after thrombectomy according to FLAIR vascular hyperintensities-DWI mismatch. Eur Radiol 29(10):5567–5576
Legrand L, Tisserand M, Turc G et al (2016) Fluid-attenuated inversion recovery vascular hyperintensities-diffusion-weighted imging mismatch identifies acute stroke patients most likely to benefit from recanalization. Stroke 47:424–427
Sakuta K, Saji N, Aoki J et al (2016) Decrease of hyperintense vessels on fluid-attenuated inversion recovery predicts good outcome in t-PA patients. Cerebrovasc Dis 41:211–218
Liu W, Yin Q, Yao L et al (2012) Decreased hyperintense vessels on FLAIR images after endovascular recanalization of symptomatic internal carotid artery occlusion. Eur J Radiol 81:1595–1600
Shrout PE, Fleiss JL (1979) Intra-class correlations: uses in assessing rater reliability. Psychol Bull 86:420–428
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Lu SS, Cao YZ, Su CQ et al (2021) Hyperperfusion on arterial spin labeling MRI predicts the 90-day functional outcome after mechanical thrombectomy in ischemic stroke. J Magn Reson Imaging 53(6):1815–1822
Ospel JM, Hill MD, Menon BK et al (2021) Strength of association between infarct volume and clinical outcome depends on the magnitude of infarct size: results from the ESCAPE-NA1 Trial. AJNR Am J Neuroradiol. 42(8):1375–1379
Nannoni S, Ricciardi F, Strambo D et al (2021) Correlation between ASPECTS and core volume on CT perfusion: impact of time since stroke onset and presence of large-vessel occlusion. AJNR Am J Neuroradiol 42(3):422–428
Albers GW, Wald MJ, Mlynash M et al (2019) Automated alculation of Alberta Stroke Program Early CT Score: validation in patients with large emispheric infarct. Stroke 50:3277–3279
Legrand L, Tisserand M, Turc G et al (2015) Do FLAIR vascular hyperintensities beyond the DWI lesion represent the ischemic penumbra? AJNR Am J Neuroradiol 36:269–274
Mahdjoub E, Turc G, Legrand L et al (2015) Do fluid-attenuated inversion recovery vascular hyperintensities represent good collaterals before reperfusion therapy? AJNR Am J Neuroradiol 39:77–83
Perez de la Ossa N, Hernandez-Perez M, Domenech S et al (2012) Hyperintensity of distal vessels on FLAIR is associated with slow progression of the infarction in acute ischemic stroke. Cerebrovasc Dis 34:376–384
De Figueiredo MM, Júnior EA, Alves MAM et al (2017) Fluid-attenuated inversion recovery vascular hyperintensities in patients with transient ischemic attack. J Stroke Cerebrovasc Dis 26(10):2412–2415
Aoki J, Suzuki K, Suda S et al (2020) Negative-FLAIR vascular hyperintensities serve as a marker of no recanalization during hospitalization in acute stroke. J Clin Neurosci 72:233–237
Grosch AS, Kufner A, Boutitie F et al (2021) Extent of FLAIR hyperintense vessels may modify treatment effect of thrombolysis: a post hoc analysis of the WAKE-UP Trial. Front Neurol 11:623881
Funding
This research is funded by National Natural Science Foundation of China (81971613 to Hai-Bin Shi and 82171907 to Shan-Shan Lu).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Fei-Yun Wu.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• case-control study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, XQ., Shen, GC., Ma, G. et al. Prognostic value of post-treatment fluid-attenuated inversion recovery vascular hyperintensity in ischemic stroke after endovascular thrombectomy. Eur Radiol 32, 8067–8076 (2022). https://doi.org/10.1007/s00330-022-08886-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08886-1