Abstract
In patients with drug-resistant epilepsy, difficulties in identifying the epileptogenic zone are well known to correlate with poorer clinical outcomes post-surgery. The integration of PET and MRI in the presurgical assessment of pediatric patients likely improves diagnostic precision by confirming or widening treatment targets. PET and MRI together offer superior insights compared to either modality alone. For instance, PET highlights abnormal glucose metabolism, while MRI precisely localizes structural anomalies, providing a comprehensive understanding of the epileptogenic zone. Furthermore, both methodologies, whether utilized through simultaneous PET/MRI scanning or the co-registration of separately acquired PET and MRI data, present unique advantages, having complementary roles in lesional and non-lesional cases. Simultaneous FDG-PET/MRI provides precise co-registration of functional (PET) and structural (MR) imaging in a convenient one-stop-shop approach, which minimizes sedation time and reduces radiation exposure in children. Commercially available fusion software that allows retrospective co-registration of separately acquired PET and MRI images is a commonly used alternative. This review provides an overview and illustrative cases that highlight the role of combining 18F-FDG-PET and MRI imaging and shares the authors’ decade-long experience utilizing simultaneous PET/MRI in the presurgical evaluation of pediatric epilepsy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epilepsy is a disorder characterized by recurrent seizures, reflecting underlying brain dysfunction from a variety of unifactorial or multifactorial causes. Approximately 1.2% of the United States population has active epilepsy, which translates to 3.4 million individuals within the U.S. population, with approximately 470,000 identified as children [1]. Globally, epilepsy is estimated to affect 50 million people worldwide and is one of the most common neurological disorders impacting all ages [2], with an overall lifetime prevalence of epilepsy of 7.60 per 1000 population (95% CI, 6.17–9.38) [3]. The correct classification of the etiology of epilepsy is crucial because it determines potential therapies and correlates strongly with outcomes. The original seizure and epilepsy classification [4] by the International League Against Epilepsy (ILAE) was revised in 2017 to incorporate advances in our understanding of epilepsy. The revised framework allows for an expanded three-level diagnostic classification of seizure type, epilepsy type, and epilepsy syndrome and combines etiology with each stage, given the interrelated implications between cause and treatment [5, 6].
Despite the wide range of antiseizure medications developed over the last decades to control epilepsy, approximately 30% of patients have drug-resistant epilepsy and remain refractory to common pharmacological treatments [7]. The ILAE defines drug-resistant epilepsy as the failure of adequate trials of two tolerated, appropriately chosen, and used antiseizure drug schedules, whether as monotherapies or in combination, to achieve sustained seizure freedom [8]. Patients with pharmacoresistance have increased risks of seizures, death, injury, psychosocial dysfunction, and reduced quality of life [9, 10]. Early and effective treatment for drug-resistant epilepsy is essential in pediatric patients since seizure control grants them an improved quality of life and allows better integration into society [11, 12]. In patients who meet the ILAE criteria for drug-resistant epilepsy, surgery has emerged as one of the best and most effective treatment options.
In pediatric epilepsy, a multidisciplinary team is vital for comprehensive evaluation and surgical planning. Neurosurgeons, neuroradiologists, neuropsychologists, and neurophysiologists collaborate to precisely localize epileptogenic zones, reducing risks and improving outcomes. Epilepsy surgery has a reported success rate of 55–80%, depending on the type of surgery performed and population [13,14,15]. The data for pediatric patients also supports the role of neurosurgical treatment of drug-resistant epilepsy with a good seizure outcome, defined as Engel class I or II, achieved in up to 85% of patients [16]. Patients with drug-resistant focal seizures have multiple surgical treatment options, which include open surgical resection, minimally invasive ablations, both open and minimally invasive disconnection procedures, radiofrequency ablation, and laser interstitial thermal therapy [17, 18]. Increasingly, laser interstitial thermal therapy is being adopted in the pediatric epilepsy population [19, 20] as a safe and effective tool for the ablation of deep epileptogenic lesions such as focal cortical hypothalamic hamartoma, periventricular nodular heterotopia, focal cortical dysplasia, tuberous sclerosis complex, and subependymal giant cell astrocytoma [21] as well MRI-negative epilepsy. In addition, studies have shown laser interstitial thermal therapy to be a less-invasive surgical alternative to open craniotomy for corpus callosotomy with good results in pediatric patients [22, 23].
The increased utilization of robotic-assisted neurosurgery offers significant advantages of enhanced precision and a minimally invasive approach. Integrating the Robotic Stereotactic Assistance (ROSA) (Medtech, Montpellier, France) provided substantial benefits in pediatric epilepsy surgery [24, 25], such as better epileptogenic focus localization and comprehensive, minimally invasive procedures. One such procedure is stereoelectroencephalography (SEEG), a minimally invasive approach for epilepsy localization, in which the electrode placement demonstrates lower complication risks compared to the subdural grid and strip electrodes [26, 27]. In addition, ROSA allows the integration of imaging modalities SPECT, PET, CT, MRI, and PET/MRI, enhancing epileptogenic focus localization (Fig. 1) and emphasizing the importance of a multidisciplinary approach and multimodal imaging in neurosurgery [28].
A 12-year-old male with drug-resistant epilepsy and non-lesional MRI underwent multimodal imaging for presurgical evaluation. a Axial FDG-PET scan shows marked hypometabolism in the right parietal lobe (arrow). b Ictal SPECT/CT brain perfusion fused with a T1-weighted MRI image revealed focal increased cerebral blood flow, representing the epileptogenic focus (arrow). c Functional MRI illustrates cortical activation in the primary motor cortex (arrow) during bilateral foot tapping. d–g Stereoelectroencephalography (SEEG) electrodes (blue dots) were placed to target the findings on FDG-PET (d) and ictal scans (e) to confirm a non-lesional MRI focus and avoid motor cortex (f) during radiofrequency ablation. This case illustrates the essential role multimodality imaging plays in surgical planning for pediatric epilepsy
Functional nuclear medicine methods, like PET and SPECT, are crucial for evaluating the epileptogenic focus [29]. While SPECT provides superior temporal resolution for identifying seizure onset zones, PET offers broader availability but may lack specificity. Integrating modalities such as SPECT, PET, fMRI, and high-resolution MRI can guide surgical procedures and enhance outcomes. PET and SPECT results can confirm hypotheses, suggest additional areas for investigation, or indicate deeper complexity, aiding in surgical decision-making. This interdisciplinary approach requires careful coordination and expertise to ensure optimal patient care and outcomes.
The importance of accurate seizure localization
The success of epilepsy surgery depends on the precise identification of the epileptogenic zone. This task can be particularly challenging in patients with diffuse injury or multifocal abnormalities. Further, the identification of eloquent areas is of utmost importance to minimize postoperative deficits. Temporal lobe epilepsy is more common in adults than in pediatric patients and is often associated with detectable anatomical abnormalities on MRI, aiding in diagnosis and contributing to favorable seizure outcomes [30, 31]. Conversely, extratemporal lobe epilepsy (ETLE), prevalent among children, poses diagnostic and therapeutic challenges. The diffuse nature of ETLEs, frequently MRI-negative, with epileptic foci sometimes located within or next to the eloquent cortex, makes the preoperative assessment particularly complex. Functional imaging studies become crucial for seizure localization and surgical planning in such cases.
Identification of the epileptogenic zone is typically accomplished through a workflow of multiple assessments of evaluating seizure semiology, imaging both structural and functional, and, when required, invasive electrophysiological diagnostics. Clinical assessment with video electroencephalogram monitoring is an initial, non-invasive approach for lateralization and localization of seizures. Inherent limitations in the spatial resolution of scalp electroencephalography (EEG) can fail to localize the seizure foci or generate misleading data [32, 33]. This limitation is especially true for seizures arising from deep lesions, rapidly spreading seizures, insular onset, or associated with mesial temporal sclerosis. Imaging, both structural (MRI) and functional (PET), plays an important role in the identification of the epileptogenic zone in patients with drug-resistant epilepsy. The utility of MRI alone is limited in epilepsy types not associated with structural lesions, such as typical primary generalized epilepsy, benign focal epilepsies of childhood, and early onset childhood epilepsy with occipital spikes [34]. PET has the ability to highlight abnormal glucose metabolism, while MRI can detect structural anomalies, synergistically enhancing the identification of epileptogenic zones in pediatric epilepsy. The co-registration of PET and MRI by a PET/MRI scanner or post-hoc fusion of separately acquired scans is beneficial in identifying subtle anatomic and metabolic abnormalities and improves diagnostic accuracy. This information complements other neuroimaging techniques like MEG and perfusion SPECT used for localization of epileptogenic foci and BOLD fMRI to map brain functions, essential for surgical planning and minimizing post-surgery deficits [35, 36]. The advances in neuroimaging methods and a multidisciplinary approach combining anatomical and functional imaging studies have significantly improved management strategies for drug-resistant epilepsy in the pediatric population and have become key components of a comprehensive presurgical planning process to achieve the surgical goal of complete lesion resection, disconnection, or ablation without compromising eloquent functions [37]. Invasive intracranial EEG monitoring remains the gold standard for the localization of epileptogenic foci and functional mapping in epilepsy surgery. It is used to confirm or refute hypotheses of seizure organization and resolve ambiguities and functional specificities of the seizure zones to enable optimal surgical decision-making. Neuroimaging has shown promise to reduce the use of invasive intracranial EEG when there is concordance and no ambiguity in the semiology and imaging data. When intracranial EEG is required, neuroimaging can assist in the planning and placement of electrodes. The principal method for intracranial EEG monitoring in patients with drug-resistant focal epilepsy is SEEG [38], driven by advances in robotic surgery systems and the need to mitigate adverse effects on patients. This minimally invasive technique facilitates diagnosis and has demonstrated a reduction in complications compared to open craniotomy despite inserting electrodes deep into the brain tissue [39, 40].
The role of anatomic and functional imaging in pediatric epilepsy
Magnetic resonance imaging
The odds of being seizure-free after surgery are two to three times higher in lesional epilepsy than in patients with non-lesional refractory epilepsy [41, 42]. MRI is the preferred method for identifying anatomic abnormalities in children with epilepsy. Multiple etiological factors have been identified as potential causes of lesional epilepsy, such as tumors, mesial temporal sclerosis, and vascular or cortical malformations. Notably, malformation of cortical development, including focal cortical dysplasia and neurodevelopmental tumors, represents the leading causes of drug-resistant pediatric epilepsy cases. Although some malformations have a somatic or germline genetic basis, identifying subtle structural lesions generally requires MRI studies using specific epilepsy protocols [43]. Using 3 Tesla MR systems was shown to reclassify approximately 5% of the patients previously diagnosed with cryptogenic epilepsy on 1.5 Tesla MR systems [44]. Even with the utilization of 3 Tesla MRI, no MRI abnormalities are identified in about 30–40% of patients with non-lesional refractory epilepsy [45].
Functional and molecular imaging
[18F]FDG-PET
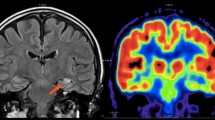
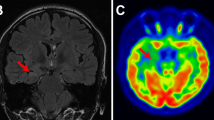
In the absence of an identifiable lesion on imaging, it is more difficult to identify putative boundaries of the seizure onset zone. Without clear boundaries, the extent of resection may be more variable. For this reason, functional imaging plays a crucial role in MRI-negative epilepsy. Multiple studies [46,47,48] have demonstrated that 18-fluoro-2-deoxyglucose (FDG)-PET effectively identifies the epileptogenic zone in cases of negative MRI (Fig. 2) or subtle findings on MRI (Fig. 3). This is particularly relevant in pediatric patients with incomplete myelinization, which creates challenges in the identification of some lesions. FDG-PET correctly lateralized the seizure focus in 87% of patients with temporal lobe epilepsy and normal conventional imaging [49]. Furthermore, patients with positive FDG-PET but negative MRI can still have excellent surgical outcomes [50].
A 3-year-old female with complex partial epilepsy showing seizure lateralization to the right centroparietal region on EEG. a Axial T1-weighted MRI shows no structural abnormality. b Axial FDG-PET and (c–d) 3D statistical parametric maps of FDG-PET demonstrate marked focal hypometabolism in the right parieto-occipital lobe (white arrows), consistent with an epileptogenic zone, and additional asymmetric decreased FDG in the mesial temporal lobe (black arrows), likely related to inhibitory neural network dysfunction. The patient underwent MRI-guided laser interstitial thermal therapy to ablate the hypometabolic focus, and pathology results revealed cortical dysplasia type II
An 18-year-old male with drug-resistant epilepsy. a Axial FLAIR image shows hypomyelination and indistinctness of the gray-white matter junction throughout the left cerebral hemisphere (arrows). b–c Axial FDG-PET reveals diffuse left hemisphere hypometabolism (arrows) related to extensive developmental cortical malformations. These malformations were overlooked in the two prior MRI examinations, likely due to diffuse involvement
Despite the development of numerous radiotracers, FDG remains the most widely used PET tracer in epilepsy, given its relatively long half-life of 110 min and wide availability. FDG is an analog of glucose that accumulates in tissues proportional to their glucose metabolism, with peak uptake occurring approximately 30 min after injection [51]. For epilepsy, FDG-PET is performed in an interictal (between seizures) interval and demonstrates epileptogenic foci as areas of hypometabolism.
Reliable interpretation of FDG-PET requires standardized protocols to ensure repeatability and reproducibility between examinations. Patients should be comfortably positioned in a quiet, dimly lit room for at least 15 min before FDG administration and during the uptake phase, typically lasting 30 min. Patients should refrain from speaking, reading, listening to music, or engaging in any activity as much as possible throughout this period, depending on their age and level of cooperation. Maintaining wakefulness with open eyes prevents a decrease in the metabolism of visual networks, particularly the occipital cortex [52]. Ensuring clear communication with the patient and family before the scheduled appointment and encouraging the use of comfort techniques can help children cooperate with these instructions. Younger patients may have increased difficulty adhering to standardized protocol due to their age or level of understanding. In such cases, sedation can be employed to facilitate the procedure. Several studies have demonstrated that sedation and anesthesia involving propofol and sevoflurane decrease regional or global glucose metabolism rates [53, 54]. This decrease can result in diminished FDG uptake and the potential for misleading image interpretation. In pediatric patients affected by epilepsy, sedation/anesthesia administration should be performed at least 30–60 min after the FDG injection to minimize brain metabolic changes [55].
FDG-PET hypometabolism is seen in 60–90% of adults and children with temporal lobe epilepsy and 30–60% with extratemporal lobe epilepsy [56, 57]. Hypometabolism confined to the epileptogenic zone has been associated with favorable postoperative outcomes in patients with temporal lobe epilepsy but less significantly correlated in extratemporal lobe epilepsy [58]. Discordant results between the PET localization and seizure onset zone detected by EEG and clinical semiology are associated with poorer outcomes [59]. Overall, FDG-PET exhibits strong predictive value for both postsurgical outcomes and cognition, with better outcomes linked to a more restricted extent of hypometabolism [60, 61].
The EANM procedure guidelines for brain PET imaging state that close monitoring with continuous EEG recording is essential for presurgical evaluation, commencing pre-injection and persisting until at least 20 min after radiopharmaceutical administration [52]. This practice ensures that FDG is not administered during recent ictal or interictal stages, preventing regions of hypermetabolism during ictal events (Fig. 4) or post-ictal hypo-metabolism unrelated to epileptogenic focus onset (Fig. 5) leading to inaccurate interpretations of FDG-PET.
A 17-month-old female with phosphatase and tensin homolog (PTEN) associated with hemimegalencephaly. a Axial T1-weighted MRI image shows right hemisphere cortical thickening (thick arrows) and ipsilateral ventricular dilatation (thin arrow), consistent with hemimegalencephaly. b–c Axial FDG-PET shows intense hypermetabolism in the right hemisphere cortex (arrows) due to several seizure episodes during the FDG uptake phase
A 3-year-old male with refractory seizures. a Axial FLAIR hyperintensity in the left frontal gyrus is suspicious for type II focal cortical dysplasia (arrow). b–c Recent ictal events result in diffuse cerebral hypometabolism on axial FDG-PET, with superimposed patchy areas of more focally decreased uptake (arrows). This complex pattern compromises the reliability of image interpretation
[18F]FDG-PET image interpretation
An FDG-PET study is normally acquired in the interictal state and is associated with regional reduced glucose metabolism that generally extends beyond the epileptogenic focus and subsequent neural networks [62, 63]. The exact mechanism underlying hypometabolism is poorly understood. It may be multifactorial, including the hypothesis that the widespread hypometabolism observed in PET may result from a protective inhibitory effect due to synaptic plasticity. This concept aligns with hypometabolism in the seizure onset zone and the network compromise during an ictal phase, as proposed by Kumar et al. [64]. This inhibitory mechanism can also explain the presence of additional hypometabolism non-related to the epileptogenic focus in the ipsilateral frontal lobe, parietal, and basal ganglia (Fig. 6). However, contralateral hypometabolism may also indicate bilateral pathology and requires correlation with anatomical abnormalities on MRI (Figs. 7 and 8). Another manifestation of decreased metabolic activity can be observed in crossed cerebellar diaschisis (CCD), characterized by non-lesional asymmetrical cerebellar hypometabolism in the setting of supratentorial parenchymal abnormality (Fig. 9). This is linked to prolonged excitatory synaptic activity along the cortico-ponto-cerebellar pathway, which is a distinct mechanism from CCD related to stroke resulting from acute disruptions in blood supply, altering cerebral blood flow and metabolism [65]. The clinical importance of identifying these findings can assist the reader in carefully interrogating the supratentorial brain as a potential cause.
A 17-year-old female with a history of drug-resistant epilepsy with an increase in seizure frequency. a Coronal T2-weighted MRI image shows a left temporal hyperintense cortical-based non-enhancing lesion (arrows). b The lesion shows an interval increase in size on subsequent MRI (arrow), raising concern for malignancy. c–d Coronal FDG-PET demonstrates marked hypometabolism in the left temporal lobe (thick arrows) consistent with biopsy-proven low-grade glioma. Mildly decreased metabolic activity in the contralateral normal temporal lobe (thin arrows), most likely related to neural network inhibition
An 8-year-old male with epilepsy, global developmental delay, and dysmorphic features with 1q43 microdeletion. a Coronal T1-weighted MRI image revealed cortical thickening of the Sylvian fissures (arrows). b Sagital T1-weighted MRI image shows an abnormal configuration of the Sylvian fissures extending almost to the vertex (arrow). c–d Symmetric moderate hypometabolism on FDG-PET along the Sylvian fissures (arrows). This case highlights the importance of correlating metabolic findings with structural imaging for accurate interpretation
An 18-year-old male with severe intractable epilepsy. a Coronal FLAIR image shows diffuse thickened gyri and indistinct gray-white differentiation involving the left cerebral hemisphere (arrows) corresponding to marked diffusely decreased metabolic activity (arrows) (b–c). In addition, there is severely decreased FDG avidity in the contralateral cerebellar hemisphere (thin arrows) with no associated morphological abnormalities on MRI consistent with crossed cerebellar diaschisis in the setting of hemimegalencephaly
In clinical practice, FDG PET interpretation for epilepsy predominantly relies on visual analysis. In children, the interpretation of FDG-PET images requires a thorough understanding of the age-related metabolic differences between children and adults for correct interpretation and diagnosis. Children exhibit dynamic metabolic processes in their developing brains, characterized by rapid growth, synaptic development, and ongoing myelination processes, leading to variations in glucose utilization patterns compared to adult patients. In children, the mean standardized uptake value (SUVmean) is impacted by age, sex, and brain structure, with most brain regions showing a relatively monotonic increase from infancy to early adolescence (< 13 years old), followed by stable uptake to 17 years old [66]. Visual inspection of FDG-PET images is subjective and dependent on observer expertise, and thus, semiquantitative and quantitative methods are gaining importance. These methods encompass a range of techniques, including the application of computer algorithms to identify areas of abnormal metabolism. Three-dimensional stereotactic surface projections (3D-SSP) and statistical parametric mapping (SPM) enable the standardization of individual brains to a common coordinate system, facilitating precise comparison and analysis of brain structures and functions across different patients and studies, and are utilized to detect subtle changes or abnormalities in metabolism. Quantitative methods in pediatric patients face challenges related to age-dependent metabolic variations and the need for an age-appropriate normal database [67, 68]. It is imperative to correlate quantitative results with visual analysis to ensure accurate validation and interpretation.
When reviewing PET and MRI scans, whether acquired on a simultaneous PET/MRI scanner or retrospectively co-registered, attention to the images used for attenuation correction (CT for PET/CT, or μ-maps for PET/MRI) should be systematically checked. Attenuation artifacts might occur in the presence of electrodes for EEG monitoring or ventricular shunt reservoirs, potentially leading to misinterpretation. Careful consideration of these artifacts is crucial for ensuring the accuracy and reliability of the imaging data for diagnostic and treatment planning (Fig. 10).
A 10-year-old male with intractable epilepsy in the setting of tuberous sclerosis. a Axial T1-weighted MRI image shows artifacts from the patient’s shunt reservoir (arrow). b FDG-PET attenuated-corrected images and 3D statistical parametric map (c) show a large right frontotemporal region of marked hypometabolism (arrows). However, axial FDG-PET non-attenuated images (d) and 3D statistical parametric map (e) do not demonstrate metabolic abnormalities (arrows). This case illustrates the need to review non-attenuated images, as attenuation correction artifacts can lead to inaccurate quantification of radiotracer uptake
PET/MRI
Our institution acquired a simultaneous PET/MRI scanner in 2011 (Biograph mMR, Siemens, Healthineers, Erlangen, Germany) consisting of a 3T MRI with a PET insert. We have performed approximately 260 pediatric PET/MRI examinations in patients ≤ 20 years old, of which 67% were neuroimaging dedicated to the presurgical evaluation of epilepsy. While FDG-PET and MRI brain protocols may seem straightforward and easily transferable, variability exists among epilepsy protocols used across institutions and research groups, including differences in patient preparation, imaging parameters, MRI sequences, and acquisition time. The ongoing development of consensus-based pediatric imaging guidelines will further harmonize epilepsy evaluation and clinical applications.
PET/MRI combines metabolic and anatomical data, enhancing diagnostic accuracy and confidence over separate MRI and PET scans [69], improving the detection of focal epileptogenic lesions, and enhancing surgical success rates, especially in complex cases including non-lesional MRI findings or subtle cortical malformations [70,71,72,73]. Furthermore, FDG-PET facilitates the identification of additional ipsilateral (Fig. 11) abnormalities that may be overlooked on MRI and evaluates contralateral metabolic functional integrity in the setting of extensive hemispheric injury or malformations (Fig. 12), potentially shifting the surgical approach from focal resection for localized lesions to considering hemispheric disconnection in diffuse etiologies.
A 10-year-old female with refractory complex partial seizures. a Coronal FLAIR images reveal right mesial temporal sclerosis (thick arrow) and subtle cortical thickening with decreased gray-white matter differentiation in the right insula (thin arrow). b–c Coronal FDG-PET images show marked hypometabolism involving these regions (thick and thin arrows), suggesting a dual pathology, confirmed by biopsy, which demonstrated gliosis predominantly in area CA1, in the setting of hippocampal sclerosis and focal cortical dysplasia
A 2-year-old male born at 27 gestational weeks with prior intracranial hemorrhage and seizures. a Coronal T1-weighted MRI image shows severe left cerebral atrophy (arrows). b–c FDG-PET and the corresponding 3D statistical parametric map reveal left hemispheric hypometabolism and no significant metabolic abnormalities in the right cerebral hemisphere. These findings exclude contralateral hemispheric compromise before posterior quadrant disconnection
The extension and degree of hypometabolism associated with malformations of cortical development vary depending on the specific type and extension. Structural malformations of cortical development severity on MRI ranges from fewer broad gyri (pachygyria), numerous small and fused gyri, often associated with an irregular cortical folding (polymicrogyria) (Fig. 13), focal thickening or thinning of the cortex, blurring of the gray-white matter junction, and abnormal gyral patterns (focal cortical dysplasia) (Fig. 14) and gray matter heterotopias (Fig. 15). In patients with extensive cortical malformations, like hemimegalencephaly, FDG-PET plays a crucial role in identifying the additional epileptogenic zones (Fig. 16), providing essential information for surgical planning.
A 3-year-old male with focal and secondarily generalized seizures. a Axial T1-weighted MRI images show a small left insula and abnormal small gyri (arrows). b–c Axial FDG-PET shows a markedly diffuse hypometabolism (arrows), facilitating the identification of polymicrogyria in the setting of unilateral perisylvian syndrome
A 9-year-old male with drug-resistant epilepsy, developmental delay, and autism. a Coronal T2-weighted images show a subtle loss of gray-white matter differentiation in the right temporal lobe (arrow). b–c Coronal FDG-PET images show markedly decreased metabolic activity in the left temporal lobe, aiding in identifying this subtle malformation retrospectively seen on the initial MRI interpretation. This case illustrates the complementary role of FDG-PET and MRI in identifying the epileptogenic focus associated with subtle anatomic abnormalities
A 17-year-old male with severe, intractable epilepsy and hemimegalencephaly. a Axial T2-weighted image show extensive cortical malformation in the left cerebral hemisphere, more pronounced in the parietal and occipital lobes (arrows). b–c Axial FDG-PET demonstrates marked hypometabolism associated with extensive cortical malformation
In simultaneous PET/MRI systems, the solid-state PET detectors are located between the MRI body and gradient coils in a single integrated scanner. This allows for the simultaneous acquisition of MR and PET data, offering the advantages of more accurate spatial registration of PET and MRI [74], a single spatial frame of reference, and minimal motion artifacts. The patient experience is enhanced with a one-stop visit that simultaneously acquires two stand-alone imaging modalities, reducing scan time and the need for multiple visits. In pediatric patients, radiation exposure and sedation risks are of greater concern than for adults, given that children are more susceptible to radiation effects and associated secondary cancers [75] and the potential risks of sedation-related neurotoxicity [76]. Simultaneous PET/MRI acquisition has been shown to reduce sedation time and the number of sedation administrations in children [77]. The decrease in ionizing radiation in PET/MRI compared to PET/CT is due to the replacement of CT by MR for anatomical co-registration and utilizing the longer imaging times to decrease radiotracer doses without significantly compromising image quality [78]. Despite these benefits, the widespread adoption of PET/MRI is hindered by its high cost and limited availability in clinical settings. Additionally, the complexity of integrated systems may require specialized expertise for operation and readers with training in nuclear medicine and MRI, which further contributes to their restricted accessibility.
As imaging techniques evolve, alternative PET and MRI fusion approaches have emerged. Using software-based algorithms, retrospective co-registration of separately acquired PET and MRI images is feasible, offering the flexibility of using existing scanners to achieve PET/MRI fusion [79]. There are concerns that these methods may introduce errors related to image registration accuracy, potentially compromising the reliability of the fused images [80, 81]. Furthermore, software co-registration may delay data interpretation and patient management, as they involve retrospective integration of prior images and reports with different time points, a concern in pediatric patients. The choice between PET/MRI integration and retrospective co-registration depends on cost, availability, and clinical workflow. Both methods aim to integrate PET and MRI data to enhance diagnostic accuracy in pediatric epilepsy.
Conclusion
The integration of functional PET and MRI, whether through simultaneous PET/MRI or retrospectively co-registered via software packages, in the presurgical evaluation of pediatric epilepsy represents an important tool for localizing epileptogenic zones in drug-resistant cases. The presence of hypometabolism on interictal 18F-FDG PET may aid in detecting lesions on MRI or confirm an epileptogenic focus on a normal MRI. Achieving good surgical outcomes from epilepsy surgery depends on multimodality imaging to increase diagnostic confidence and accuracy in delineating epileptogenic zones, as they play a pivotal role in maximizing the effectiveness of surgery and reducing postoperative deficits.
Data availability
All authors ensure the data and materials presented comply with field standards. The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
N/A.
References
Zack MM, Kobau R (2017) National and state estimates of the numbers of adults and children with active epilepsy - United States, 2015. MMWR Morb Mortal Wkly Rep 66:821–825
World Health Organization (2019) Epilepsy: a public health imperative. Summary. World Health Organization, Geneva. (WHO/MSD/MER/19.2). Licence: CC BY-NC-SA 3.0 IGO. https://www.who.int/publications/i/item/epilepsy-a-public-health-imperative
Sander JW (2003) The epidemiology of epilepsy revisited. Curr Opin Neurol 16:165–170
CoCaTotILA E (1981) Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the commission on classification and terminology of the international league against epilepsy. Epilepsia 22:489–501
Fisher RS (2017) The new classification of seizures by the International League Against Epilepsy 2017. Curr Neurol Neurosci Rep 17:48
Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, Hirsch E, Jain S, Mathern GW, Moshe SL, Nordli DR, Perucca E, Tomson T, Wiebe S, Zhang YH, Zuberi SM (2017) ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 58:512–521
Picot MC, Baldy-Moulinier M, Daures JP, Dujols P, Crespel A (2008) The prevalence of epilepsy and pharmacoresistant epilepsy in adults: a population-based study in a Western European country. Epilepsia 49:1230–1238
Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshe SL, Perucca E, Wiebe S, French J (2010) Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE commission on therapeutic strategies. Epilepsia 51:1069–1077
Tellez-Zenteno JF, Nguyen R, Hernadez-Ronquillo L (2010) Injuries, accidents and mortality in epilepsy: a review of its prevalence risk factors and prevention. Rev Invest Clin 62:466–479
Mahler B, Carlsson S, Andersson T, Tomson T (2018) Risk for injuries and accidents in epilepsy: a prospective population-based cohort study. Neurology 90:e779–e789
Pittau F, Grouiller F, Spinelli L, Seeck M, Michel CM, Vulliemoz S (2014) The role of functional neuroimaging in pre-surgical epilepsy evaluation. Front Neurol 5:31
Hirsch LJ (2012) Long-term outcome after epilepsy surgery: relapsing, remitting disorder? Epilepsy Curr 12:140–142
Jette N, Wiebe S (2013) Update on the surgical treatment of epilepsy. Curr Opin Neurol 26:201–207
Ivanovic J, Larsson PG, Ostby Y, Hald J, Krossnes BK, Fjeld JG, Pripp AH, Alfstad KA, Egge A, Stanisic M (2017) Seizure outcomes of temporal lobe epilepsy surgery in patients with normal MRI and without specific histopathology. Acta Neurochir (Wien) 159:757–766
Engel J Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, Sperling MR, Gardiner I, Erba G, Fried I, Jacobs M, Vinters HV, Mintzer S, Kieburtz K, Early Randomized Surgical Epilepsy Trial Study G (2012) Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA 307:922–930
Ormond DR, Clusmann H, Sassen R, Hoppe C, Helmstaedter C, Schramm J, Grote A (2019) Pediatric temporal lobe epilepsy surgery in Bonn and review of the literature. Neurosurgery 84:844–856
Smyth MD, Vellimana AK, Asano E, Sood S (2017) Corpus callosotomy-open and endoscopic surgical techniques. Epilepsia 58(Suppl 1):73–79
Roland JL, Akbari SHA, Salehi A, Smyth MD (2019) Corpus callosotomy performed with laser interstitial thermal therapy. J Neurosurg 134:314–322
Remick M, McDowell MM, Gupta K, Felker J, Abel TJ (2020) Emerging indications for stereotactic laser interstitial thermal therapy in pediatric neurosurgery. Int J Hyperthermia 37:84–93
Arocho-Quinones EV, Lew SM, Handler MH, Tovar-Spinoza Z, Smyth MD, Bollo RJ, Donahue D, Perry MS, Levy M, Gonda D, Mangano FT, Kennedy BC, Storm PB, Price AV, Couture DE, Oluigbo C, Duhaime AC, Barnett GH, Muh CR, Sather MD, Fallah A, Wang AC, Bhatia S, Eastwood D, Tarima S, Graber S, Huckins S, Hafez D, Rumalla K, Bailey L, Shandley S, Roach A, Alexander E, Jenkins W, Tsering D, Price G, Meola A, Evanoff W, Thompson EM, Brandmeir N, Pediatric Stereotactic Laser Ablation W (2023) Magnetic resonance imaging-guided stereotactic laser ablation therapy for the treatment of pediatric epilepsy: a retrospective multiinstitutional study. J Neurosurg Pediatr 31:551
Aum DJ, Reynolds RA, McEvoy SD, Wong M, Roland JL, Smyth MD (2023) Laser interstitial thermal therapy compared with open resection for treating subependymal giant cell astrocytoma. J Neurosurg Pediatr 1:1–10
Caruso JP, Janjua MB, Dolce A, Price AV (2021) Retrospective analysis of open surgical versus laser interstitial thermal therapy callosotomy in pediatric patients with refractory epilepsy. J Neurosurg Pediatr 27:420–428
Aum DJ, Reynolds RA, McEvoy S, Tomko S, Zempel J, Roland JL, Smyth MD (2023) Surgical outcomes of open and laser interstitial thermal therapy approaches for corpus callosotomy in pediatric epilepsy. Epilepsia 64:2274–2285
Roland JL, Smyth MD (2019) Recent advances in the neurosurgical treatment of pediatric epilepsy: JNSPG 75th Anniversary Invited Review Article. J Neurosurg Pediatr 23:411–421
Nelson JH, Brackett SL, Oluigbo CO, Reddy SK (2020) Robotic stereotactic assistance (ROSA) for pediatric epilepsy: a single-center experience of 23 consecutive cases. Children (Basel) 7:94
Johnston JM Jr, Mangano FT, Ojemann JG, Park TS, Trevathan E, Smyth MD (2006) Complications of invasive subdural electrode monitoring at St. Louis Children’s Hospital, 1994–2005. J Neurosurg 105:343–347
Gonzalez-Martinez J, Mullin J, Vadera S, Bulacio J, Hughes G, Jones S, Enatsu R, Najm I (2014) Stereotactic placement of depth electrodes in medically intractable epilepsy. J Neurosurg 120:639–644
Jin L, Choi JY, Bulacio J, Alexopoulos AV, Burgess RC, Murakami H, Bingaman W, Najm I, Wang ZI (2021) Multimodal image integration for epilepsy presurgical evaluation: a clinical workflow. Front Neurol 12:709400
Ponisio MR, Zempel JM, Day BK, Eisenman LN, Miller-Thomas MM, Smyth MD, Hogan RE (2021) The role of SPECT and PET in epilepsy. AJR Am J Roentgenol 216:759–768
McIntosh AM, Kalnins RM, Mitchell LA, Fabinyi GC, Briellmann RS, Berkovic SF (2004) Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain 127:2018–2030
Deleo F, Garbelli R, Milesi G, Gozzo F, Bramerio M, Villani F, Cardinale F, Tringali G, Spreafico R, Tassi L (2016) Short- and long-term surgical outcomes of temporal lobe epilepsy associated with hippocampal sclerosis: Relationships with neuropathology. Epilepsia 57:306–315
Spencer SS, Williamson PD, Bridgers SL, Mattson RH, Cicchetti DV, Spencer DD (1985) Reliability and accuracy of localization by scalp ictal EEG. Neurology 35:1567–1575
Ramantani G, Maillard L, Koessler L (2016) Correlation of invasive EEG and scalp EEG. Seizure 41:196–200
Cendes F, Theodore WH, Brinkmann BH, Sulc V, Cascino GD (2016) Neuroimaging of epilepsy. Handb Clin Neurol 136:985–1014
Gaillard WD, Balsamo L, Xu B, McKinney C, Papero PH, Weinstein S, Conry J, Pearl PL, Sachs B, Sato S, Vezina LG, Frattali C, Theodore WH (2004) fMRI language task panel improves determination of language dominance. Neurology 63:1403–1408
Ota T, Kamada K, Kawai K, Yumoto M, Aoki S, Saito N (2011) Refined analysis of complex language representations by non-invasive neuroimaging techniques. Br J Neurosurg 25:197–202
Shaikh Z, Torres A, Takeoka M (2019) Neuroimaging in pediatric epilepsy. Brain Sci 9:190
Gavvala J, Zafar M, Sinha SR, Kalamangalam G, Schuele S, American Seeg Consortium sbTACNS (2022) Stereotactic EEG practices: a survey of United States tertiary referral epilepsy centers. J Clin Neurophysiol 39:474–480
Goldstein HE, Youngerman BE, Shao B, Akman CI, Mandel AM, McBrian DK, Riviello JJ, Sheth SA, McKhann GM, Feldstein NA (2018) Safety and efficacy of stereoelectroencephalography in pediatric focal epilepsy: a single-center experience. J Neurosurg Pediatr 22:444–452
Tandon N, Tong BA, Friedman ER, Johnson JA, Von Allmen G, Thomas MS, Hope OA, Kalamangalam GP, Slater JD, Thompson SA (2019) Analysis of morbidity and outcomes associated with use of subdural grids vs stereoelectroencephalography in patients with intractable epilepsy. JAMA Neurol 76:672–681
Tellez-Zenteno JF, Hernandez Ronquillo L, Moien-Afshari F, Wiebe S (2010) Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res 89:310–318
Widjaja E, Jain P, Demoe L, Guttmann A, Tomlinson G, Sander B (2020) Seizure outcome of pediatric epilepsy surgery: systematic review and meta-analyses. Neurology 94:311–321
Gaillard WD, Chiron C, Cross JH, Harvey AS, Kuzniecky R, Hertz-Pannier L, Vezina LG, CfNSfP I (2009) Guidelines for imaging infants and children with recent-onset epilepsy. Epilepsia 50:2147–2153
Winston GP, Micallef C, Kendell BE, Bartlett PA, Williams EJ, Burdett JL, Duncan JS (2013) The value of repeat neuroimaging for epilepsy at a tertiary referral centre: 16 years of experience. Epilepsy Res 105:349–355
Mellerio C, Labeyrie MA, Chassoux F, Roca P, Alami O, Plat M, Naggara O, Devaux B, Meder JF, Oppenheim C (2014) 3T MRI improves the detection of transmantle sign in type 2 focal cortical dysplasia. Epilepsia 55:117–122
Henry TR, Sutherling WW, Engel J Jr, Risinger MW, Levesque MF, Mazziotta JC, Phelps ME (1991) Interictal cerebral metabolism in partial epilepsies of neocortical origin. Epilepsy Res 10:174–182
da Silva EA, Chugani DC, Muzik O, Chugani HT (1997) Identification of frontal lobe epileptic foci in children using positron emission tomography. Epilepsia 38:1198–1208
Schlaug G, Antke C, Holthausen H, Arnold S, Ebner A, Tuxhorn I, Jancke L, Luders H, Witte OW, Seitz RJ (1997) Ictal motor signs and interictal regional cerebral hypometabolism. Neurology 49:341–350
Carne RP, O’Brien TJ, Kilpatrick CJ, MacGregor LR, Hicks RJ, Murphy MA, Bowden SC, Kaye AH, Cook MJ (2004) MRI-negative PET-positive temporal lobe epilepsy: a distinct surgically remediable syndrome. Brain 127:2276–2285
Tomas J, Pittau F, Hammers A, Bouvard S, Picard F, Vargas MI, Sales F, Seeck M, Garibotto V (2019) The predictive value of hypometabolism in focal epilepsy: a prospective study in surgical candidates. Eur J Nucl Med Mol Imaging 46:1806–1816
Hays MT, Watson EE, Thomas SR, Stabin M (2002) MIRD dose estimate report no. 19: radiation absorbed dose estimates from (18)F-FDG. J Nucl Med 43:210–214
Guedj E, Varrone A, Boellaard R, Albert NL, Barthel H, van Berckel B, Brendel M, Cecchin D, Ekmekcioglu O, Garibotto V, Lammertsma AA, Law I, Penuelas I, Semah F, Traub-Weidinger T, van de Giessen E, Van Weehaeghe D, Morbelli S (2022) EANM procedure guidelines for brain PET imaging using [(18)F]FDG, version 3. Eur J Nucl Med Mol Imaging 49:632–651
Kaisti KK, Metsahonkala L, Teras M, Oikonen V, Aalto S, Jaaskelainen S, Hinkka S, Scheinin H (2002) Effects of surgical levels of propofol and sevoflurane anesthesia on cerebral blood flow in healthy subjects studied with positron emission tomography. Anesthesiology 96:1358–1370
Song XX, Yu BW (2015) Anesthetic effects of propofol in the healthy human brain: functional imaging evidence. J Anesth 29:279–288
Tian M, Watanabe Y, Kang KW, Murakami K, Chiti A, Carrio I, Civelek AC, Feng J, Zhu Y, Zhou R, Wu S, Zhu J, Ding Y, Zhang K, Zhang H, Molecular Imaging-based Precision Medicine Task Group of AFP (2021) International consensus on the use of [(18)F]-FDG PET/CT in pediatric patients affected by epilepsy. Eur J Nucl Med Mol Imaging 48:3827–3834
Sadzot B, Debets RM, Maquet P, van Veelen CW, Salmon E, van Emde BW, Velis DN, van Huffelen AC, Franck G (1992) Regional brain glucose metabolism in patients with complex partial seizures investigated by intracranial EEG. Epilepsy Res 12:121–129
Gaillard WD, Fazilat S, White S, Malow B, Sato S, Reeves P, Herscovitch P, Theodore WH (1995) Interictal metabolism and blood flow are uncoupled in temporal lobe cortex of patients with complex partial epilepsy. Neurology 45:1841–1847
Lagarde S, Boucekine M, McGonigal A, Carron R, Scavarda D, Trebuchon A, Milh M, Boyer L, Bartolomei F, Guedj E (2020) Relationship between PET metabolism and SEEG epileptogenicity in focal lesional epilepsy. Eur J Nucl Med Mol Imaging 47:3130–3142
Willmann O, Wennberg R, May T, Woermann FG, Pohlmann-Eden B (2007) The contribution of 18F-FDG PET in preoperative epilepsy surgery evaluation for patients with temporal lobe epilepsy: a meta-analysis. Seizure 16:509–520
Guedj E, Bonini F, Gavaret M, Trebuchon A, Aubert S, Boucekine M, Boyer L, Carron R, McGonigal A, Bartolomei F (2015) 18FDG-PET in different subtypes of temporal lobe epilepsy: SEEG validation and predictive value. Epilepsia 56:414–421
Vinton AB, Carne R, Hicks RJ, Desmond PM, Kilpatrick C, Kaye AH, O’Brien TJ (2007) The extent of resection of FDG-PET hypometabolism relates to outcome of temporal lobectomy. Brain 130:548–560
Rathore C, Dickson JC, Teotonio R, Ell P, Duncan JS (2014) The utility of 18F-fluorodeoxyglucose PET (FDG PET) in epilepsy surgery. Epilepsy Res 108:1306–1314
Mendes Coelho VC, Morita ME, Amorim BJ, Ramos CD, Yasuda CL, Tedeschi H, Ghizoni E, Cendes F (2017) Automated online quantification method for (18)F-FDG positron emission tomography/CT improves detection of the epileptogenic zone in patients with pharmacoresistant epilepsy. Front Neurol 8:453
Kumar A, Semah F, Chugani HT, Theodore WH (2012) Epilepsy diagnosis: positron emission tomography. Handb Clin Neurol 107:409–424
Kudr M, Krsek P, Marusic P, Tomasek M, Trnka J, Michalova K, Jaruskova M, Sanda J, Kyncl M, Zamecnik J, Rybar J, Jahodova A, Mohapl M, Komarek V, Tichy M (2013) SISCOM and FDG-PET in patients with non-lesional extratemporal epilepsy: correlation with intracranial EEG, histology, and seizure outcome. Epileptic Disord 15:3–13
Cruz-Cortes A, Avendano-Estrada A, Alcauter S, Nunez-Enriquez JC, Rivera-Bravo B, Olarte-Casas MA, Avila-Rodriguez MA (2023) Semiquantitative analysis of cerebral [(18)F]FDG-PET uptake in pediatric patients. Pediatr Radiol 53:2574
Muzik O, Chugani DC, Juhasz C, Shen C, Chugani HT (2000) Statistical parametric mapping: assessment of application in children. Neuroimage 12:538–549
Kumar A, Juhasz C, Asano E, Sood S, Muzik O, Chugani HT (2010) Objective detection of epileptic foci by 18F-FDG PET in children undergoing epilepsy surgery. J Nucl Med 51:1901–1907
Kikuchi K, Togao O, Yamashita K, Momosaka D, Nakayama T, Kitamura Y, Kikuchi Y, Baba S, Sagiyama K, Ishimatsu K, Kamei R, Mukae N, Iihara K, Suzuki SO, Iwaki T, Hiwatashi A (2021) Diagnostic accuracy for the epileptogenic zone detection in focal epilepsy could be higher in FDG-PET/MRI than in FDG-PET/CT. Eur Radiol 31:2915–2922
Oldan JD, Shin HW, Khandani AH, Zamora C, Benefield T, Jewells V (2018) Subsequent experience in hybrid PET-MRI for evaluation of refractory focal onset epilepsy. Seizure 61:128–134
Flaus A, Mellerio C, Rodrigo S, Brulon V, Lebon V, Chassoux F (2021) (18)F-FDG PET/MR in focal epilepsy: a new step for improving the detection of epileptogenic lesions. Epilepsy Res 178:106819
Salamon N, Kung J, Shaw SJ, Koo J, Koh S, Wu JY, Lerner JT, Sankar R, Shields WD, Engel J Jr, Fried I, Miyata H, Yong WH, Vinters HV, Mathern GW (2008) FDG-PET/MRI coregistration improves detection of cortical dysplasia in patients with epilepsy. Neurology 71:1594–1601
Chassoux F, Rodrigo S, Semah F, Beuvon F, Landre E, Devaux B, Turak B, Mellerio C, Meder JF, Roux FX, Daumas-Duport C, Merlet P, Dulac O, Chiron C (2010) FDG-PET improves surgical outcome in negative MRI Taylor-type focal cortical dysplasias. Neurology 75:2168–2175
Rakheja R, DeMello L, Chandarana H, Glielmi C, Geppert C, Faul D, Friedman KP (2013) Comparison of the accuracy of PET/CT and PET/MRI spatial registration of multiple metastatic lesions. AJR Am J Roentgenol 201:1120–1123
Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB, Giles GG, Wallace AB, Anderson PR, Guiver TA, McGale P, Cain TM, Dowty JG, Bickerstaffe AC, Darby SC (2013) Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 346:f2360
Kamat PP, Sulton C, Kudchadkar SR, McCracken CE, Nguyen KM, Simoneaux SF, Mallory MD, Simon HK (2019) Procedural sedation outside the operating room and potential neurotoxicity: analysis of an at-risk pediatric population. Acad Pediatr 19:978–984
States LJ, Reid JR (2020) Whole-body PET/MRI applications in pediatric oncology. AJR Am J Roentgenol 215:713–725
Soret M, Maisonobe JA, Desarnaud S, Bergeret S, Causse-Lemercier V, Berenbaum A, Rozenblum L, Habert MO, Kas A (2022) Ultra-low-dose in brain 18F-FDG PET/MRI in clinical settings. Sci Rep 12:15341
Haddadpour M, Daneshvar S, Seyedarabi H (2017) PET and MRI image fusion based on combination of 2-D Hilbert transform and IHS method. Biomed J 40:219–225
Monti S, Cavaliere C, Covello M, Nicolai E, Salvatore M, Aiello M (2017) An evaluation of the benefits of simultaneous acquisition on PET/MR coregistration in head/neck imaging. J Healthc Eng 2017:1
Brock KK, Mutic S, McNutt TR, Li H, Kessler ML (2017) Use of image registration and fusion algorithms and techniques in radiotherapy: report of the AAPM Radiation Therapy Committee Task Group No. 132. Med Phys 44:e43–e76
Author information
Authors and Affiliations
Contributions
MRP conceived, supervised, collected data, and drafted the initial manuscript. JMZ collected data and edited the manuscript. JPW supervised and edited the manuscript. JTW, SDM, JLR, and SRT edited the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Retrospective study IRB was obtained.
Consent to participate
N/A.
Consent for publication
All authors consent to the publication of this data.
Conflicts of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ponisio, M.R., Zempel, J.M., Willie, J.T. et al. FDG-PET/MRI in the presurgical evaluation of pediatric epilepsy. Pediatr Radiol 54, 1589–1602 (2024). https://doi.org/10.1007/s00247-024-06011-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-024-06011-6