Abstract
Objectives
Preoperative 18F-fluoro-2-deoxy-d-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) is controversial to assess lymph node (LN) staging in patients with invasive bladder cancer. We proposed to use the maximum standardized uptake value (SUVmax) associated with axial-based LN size to improve the detection of regional LN metastasis.
Methods
From May 2015 to May 2017, we prospectively included patients with urothelial bladder cancer who underwent radical cystectomy with extended pelvic LN dissection. All patients underwent preoperative 18F-FDG PET/CT staging before surgery. The gold standard comparator was the pathological examination of resected LNs. The data were reported on a regional per area- and patient-based model according to SUVmax values and axial-based LN size criteria.
Results
In total, 1012 LNs were identified in 61 patients with clinically localized invasive bladder cancer who underwent radical cystectomy and extended pelvic LN dissection. Loco-regional involvement of 24 LN areas was confirmed in 17 patients. In per area analysis, diagnostic accuracy of PET/CT and CT alone were respectively 84% and 78% (p = 0.039). On patient-based analysis, combined PET/CT correctly classified pelvic LN status in 5/61 (+ 8%) additional patients using optimal thresholds compared to CT alone, with accuracies of 82% and 74%, respectively (p = 0.13).
Conclusion
Combining SUVmax and axial-based LN size criteria using 18F-FDG PET/CT improved the diagnostic accuracy for preoperative LN staging in patients with invasive bladder cancer, in per area analysis.
Key Points
• Combining metabolical and morphological features using18F-FDG PET/CT improves the detection of malignant lymph node in patients with bladder cancer.
• 18 F-FDG PET/CT may help for initial staging of patients with muscle invasive bladder cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bladder cancer is one of the most common and lethal cancer worldwide, with approximately 430,000 new cases and more than 165,000 related deaths per year, a figure anticipated to double in the near future [1, 2]. Although a strong male predominance is observed with three-quarters of all bladder cancer cases occurring in men, some increasing trends have been seen in women [3]. Highest incidence rates affect Southern and Western Europe, North America, and as well certain countries in Northern Africa or Western Asia. At diagnosis, 25% of patients have muscle-invasive bladder cancer (MIBC), characterized by high rates of metastasis and 5-year survival < 50% despite radical surgery [4]. Among patients with MIBC, evaluation of pelvic lymph node (LN) involvement is a critical step to select the optimal treatment strategy. Pelvic LN involvement is one of the most pejorative prognostic factors for overall survival [5]. Patients with regional LN invasion reportedly benefit from frontline cisplatin-based chemotherapy alone, whereas perioperative chemotherapy associated with radical cystectomy (RC) and extended pelvic lymph node dissection (ePLND) are the gold-standard treatment for patients with T2-T4aN0M0 bladder cancer.
Several studies evidenced the ability of 18F-fluoro-2-deoxy-d-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) to detect more distant metastases in comparison to CT alone, and its potential impact on management of patients with muscle invasive bladder cancer [6,7,8]. Nevertheless, currently, there is no evidence supporting the routine use of PET in the nodal staging of MIBC. Therefore, the use of abdomen computed tomography (CT) or magnetic resonance imaging (MRI) has been recommended for evaluation of pelvic LN involvement. Solely based on morphological criteria, LNs are considered to be abnormal if larger than 8 mm in the short-axis diameter [9]. With a sensitivity of 45.5% and a specificity of 91.5%, this diagnostic criterion remains dramatically imperfect, leading to the allocation of unnecessary treatment [10]. Several studies have evaluated the reliability of 18F-FDG PET/CT for the detection of malignant pelvic LNs in MIBC [10,11,12,13,14,15,16,17,18,19]. Most demonstrated a minimal advantage of 18F-FDG PET/CT over CT alone. However, the heterogeneous design of these studies did not allow reliable conclusions to be drawn. Arguably, various cut-off values have been used for the analyses of CT images, such as LNs larger than 10 mm [10, 13, 14, 17] or 8 mm [10, 12, 19] in short-axis or 10 mm in long axis [11, 18]. Heterogeneous methods have also been used for PET/CT analyses, by using different cut-offs of maximal standardized uptake values (SUVmax) [14, 18,19,20] or no objective cut-off [10,11,12, 15, 16]. None of those studies evaluated combined analysis taking into account both the PET and the CT data. Two recent studies highlighted the major impact of the threshold values on diagnostic performances for the size on CT [10] and for the SUVmax on 18F-FDG PET [20]. Owing to the previous findings in our retrospective cohort [13], we hypothesized that combining the use of quantification with SUVmax and the morphological features of axial-based LN size criteria may increase the detection of regional LN metastasis in bladder cancer (there was no overlap in the patients between this previous study and the current one).
The objective of our study was to compare the accuracy of prospectively acquired 18F-FDG PET/CT to the accuracy of CT alone for preoperative regional LN staging in patients with MIBC.
Materials and methods
Patient population
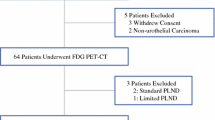
Between May 2015 and May 2017, a total of 112 patients with MIBC at diagnosis (≥ pT2 proven by transurethral resection of the bladder tumor) were prospectively referred to our Nuclear Medicine Department for baseline staging. All patients underwent 18F-FDG PET combined with CT scan, as a standard of care. Inclusion criteria were age ≥ 18 years old, no previous pelvic or genitourinary cancer, and no distant metastasis on conventional imaging. A total of 51 patients were excluded based on the following exclusion criteria (Fig. 1): distant metastasis detected with 18F-PET/CT (n = 24), prior neoadjuvant chemotherapy (NAC) (n = 14), non-operable patient or patient’s refusal of surgery (n = 6), lost to follow-up (n = 5), unilateral PLND because of macroscopic LN involvement (n = 1), hematological disease preventing pelvic LN interpretation (n = 1). Hence, the study population consisted of 61 patients with clinically localized MIBC without prior NAC. This study was approved by a national ethical committee. All the patients included provided written informed consent.
Equipment and 18F-FDG PET/CT protocol
A 6-h fasting and capillary glycemia less than 12 mM/L were required before intravenous injection of 18F-FDG (4 MBq/kg). Patients without continence disorders or contraindication received an intravenous injection of furosemide (20 mg) immediately after administration of 18F-FDG. After voiding, PET and CT images were acquired 60-min post-injection, from vertex to mid-thigh, in supine position, arms above the head, on an integrated Discovery 610 Elite PET/CT system (GE Healthcare). Iterative reconstruction with 32 subgroups and 2 iterations was used with a matrix of 256 for PET. No CT contrast agent was used. Parameters for CT images were 120 kV, 180 mAs, slice thickness 2.5 mm, pitch 1.3, and matrix of 512.
Image analysis
Two pelvic LN areas were defined: a right one and a left one, each one including the ipsilateral external iliac, obturator, common iliac, and internal iliac regions. For each patient, PET/CT images were interpreted by two experienced nuclear medicine physicians (AG and JFG), who recorded the size in short-axis of the largest LN in the area and the SUVmax of the most intense LN in the area. In case of discrepancy, both investigators were asked to perform a new analysis allowing to reach a consensus at second reading. A pelvic area was considered “LN-positive” (1) on CT alone, if ≥ 1 LN short-axis diameter was >8 mm [9, 10]; (2) on PET alone, if ≥ 1 LN had an SUVmax > 2; (3) on combined PET/CT analysis, if ≥ 1 LN short-axis diameter was > 8 mm with SUVmax > 2, or ≥ 1 LN short-axis diameter was > 10 mm regardless of the SUV max, or SUVmax was > 4 regardless of the LN size. Otherwise, the area was considered “LN-negative.” For this latest integrated interpretation of PET/CT, we combined optimal thresholds from SUVmax and axial-based LN size criteria, in order to maximize accuracy. According to data from the literature, short-axis > 8 mm [10] or SUVmax > 2 [20] provided higher sensitivities, whereas short-axis > 10 mm [10] or SUVmax > 4 [20] provided higher specificities.
On a patient-based model, the patient status was considered “LN-positive” if at least one of the pelvic LN areas was “LN-positive.” If not, the patient was considered “LN-negative”.
Surgical management
Preoperative clinical staging included bimanual examination, cystoscopy, and cross-sectional radiographic assessment plus 18F-FDG PET/CT results. All patients underwent standardized open radical cystectomy and ePLND after conventional staging and 18F-FDG PET/CT. LNs were dissected along the ilio-obturator region and from the internal and external iliac arteries up to the common iliac vessels and the aortic bifurcation, according to the template defined by the European Urology Association [9]. Therefore, tissue from each anatomic field was sent separately for histologic evaluation, allowing correlation with preoperatively performed 18F-FDG PET/CT imaging.
Histopathological examination
Pathological review was centralized and performed by two uropathologists (CR and ST). Each LN was individualized from the fresh sample and processed according to the ADASP recommendations [21]. Resected LNs were fixed overnight in 4% formalin, degreased with acetone, and then completely embedded in paraffin. Samples were sectioned into 3-mm thick tissue slices and stained with hematoxylin and eosin. Step by step sections stained with hematoxylin and eosin for histologic evaluation (200-mm-thick slices) were performed to detect the presence or absence of LN metastases and micrometastases. The number of LNs positive for metastatic disease and the total number of LNs examined microscopically were reported. A pelvic area was considered malignant if one or more LN was involved. Otherwise, the area was considered as free of malignancy.
Statistical analyses
Statistical analyses were performed using MedCalc Statistical Software version 16.2.0 (MedCalc Software bvba). Diagnostic accuracies, sensitivities, and specificities of PET and combined PET/CT were compared to that of CT alone using the McNemar test. Statistical dispersion of short-axis diameter and SUVmax values is presented by median and interquartile range (IQR). LN sizes and 18F-FDG uptake were compared in malignant and non-malignant pelvic LN areas using the bilateral Wilcoxon-Mann-Whitney test. The alpha risk was fixed at α = 0.05. A sample size of 116 patients was required to provide 80% power to detect an improvement in accuracy of 10%, considering that one third of included patients would have distant metastases at time of diagnosis and would be excluded.
Results
Population characteristics
The median age was 73 years old and sex distribution was 92% men (n = 56) and 8% women (n = 5). The median time between 18F-FDG PET/CT and RC was 65 days (range 2–140). A total number of 1012 LNs were identified from 122 pelvic areas and 61 patients. The median LNs yield per patient was 17 LN (range 4–37). Sixty-two LN metastases from bladder cancer were identified in 17 patients distributed in 24 regions. Median LN density was 9% (total of 62 positive LNs among 327 excised LNs) in patients with LN involvement. The population characteristics are presented in Table 1.
LN staging with CT or PET interpretation alone
There was a statistically significant difference between the size of malignant measurable LNs (median short-axis = 7 mm, IQR 5 mm–9 mm) and LNs free of malignancy (median short-axis = 5 mm, IQR 5 mm–6 mm) (p = 0.015). Similarly, malignant measurable LNs had significantly higher SUVmax values (median SUVmax = 1.9, IQR 1.4–4.3) compared to LNs free of malignancy (median SUVmax = 1.3, IQR 1.0–1.7) (p = 0.002). In 35 regions, LNs were too small to be detected by CT and PET. Sensitivity and specificity on patient-based analysis were 41% and 86% respectively for CT, and 47% and 91% respectively for PET alone. Sensitivity and specificity on regional-based analysis were 25% and 91% respectively for CT, and 29% and 94% respectively for PET alone. Diagnostic performances for CT and PET alone are presented in Table 2. For each pelvic LN area where at least one LN was detectable, the LN with the highest SUVmax was also the largest one.
LN staging with integrated interpretation of combined PET/CT
Based on regional analysis, diagnostic accuracy of combined 18F-FDG PET/CT was 84%, and sensitivity and specificity were 29% and 97%, respectively. Compared to CT alone, diagnostic accuracy was significantly improved on regional-based analysis (p = 0.039) (Fig. 2). However, this improvement did not reach statistical significance on patient-based analysis, with a diagnostic accuracy of 82% (Table 2). The pathological status of each pelvic LN area according to axial-based LN size and SUVmax of the largest and the most intense LNs are presented in Table 3. Among the 9 patients who had no visualized LN, neither on CT, nor on PET images, 3 had pelvic LN involvement on final pathological examination.
18F-FDG-PET/CT fusion images (upper row) and CT images (lower row) of (a) a patient with and (b) a patient without pelvic LN involvement who were misclassified as LN-negative and LN-positive, respectively, based on CT. Both patients were correctly classified with combined 18F-FDG PET/CT interpretation
In the 3 false-positive areas on combined PET/CT interpretation, the largest and most intense LN was located in the obturator region. In the 7 true-positive areas, it was located in obturator region in three patients (43%), in the common iliac in two (29%), in the internal iliac in one (14%), and in the external iliac in one (14%). In the 14 patients (28 areas) excluded due to NAC, no discordance was found between combined PET/CT results and CT alone (13 “LN-positive” and 15 “LN-negative” areas).
Discussion
In this study, we analyzed 122 pelvic LN areas from 61 patients using the metabolical and the morphological information of 18F-FDG PET/CT, compared to CT alone, for the detection of pelvic LN involvement of MIBC. Based on regional analysis, we found that diagnostic accuracy with 18F-FDG PET/CT was significantly higher than with CT alone.
Regional and distant staging is of major importance in the management of MIBC, since accurate staging dramatically impacts treatment efficacy. Although multiphase contrast enhanced CT is usually used alone for MIBC staging, several studies have shown that combined 18F-FDG PET/CT is superior for detection of distant metastases in bladder cancer [5, 12]. Arguably, combined 18F-FDG PET/CT has demonstrated a positive impact on patient management [6, 7]. Nevertheless, data regarding the benefit of 18F-FDG PET/CT over CT alone in preoperative LN staging of MIBC are conflicting [10,11,12,13,14,15,16,17,18,19]. It is important to note that, in most of the previously published studies, evaluation criteria of combined PET/CT relied exclusively on PET images analysis, not taking into account anatomical information [10,11,12,13,14,15,16,17,18,19]. Furthermore, interpretation criteria were highly heterogeneous (Table 4). For instance, Jeong et al [14], Uttam et al [17], Aljabery et al [18] and Soubra et al [19] used an objective threshold of SUVmax, whereas subjective evaluation by practitioners was used for other studies [10,11,12,13, 15, 16]. Also, CT interpretation varied with objective thresholds of size in short-axis of 8 mm [10, 12, 19] or 10 mm [10, 13, 14, 17] used in some studies, and with 10 mm in the long-axis used in some others [11, 18]. As a consequence, results were also highly heterogeneous, with a diagnostic accuracy of 18F-FDG PET/CT varying from 65% [11] to 90% [19] (Table 4). We proposed that integrated interpretation of PET/CT may improve diagnostic accuracy by combining optimal thresholds from SUVmax and axial-based LN size criteria. Vind-Kezunovic et al [20] recently reported the importance of the SUVmax threshold value to increase PET diagnostic performances for pelvic LN staging. In this study, the sensitivity and specificity were 79.4% and 66.5%, respectively, when LNs were considered malignant if SUVmax was > 2. The sensitivity and specificity were 61.8% and 84.5%, respectively, with a threshold value of SUVmax > 4. Pichler et al [10] showed that CT for pelvic LN staging of MIBC had 45.5% sensitivity and 91.5% specificity when the LN short-axis criterion was > 8 mm, and 27.3% and 96.6%, respectively, when the LN short-axis threshold was set at 10 mm. Based on these recent studies, we selected optimal thresholds values for SUVmax and axial-based LN size. In our protocol study, a pelvic LN area was considered “LN-positive” with combine 18F-FDG PET/CT when ≥ 1 LN had SUVmax > 4, or short-axis diameter > 10 mm, or ≥ 1 LN had SUVmax > 2 and short-axis diameter > 8 mm. Based on regional analysis, we demonstrated that combining metabolic and anatomic features by PET/CT enhances the diagnostic accuracy compared to CT alone. On patient-based analysis, the improvement was not statistically significant (p = 0.13), probably because of the small sample size. Sensitivity, specificity, and accuracy in our study were 41%, 86%, and 74%, respectively for CT alone, and 47%, 91%, and 79% respectively for PET alone, on patient-based analysis (Table 3). It matched with polled diagnostic performances of previously published studies (Table 4). However, our integrated PET/CT interpretation permitted to correctly identify the pelvic LN status in 5/61 (+ 8%) more patients than CT alone, and 3/61 (+ 5%) more patients than PET reading alone.
Previous studies reported no advantage of 18F-FDG PET/CT for local staging of bladder tumors, since the presence of radioactive urine in the bladder dramatically limits its performances [22]. On the contrary, recent studies highlighted the role of functional multiparametric MRI to detect bladder malignancy [23] and accurately assess bladder tumor invasion depth [24, 25]. Altogether, these data further reinforce the promising role of functional imaging in loco-regional staging of bladder cancer.
Our study has some limits. First, CT and PET performances for regional LN staging may have been underestimated because of the use of unenhanced CT and the lack of “time of flight” technology on the PET system. On the other hand, diuretic administration prevented radioactive urine accumulation in the ureter to be mistakenly reported as lymph node metastasis [26]. Second, patients with neoadjuvant chemotherapy were excluded. Third, regional-based analysis is a simplified evaluation compared to per area analysis according to the template of ePLND, and we did not perform imaging-to-pathology matching for each LN. Furthermore, the number of LN removed was inferior to 10 LNs in 11 patients, limiting the accuracy of LN staging [11]. Finally, for 6 patients, the delay between 18F-FDG PET/CT and surgery was longer than 90 days (maximum time period recommended by the EAU [9]). Among them, 3 patients had LN involvement at histopathological analysis, whereas CT, PET, and combined PET/CT were normal. If malignant cells have spread to pelvic LN during this delay, accuracies for each imaging strategy could have been equally underestimated.
The presence of extravesical disease, including isolated pelvic LNs, is correlated with poor prognosis and dramatic shortening of overall survival [4]. Identifying those patients is a critical step in terms of therapeutic management. Thus, every effort must be made at time of diagnosis to identify LN involvement and provide full-course induction systemic chemotherapy to LN-positive non-metastatic patients. The high specificity (≥ 95%) of combined PET/CT to detect malignant pelvic LNs, both on regional-based and patients-based analyses, helps to select the right patients for systemic chemotherapy rather than useless radical cystectomy. In the new era of immunotherapy, 18F-FDG PET/CT should also be assessed for predicting and monitoring treatment response [27, 28]. As a conclusion, integrated interpretation of 18F-FDG PET/CT using optimal thresholds for SUVmax and axial-based LN size improved diagnostic accuracy for baseline LN staging in MIBC, compared to CT alone, on regional-based analysis. 18F-FDG PET/CT may contribute to stratify therapeutic decision and individual treatments.
Abbreviations
- ePLND:
-
Extended pelvic lymph node dissection
- IQR:
-
Interquartile range
- LN:
-
Lymph node
- MIBC:
-
Muscle-invasive bladder cancer
- NAC:
-
Neoadjuvant chemotherapy
- NMIBC:
-
Non-muscle-invasive bladder cancer
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- RC:
-
Radical cystectomy
- SUVmax :
-
Maximal standardized uptake value
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Sanli O, Dobruch J, Knowles MA et al (2017) Bladder cancer. Nat Rev Dis Primers 3:17022
Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F (2017) Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol 71(1):96–108
Kamat AM, Hahn NM, Efstathiou JA et al (2016) Bladder cancer. Lancet 388(10061):2796–2810
Hautmann RE, de Petriconi RC, Pfeiffer C, Volkmer BG (2012) Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long-term results in 1100 patients. Eur Urol 61(5):1039–1047
Apolo AB, Riches J, Schöder H et al (2010) Clinical value of fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in bladder cancer. J Clin Oncol 28(25):3973–3978
Mertens LS, Fioole-Bruining A, Vegt E, Vogel WV, van Rhijn BW, Horenblas S (2013) Impact of (18) F-fluorodeoxyglucose (FDG)-positron-emission tomography/computed tomography (PET/CT) on management of patients with carcinoma invading bladder muscle. BJU Int 112(6):729–734
Yang Z, Cheng J, Pan L et al (2012) Is whole-body fluorine-18 fluorodeoxyglucose PET/CT plus additional pelvic images (oral hydration-voiding-refilling) useful for detecting recurrent bladder cancer? Ann Nucl Med 26(7):571–577
Alfred Witjes J, Lebret T, Compérat EM et al (2017) Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol 71(3):462–475
Pichler R, De Zordo T, Fritz J et al (2017) Pelvic lymph node staging by combined18F-FDG-PET/CT imaging in bladder cancer prior to radical cystectomy. Clin Genitourin Cancer 15(3):e387–e395
Hitier-Berthault M, Ansquer C, Branchereau J et al (2013) 18 F-fluorodeoxyglucose positron emission tomography-computed tomography for preoperative lymph node staging in patients undergoing radical cystectomy for bladder cancer: a prospective study. Int J Urol 20(8):788–796
Goodfellow H, Viney Z, Hughes P et al (2014) Role of fluorodeoxyglucose positron emission tomography (FDG PET)-computed tomography (CT) in the staging of bladder cancer. BJU Int 114(3):389–395
Rouanne M, Girma A, Neuzillet Y et al (2014) Potential impact of 18F-FDG PET/CT on patients selection for neoadjuvant chemotherapy before radical cystectomy. Eur J Surg Oncol 40(12):1724–1730
Jeong IG, Hong S, You D, Hong JH, Ahn H, Kim CS (2015) FDG PET-CT for lymph node staging of bladder cancer: a prospective study of patients with extended pelvic lymphadenectomy. Ann Surg Oncol 22(9):3150–3156
Swinnen G, Maes A, Pottel H et al (2010) FDG-PET/CT for the preoperative lymph node staging of invasive bladder cancer. Eur Urol 57(4):641–647
Kibel AS, Dehdashti F, Katz MD et al (2009) Prospective study of [18F] fluorodeoxyglucose positron emission tomography/computed tomography for staging of muscle-invasive bladder carcinoma. J Clin Oncol 27(26):4314–4320
Uttam M, Pravin N, Anish B, Nandita K, Arup M (2016) Is [F-18]-fluorodeoxyglucose FDG-PET/CT better than ct alone for the preoperative lymph node staging of muscle invasive bladder cancer? Int Braz J Urol 42(2):234–241
Aljabery F, Lindblom G, Skoog S et al (2015) PET/CT versus conventional CT for detection of lymph node metastases in patients with locally advanced bladder cancer. BMC Urol 15:87
Soubra A, Hayward D, Dahm P et al (2016) The diagnostic accuracy of 18F-fluorodeoxyglucose positron emission tomography and computed tomography in staging bladder cancer: a single-institution study and a systematic review with meta-analysis. World J Urol 34(9):1229–1237
Vind-Kezunovic S, Bouchelouche K, Ipsen P, Høyer S, Bell C, Bjerggaard Jensen J (2017) Detection of lymph node metastasis in patients with bladder cancer using maximum standardised uptake value and18F-fluorodeoxyglucose positron emission tomography/computed tomography: results from a high-volume centre including long-term follow-up. Eur Urol Focus. https://doi.org/10.1016/j.euf.2017.06.005
ADASP Committee. The Association of Directors of Anatomic and Surgical Pathology (2001) ADASP recommendations for processing and reporting of lymph node specimens submitted for evaluation of metastatic disease. Mod Pathol 14(6):629–632
Société Française de Médecine Nucléaire et d’Imagerie Moléculaire (2018) Recommandations de bonne pratique clinique pour l’utilisation de la TEP en cancérologie. Available via https://www.sfmn.org/drive/SFMN/GUIDES%20DE%20PROCEDURES/GuidesEtRecommandation_PublicWeb/RBP%20utilisation%20TEP%20en%20cancerologie%20thesaurus_V1.pdf. Accessed May 2018
Nguyen HT, Shah ZK, Mortazavi A et al (2017) Non-invasive quantification of tumour heterogeneity in water diffusivity to differentiate malignant from benign tissues of urinary bladder: a phase I study. Eur Radiol 27(5):2146–2152
Lee M, Shin SJ, Oh YT et al (2017) Non-contrast magnetic resonance imaging for bladder cancer: fused high b value diffusion-weighted imaging and T2-weighted imaging helps evaluate depth of invasion. Eur Radiol 27(9):3752–3758
Panebianco V, De Berardinis E, Barchetti G et al (2017) An evaluation of morphological and functional multi-parametric MRI sequences in classifying non-muscle and muscle invasive bladder cancer. Eur Radiol 27(9):3759–3766
Derlin T, Weiberg D, von Klot C et al (2016) 68Ga-PSMA I&T PET/CT for assessment of prostate cancer: evaluation of image quality after forced diuresis and delayed imaging. Eur Radiol 26:4345–4353
Davarpanah NN, Yuno A, Trepel JB, Apolo AB (2017) Immunotherapy: a new treatment paradigm in bladder cancer. Curr Opin Oncol. https://doi.org/10.1097/CCO.0000000000000366
Rijnders M, de Wit R, Boormans JL, Lolkema MPJ, van der Veldt AAM (2017) Systematic review of immune checkpoint inhibition in urological cancers. Eur Urol 72(3):411–423
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Antoine Girard.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• diagnostic study
• performed at one institution
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Antoine Girard and Mathieu Rouanne are first co-authors.
Rights and permissions
About this article
Cite this article
Girard, A., Rouanne, M., Taconet, S. et al. Integrated analysis of 18F-FDG PET/CT improves preoperative lymph node staging for patients with invasive bladder cancer. Eur Radiol 29, 4286–4293 (2019). https://doi.org/10.1007/s00330-018-5959-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5959-0