Abstract
Objectives
The aim of this meta-analysis was to estimate the clinical use value of 11C-FMZ and 18F-FDG in PET for the localization of epileptogenic zone and to provide evidence for practitioners’ clinical decision-making.
Methods
We searched PubMed and Embase in a time frame from inception to May 31, 2020. Studies utilizing FMZ or FDG-PET or FDG-PET/MRI used in patients with epilepsy, with EEG or surgical outcomes as the gold standard and corresponding outcomes such as concordance rates of PET or PET/MRI scan compared with reference standard, absolute numbers of participants with true-positive (TP), false-positive (FP), true-negative (TN), and false-negative (FN) results in FDG or FMZ PET. Pooled concordance rates, overall sensitivity, and specificity of 11C-FMZ-PET and 18F-FDG-PET were calculated.
Results
In total, 44 studies met the inclusion criteria. The pooled concordance rates of FDG-PET, FMZ-PET, and FDG-PET/MRI coregistration compared with reference standard were 0.67 (95% CI: 0.60–0.73), 0.75 (95% CI: 0.57–0.93), and 0.93 (95% CI: 0.89–0.97), respectively. The concordance rate of 18F-FDG-PET in patients with temporal lobe epilepsy (TLE) was 0.79 (0.63; 0.92). The overall sensitivity and specificity of 18F-FDG-PET were 0.66 (95% CI: 0.58–0.73) and 0.71 (95% CI: 0.63–0.78), respectively. 11C-FMZ-PET displayed an overall sensitivity of 0.62 (95% CI: 0.49–0.73) and specificity of 0.73 (95% CI: 0.59–0.84).
Conclusions
Both 11C-FMZ PET and 18F-FDG PET are the choice of modalities for the localization of epileptogenic zone, especially when coregistered with MRI.
Key Points
• 11 C-FMZ-PET may be more helpful than 18 F-FDG-PET in the localization of epilepsy foci.
• Coregistration of FDG-PET and MRI is recommended in the localization of epileptogenic zone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epilepsy is one of the most common and serious chronic cerebral disorders, which affects over 70 million people worldwide [1, 2]. Almost 80% of patients with epilepsy reside in low- and middle-income countries or districts [3]. Among infants < 1 year old and people > 50 years old, the performances of epilepsy which appear a bimodal distribution with two peaks are more implicated than people in other age groups [4, 5]. Clinically, epileptic seizures vary widely in manifestation from abnormal sensations to motor symptoms.
Epilepsy is a multi-symptom disease with complex risk factors and in many cases has a strong inherited tendency, instead of a situation with a single cause and a single expression [3, 6]. For decades, the etiology, pathophysiology, and antiepileptic drugs (AEDs) are continually being explored and investigated [2, 7,8,9]. Currently, AEDs are effective in only about two-thirds of patients in developed countries, and despite being available of more than 25 medications worldwide, only a few of them are considered first-line [10]. Furthermore, epilepsy surgery is considered to be the most efficacious way to attain long-term seizure freedom, but it has been confined to individuals with drug-resistant epilepsy and still underused [11,12,13,14].
Nowadays, the insufficient understanding and absence of specific biomarkers of the epileptogenic process are the major constraints in the research and development of new AEDs that are able to prevent the underlying disease or improve prognosis [15]. The rapid advances in neuroimaging modalities have expanded our chances to investigate the disease of epilepsy by means of noninvasive research modalities. The field of neuroimaging has been remarkably developed in recent years. Neuroimaging techniques used in clinical settings for the assessment of patients with epilepsy include but not limited to non-contrast computerized tomography (CT), structural and functional MRI (fMRI), electroencephalograph combined with fMRI (EEG/fMRI), MR spectroscopy (MRS), positron emission tomography (PET), single-photon emission computed tomography (SPECT), and magnetoencephalography (MEG) [16]. The utilities of these modalities depend on specific circumstances and clinical questions to be addressed [17]. Currently, chronic intracranial EEG monitoring remains the gold standard in defining epileptic foci, but it also has boundedness and its application is highly dependent on other localization information [18, 19].
PET is manifested to be an available noninvasive method to guide intracranial electrode placement, and it can also reduce the number of patients requiring invasive EEG [20]. PET also plays a very important role in the evaluation of epilepsy. The most commonly used PET tracer in epilepsy is [18F]2-fluoro-2-deoxy-d-glucose (18F-FDG), usually performed in the seizure-free interval and aimed at the identification of cerebral regions with decreased glucose metabolism; it is considered to partially reflect the reduction of synaptic activity [21]. However, the epileptic areas are commonly smaller than the hypometabolic regions [18, 22, 23]. Besides 18F-FDG-PET, the GABA-A receptor ligand 11C-flumazenil (11C-FMZ) has displayed promising results in epileptic foci localization and lateralization [15, 24, 25]. Other potential PET tracers for detecting epileptic regions include [11C]a-methyl-L-tryptophan (AMT) which detects tryptophan metabolism, most recently 5-hydroxytryptamine type 1A (5-HT-1A) receptor ligands, and other radioligands that bind to opioid, histamine, N-methyl-d-aspartate (NMDA), “peripheral benzodiazepine” or acetylcholine receptors. Although the clinical application of most of them in epilepsy has not been systematically established, they have very important research value.
However, to our knowledge, only a few studies limited to small sample size directly compared the clinical performance of 18F-FDG-PET or 11C-FMZ PET compared with EEG or surgical outcome in localization of epileptogenic areas. We firstly did a meta-analysis by collating the available evidence to generate a precise estimation of the clinical utility of 18F-FDG-PET and 11C-FMZ-PET for the localization of the epileptogenic foci in patients with epilepsy, and secondly to provide evidence or clues for practitioners’ clinical decision-making and practice.
Methods
Search strategy and selection criteria
This meta-analysis was performed on the basis of the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines [26]. The research question of this study was raised in terms of PICOS, populations (participants with diagnosed epilepsy), interventions (FMZ or FDG-PET or coregistration of FDG-PET and MRI), comparators (EEG or surgical outcomes), outcomes (absolute numbers of participants with true-positive (TP), false-positive (FP), true-negative (TN), and false-negative (FN) results), and study designs (retrospective and prospective studies). The search strategy was restrictively based on the pre-designed protocol. We did a systematic search on PubMed and Embase to assay studies from inception to May 31, 2020, with articles in English considered. The following search terms were used: (11C-flumazenil OR 18F-FDG) AND (epilepsy OR epilepsies OR seizure disorder OR seizure disorders OR cryptogenic epilepsy) AND ((positron emission tomography) OR PET). We supplemented the online search with manual screen of the reference lists of all primary studies as well as relevant review articles. We considered studies using FMZ or FDG-PET or coregistration of FDG-PET and MRI for the assessment of patients with epilepsy. Inclusion criteria were as follows: FMZ or FDG-PET or FDG-PET/MRI used in participants with diagnosed epilepsy; use of EEG or surgical outcomes as the gold standard to evaluate diagnostic performance; corresponding outcomes such as concordance rates of PET or PET/MRI scan compared with the reference standard, absolute numbers of participants with TP, FP, TN, and FN results via FDG or FMZ PET. If studies recruited participants over the same period of time or from the same study center, only the research with the largest sample size or yielding the most pertinent outcomes was included to avoid duplications. Both retrospective and prospective studies were considered. Studies in abstract form, case reports, and successive cases seen in a unit were excluded.
Two independent investigators (Haiqun Xing and Meiqi Wu) conducted the process of literature search and study inclusion. When disagreement occurred, they discussed their arguments, and a third reviewer (Na Niu) was involved in case that no consensus was achieved.
Data extraction and quality assessments
Data were extracted from each selected publication by two investigators (Yanru Ma and Yimin Liu) independently. The following information were recorded: name of the first investigator, year of publication, number of participants, duration of epilepsy, age, gender ratio, type of epilepsy, reference standard, concordance rates of PET or PET/MRI compared with the reference standard, TP, FP, TN, and FN. To assess the methodological quality of the included studies and risk of bias and applicability concerns, we used the checklist of QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. This tool contains components in terms of patient selection, index test, and reference standard, as well as flow and timing.
Statistical analysis
All analysis was performed at the study level with the Stata15.0, R4.0.2 software and Review Manager 5.3 software. p < 0.05 was considered to be statistically significant. We calculated pooled concordance rates, sensitivity, specificity, positive likelihood ratio (LR) and negative LR, odds ratio (OR) with their respective 95% confidence intervals (CIs), and area under the summarized receiver operating characteristic (sROC) curves (AUCs). We used the Cochran Q and the I2 statistics to evaluate the heterogeneity of results between studies included. I2 values of 0–25%, 25–50%, 50–75%, and 75–100% indicate insignificant, low, moderate, and high heterogeneity, respectively. We created funnel plots to assess publication and related bias. Deeks’ method was used to statistically check the asymmetry of the funnel plot and detect publication bias. Moreover, subgroup analysis was performed to explore the potential sources of heterogeneity of different studies and influence analysis was used for the detection of outliers (studies) which affected the pooled results statistically.
Results
Study selection and characteristics
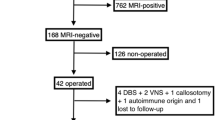
A total of 606 articles were identified from the databases searched. One hundred seventy duplicates were removed and 347 studies were excluded through an initial screening. After a full-text assessment for eligibility of the remaining 89 articles, 44 studies were identified for inclusion in this meta-analysis. No additional studies were found through the screening of references of the included full-text articles (see Fig. 1). The selected 44 studies containing a total of 2246 patients with diagnosed epilepsy. These articles were published from 1995 to 2020. More details of the studies included are shown in Table 1.
Comparison between PET and reference standard
The pooled concordance rate of FDG-PET compared with the reference standard was 0.67 (95% CI: 0.60–0.73); as for FMZ-PET, the concordance rate was 0.75 (95% CI: 0.57–0.93). Concordance rate for FDG-PET/MRI coregistration was 0.93 (95% CI: 0.89–0.97) (see Figs. 2, 3, and 4). Subgroup analysis revealed that 18F-FDG-PET showed the highest concordance rate in children 0.84 (0.75; 0.92); 11C-FMZ had the highest concordance rate in the subgroup of adults 0.92 (0.74; 1.00). In patients with TLE, the pooled concordance rate of 18F-FDG-PET was 0.79 (0.63; 0.92) (see Table 2).
Diagnostic performances of 11C-FMZ and 18F-FDG PET
Data from the 12 studies were used in the bivariate mixed-effects regression model to compute the pooled results on the basis of threshold analysis on FDG-PET (p = 0.67) and 7 studies with respect to FMZ-PET (p = 0.94) were analyzed. FDG-PET demonstrated an overall sensitivity of 0.66 (95% CI: 0.58–0.73) and specificity of 0.71 (95% CI: 0.63–0.78), with an AUC of 0.71 (95% CI: 0.67–0.75), positive LR of 2.3 (95% CI: 1.7–3.0), negative LR of 0.48 (95% CI: 0.38–0.61), and diagnostic OR of 5 (95% CI: 3–8) for the localization of epileptogenic zone in patients with epilepsy. FMZ-PET showed an overall sensitivity of 0.62 (95% CI: 0.49–0.73) and specificity of 0.73 (95% CI: 0.59–0.84), with an AUC of 0.71 (95% CI: 0.67–0.75), positive LR of 2.3 (95% CI: 1.3–4.0), negative LR of 0.52 (95% CI: 0.36–0.77), and diagnostic OR of 4 (95% CI: 2–11) (Fig. 5). The results of subgroup analysis manifested that there was no statistical significance in different subgroups of 18F-FDG-PET performance. 11C-FMZ-PET showed better diagnostic performance in the TLE subgroup and adults, respectively. Besides, pooled sensitivity and specificity for FDG-PET were 0.67 (0.55–0.79) and 0.76 (0.64–0.87) (Table 3).
Forest plots of studies included in the meta-analysis for localization of epilepsy with 18F-FDG PET (a) and 11C-FMZ PET (b). FDG-PET demonstrated an overall sensitivity of 0.66 and specificity of 0.71; heterogeneity for pooled sensitivity and specificity was statistically insignificant. FMZ-PET demonstrated an overall sensitivity of 0.62 and specificity of 0.73; heterogeneity for pooled sensitivity and specificity was statistically insignificant
Heterogeneity and quality of studies
A forest plot showed no heterogeneity for the sensitivity of 18F-FDG PET (Cochran Q = 1.82, p = 0.38, I2 = 6.9%), and there was no heterogeneity for the specificity of 18F-FDG PET (Cochran Q = 11.65, p = 0.38, I2 = 5.6%). As for the results of 11C-FMZ PET, no significant heterogeneity of sensitivity (Cochran Q = 9.13, p = 0.17, I2 = 34.3%) and specificity (Cochran Q = 9.85, p = 0.13, I2 = 37.1%) were found (see Fig. 5).
Quality assessment by QUADAS-2 scale showed that 37 studies had low risk of bias for patient selection, 2 studies had high risk of bias, and 5 studies had unclear risk of bias. Thirty-one studies had low risk of bias for index test, 3 studies had high risk of bias, and 10 studies had unclear risk of bias. Thirty-seven studies had low risk of bias for reference standard and 7 studies had unclear risk of bias. Clinical applicability concerns of each study included were also evaluated (Supplementary Figures 1 and 2).
The results of subgroup analysis demonstrated statistical difference between subgroups of population in the concordance rate analysis for FDG PET (p = 0.001). As for FMZ PET, difference was present in subgroups of duration and population (p = 0.009, p = 0.011). Subgroup analysis of diagnostic performance of 11C-FMZ-PET showed statistically significant difference in the TLE patients and adults (see Tables 2 and 3).
Influence analysis showed that no single study had significant impact on the overall outcomes in all pooled analysis.
Publication bias
Deeks’ funnel plot asymmetry tests yielded a p value of 0.59 for 18F-FDG PET and a p value of 0.24 for 11C-FMZ PET, respectively (see Fig. 6).
Discussion
PET is considered to be a neuroimaging technique that provided satisfactory insights into the molecular functioning of the brain in a living human [27]. 18F-FDG was developed to assess brain glycometabolism, which led to the original application of PET in epilepsy [28]. Although 18F-FDG is widely used in patients with epilepsy, unfortunately, it is not a desired tracer: the distribution of glucose hypometabolism regions is not related accurately to the level of hippocampal sclerosis, as results from MRI or histopathological test [29]. Since the 1990s, plentiful researches have assayed the implementation of 11C-FMZ PET in the field of epilepsy [25]. Interestingly, it was demonstrated that the increase of seizure frequency correlated inversely with the tracer intake in the frontalis piriform cortex in an 11C-FMZ PET study in epilepsy patients [30]. The site was inconsistent with the location of seizure onset and has been consistent with the results of morphometric MRI and EEG-fMRI researches [31]. Nevertheless, the tracer has not seen widely clinical utility, mainly due to its short half-life.
For decades, endeavors have been made to compare the utility of 18F-FDG and 11C-FMZ in the localization of epileptogenic zone [18, 32,33,34,35,36,37]. Nonetheless, the conclusions were less powerful to be extrapolated to clinical practice due to limited sample sizes and heterogeneity of different studies. We performed a meta-analysis to generate a more precise effect size of performances of FDG and FMZ in epilepsy localization and to provide a convincing evidence for healthcare professionals in counseling patients with epilepsy.
In this meta-analysis, we did a detailed literature search to improve the potential to capture all relevant studies as we can. Data extraction was conducted by two independent investigators using a pre-designed form. Furthermore, we assessed the heterogeneity between studies included, source of heterogeneity, quality of each study, and publication bias. The quality of individual study included in this meta-analysis was evaluated as high according to the QUADAS-2 scale. Furthermore, no significant heterogeneity between studies was observed; the analysis of threshold indicated that the results of studies can be consolidated with sufficient reasons. The influence analysis showed no single study had significant impact on the overall results. However, as we performed subgroup analysis, the results revealed that as for 11C-FMZ-PET, there was statistically significant difference in the TLE patients and adults. This contradiction may be attributed to the small numbers of studies in subgroups. Deeks’ funnel plot asymmetry tests indicated that publication bias may not affect the results between studies in either 18F-FDG or 11C-FMZ PET. This meta-analysis showed higher sensitivity of FDG-PET over FMZ-PET for the localization of epilepsy; however, the specificity of FDG-PET was lower than that of FMZ-PET. With respect to the type of epilepsy, both modalities showed better performance in patients with TLE compared with other epilepsy types. In consideration of clinical applications, given the positive and negative LRs of FMZ-PET (2.3 (95% CI: 1.3–4.0) and 0.52 (95% CI: 0.36–0.77)), it might help in excluding and confirming the localization of epilepsy foci in contrast to relevant indicators of FDG-PET. With respect to concordant detection results compared with reference standards (EEG/surgery outcome), FDG-PET/MRI coregistration reached a concordance rate of 93%; FMZ-PET manifested superior concordance rate over FDG-PET imaging. FMZ-PET showed higher concordance rate than FDG-PET.
Nevertheless, there are limitations in this meta-analysis. All analysis was performed at the study level, so we were unable to extract information at individual level based on the information in each study. Therefore, the division of subgroups for age of participants and the duration of epilepsy was deemed to be less specific. Although we detailed the inclusion and exclusion criteria, heterogeneity in studies still existed. Even though the subgroup analysis was conducted, the corresponding interpretation should be made with caution.
On the basis of our findings in this analysis, we may conclude that both 11C-FMZ PET and 18F-FDG PET can provide helpful complementary information for the localization of epileptogenic zone, especially when combined with other noninvasive technologies such as MRI. Interestingly, the recent development of 18F-FMZ, an alternative tracer of 11C-FMZ, might overcome the issues including short half-life [38]. Further assessments of potentially powerful tracers such as 18F-FMZ are needed.
Abbreviations
- 11C-FMZ:
-
11C-flumazenil
- 18F-FDG:
-
18F-2-fluoro-2-deoxy-D-glucose
- AEDs:
-
Antiepileptic drugs
- AMT:
-
a-Methyl-L-tryptophan
- AUCs:
-
Area under the sROC curves
- CIs:
-
Confidence intervals
- EEG:
-
Electroencephalograph
- FLE:
-
Frontal lobe epilepsy
- FN:
-
False negative
- FP:
-
False positive
- LR:
-
Likelihood ratio
- MRI:
-
Magnetic resonance imaging
- NMDA:
-
N-Methyl-d-aspartate
- OR:
-
Odds ratio
- PET:
-
Positron emission tomography
- PRISMA:
-
The Preferred Reporting Items for Systematic Review and Meta-analysis
- QUADAS:
-
Quality assessment of diagnostic accuracy studies
- SPECT:
-
Single-photon emission computed tomography
- sROC:
-
Summarized receiver operating characteristic curves
- TLE:
-
Temporal lobe epilepsy
- TN:
-
True negative
- TP:
-
True positive
References
Bertoglio D, Verhaeghe J, Dedeurwaerdere S, Grohn O (2017) Neuroimaging in animal models of epilepsy. Neuroscience 358:277–299. https://doi.org/10.1016/j.neuroscience.2017.06.062
Thijs RD, Surges R, O'Brien TJ, Sander JW (2019) Epilepsy in adults. Lancet 393:689–701. https://doi.org/10.1016/S0140-6736(18)32596-0
Fisher RS, Cross JH, French JA et al (2017) Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 58:522–530. https://doi.org/10.1111/epi.13670
Ngugi AK, Kariuki SM, Bottomley C, Kleinschmidt I, Sander JW, Newton CR (2011) Incidence of epilepsy: a systematic review and meta-analysis. Neurology 77:1005–1012. https://doi.org/10.1212/WNL.0b013e31822cfc90
Fiest KM, Sauro KM, Wiebe S et al (2017) Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology 88:296–303. https://doi.org/10.1212/WNL.0000000000003509
Fisher RS, Cross JH, D'Souza C et al (2017) Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia 58:531–542. https://doi.org/10.1111/epi.13671
Manford M (2017) Recent advances in epilepsy. J Neurol 264:1811–1824. https://doi.org/10.1007/s00415-017-8394-2
Wang Y, Chen Z (2019) An update for epilepsy research and antiepileptic drug development: toward precise circuit therapy. Pharmacol Ther 201:77–93. https://doi.org/10.1016/j.pharmthera.2019.05.010
Whiting P, Gupta R, Burch J et al (2006) A systematic review of the effectiveness and cost-effectiveness of neuroimaging assessments used to visualise the seizure focus in people with refractory epilepsy being considered for surgery. Health Technol Assess 10:iii–128. https://doi.org/10.3310/hta10040
Duncan JS, Sander JW, Sisodiya SM, Walker MC (2006) Adult epilepsy. Lancet 367:1087–1100. https://doi.org/10.1016/S0140-6736(06)68477-8
Jette N, Sander JW, Keezer MR (2016) Surgical treatment for epilepsy: the potential gap between evidence and practice. Lancet Neurol 15:982–994. https://doi.org/10.1016/S1474-4422(16)30127-2
Haneef Z, Stern J, Dewar S, Engel J Jr (2010) Referral pattern for epilepsy surgery after evidence-based recommendations: a retrospective study. Neurology 75:699–704. https://doi.org/10.1212/WNL.0b013e3181eee457
Englot DJ, Ouyang D, Garcia PA, Barbaro NM, Chang EF (2012) Epilepsy surgery trends in the United States, 1990-2008. Neurology 78:1200–1206. https://doi.org/10.1212/WNL.0b013e318250d7ea
Dewar SR, Pieters HC (2015) Perceptions of epilepsy surgery: a systematic review and an explanatory model of decision-making. Epilepsy Behav 44:171–178. https://doi.org/10.1016/j.yebeh.2014.12.027
Pitkanen A, Loscher W, Vezzani A et al (2016) Advances in the development of biomarkers for epilepsy. Lancet Neurol 15:843–856. https://doi.org/10.1016/S1474-4422(16)00112-5
Krumholz A, Shinnar S, French J, Gronseth G, Wiebe S (2015) Evidence-based guideline: management of an unprovoked first seizure in adults: report of the guideline development Subcommittee of the American Academy of neurology and the American Epilepsy Society. Neurology 85:1526–1527. https://doi.org/10.1212/WNL.0000000000001487
Middlebrooks EH, Ver Hoef L, Szaflarski JP (2017) Neuroimaging in Epilepsy. Curr Neurol Neurosci Rep 17:32. https://doi.org/10.1007/s11910-017-0746-x
Juhász C, Nagy F, Watson C et al (1999) Glucose and [11C]flumazenil positron emission tomography abnormalities of thalamic nuclei in temporal lobe epilepsy. Neurology 53:2037–2045. https://doi.org/10.1212/wnl.53.9.2037
Juhász C, Chugani DC, Muzik O et al (2000) Electroclinical correlates of flumazenil and fluorodeoxyglucose PET abnormalities in lesional epilepsy. Neurology 55:825–835. https://doi.org/10.1212/wnl.55.6.825
Theodore WH, Sato S, Kufta CV, Gaillard WD, Kelley K (1997) FDG-positron emission tomography and invasive EEG: seizure focus detection and surgical outcome. Epilepsia 38:81–86. https://doi.org/10.1111/j.1528-1157.1997.tb01081.x
Rocher AB, Chapon F, Blaizot X, Baron JC, Chavoix C (2003) Resting-state brain glucose utilization as measured by PET is directly related to regional synaptophysin levels: a study in baboons. NeuroImage 20:1894–1898. https://doi.org/10.1016/j.neuroimage.2003.07.002
Arnold S, Schlaug G, Niemann H et al (1996) Topography of interictal glucose hypometabolism in unilateral mesiotemporal epilepsy. Neurology 46:1422–1430. https://doi.org/10.1212/wnl.46.5.1422
Savic I, Ingvar M, Stone-Elander S (1993) Comparison of [11C]flumazenil and [18F]FDG as PET markers of epileptic foci. J Neurol Neurosurg Psychiatry 56:615–621. https://doi.org/10.1136/jnnp.56.6.615
Bankstahl M, Bankstahl JP (2017) Recent advances in radiotracer imaging hold potential for future refined evaluation of epilepsy in veterinary neurology. Front Vet Sci 4:218. https://doi.org/10.3389/fvets.2017.00218
la Fougère C, Rominger A, Förster S, Geisler J, Bartenstein P (2009) PET and SPECT in epilepsy: a critical review. Epilepsy Behav 15:50–55. https://doi.org/10.1016/j.yebeh.2009.02.025
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. https://doi.org/10.1136/bmj.b2535
Galovic M, Koepp M (2016) Advances of molecular imaging in epilepsy. Curr Neurol Neurosci Rep 16:58. https://doi.org/10.1007/s11910-016-0660-7
Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE (1979) Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol 6:371–388. https://doi.org/10.1002/ana.410060502
Hodolic M, Topakian R, Pichler R (2016) 18F-fluorodeoxyglucose and 18F-flumazenil positron emission tomography in patients with refractory epilepsy. Radiol Oncol 50:247–253. https://doi.org/10.1515/raon-2016-0032
Laufs H, Richardson MP, Salek-Haddadi A et al (2011) Converging PET and fMRI evidence for a common area involved in human focal epilepsies. Neurology 77:904–910. https://doi.org/10.1212/WNL.0b013e31822c90f2
Centeno M, Vollmar C, Stretton J et al (2014) Structural changes in the temporal lobe and piriform cortex in frontal lobe epilepsy. Epilepsy Res 108:978–981. https://doi.org/10.1016/j.eplepsyres.2014.03.001
Yankam Njiwa J, Gray KR, Costes N, Mauguiere F, Ryvlin P, Hammers A (2015) Advanced [18F]FDG and [11C]flumazenil PET analysis for individual outcome prediction after temporal lobe epilepsy surgery for hippocampal sclerosis. Neuroimage Clin 7:122–131. https://doi.org/10.1016/j.nicl.2014.11.013
Szelies B, Weber-Luxenburger G, Pawlik G et al (1996) MRI-guided flumazenil- and FDG-PET in temporal lobe epilepsy. Neuroimage 3:109–118. https://doi.org/10.1006/nimg.1996.0013
Szelies B, Weber-Luxenburger G, Mielke R et al (2000) Interictal hippocampal benzodiazepine receptors in temporal lobe epilepsy: comparison with coregistered hippocampal metabolism and volumetry. Eur J Neurol 7:393–400. https://doi.org/10.1046/j.1468-1331.2000.00077.x
Padma MV, Simkins R, White P et al (2004) Clinical utility of 11C-flumazenil positron emission tomography in intractable temporal lobe epilepsy. Neurol India 52:457–462
Komoto D, Iida K, Higaki T et al (2015) Diagnostic performance of positron emission tomography for the presurgical evaluation of patients with non-lesional intractable partial epilepsy: comparison among 18F-FDG, 11C-flumazenil, and 11C-flumazenil binding potential imaging using statistical imaging analysis. Hiroshima J Med Sci 64:51–57
Debets RM, Sadzot B, van Isselt JW et al (1997) Is 11C-flumazenil PET superior to 18FDG PET and 123I-iomazenil SPECT in presurgical evaluation of temporal lobe epilepsy? J Neurol Neurosurg Psychiatry 62:141–150. https://doi.org/10.1136/jnnp.62.2.141
Vivash L, Gregoire MC, Lau EW et al (2013) 18F-flumazenil: a γ-aminobutyric acid A-specific PET radiotracer for the localization of drug-resistant temporal lobe epilepsy. J Nucl Med 54:1270–1277. https://doi.org/10.2967/jnumed.112.107359
Acknowledgments
We thank the National Natural Science Foundation of China, Capital’s Funds for Health Improvement and Research (CFH), and CAMS Innovation Fund for Medical Sciences (CIFMS) for the financial support.
Funding
This work was sponsored in part by the National Natural Science Foundation of China (Grant No. 81571713), Capital’s Funds for Health Improvement and Research (CFH) (Grant No. 2016-2-40115), and CAMS Innovation Fund for Medical Sciences (CIFMS) (Grant Nos. 2016-I2M-4-003, 2017-I2M-3-001, 2018-I2M-3-001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Li Huo (huoli@pumch.cn).
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because this was a meta-analysis using the studies in published literature and did not analyze specific human subjects.
Ethical approval
Institutional Review Board approval was not required because this was a meta-analysis using the studies in published literature and did not analyze specific human subjects.
Study subjects or cohorts overlap
Information of study’s subjects or cohorts was extracted from previously published studies which were cited in the article.
Methodology
• meta-analysis
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 535 kb)
Rights and permissions
About this article
Cite this article
Niu, N., Xing, H., Wu, M. et al. Performance of PET imaging for the localization of epileptogenic zone in patients with epilepsy: a meta-analysis. Eur Radiol 31, 6353–6366 (2021). https://doi.org/10.1007/s00330-020-07645-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07645-4