Abstract
Objectives
To determine the relationship between the number of administrations of various gadolinium-based contrast agents (GBCAs) and increased T1 signal intensity in the globus pallidus (GP) and dentate nucleus (DN).
Methods
This retrospective study included 122 patients who underwent double-dose GBCA-enhanced magnetic resonance imaging. Two radiologists calculated GP-to-thalamus (TH) signal intensity ratio, DN-to-pons signal intensity ratio and relative change (Rchange) between the baseline and final examinations. Interobserver agreement was evaluated. The relationships between Rchange and several factors, including number of each GBCA administrations, were analysed using a generalized additive model.
Results
Six patients (4.9%) received linear GBCAs (mean 20.8 number of administration; range 15–30), 44 patients (36.1%) received macrocyclic GBCAs (mean 26.1; range 14–51) and 72 patients (59.0%) received both types of GBCAs (mean 31.5; range 12–65). Interobserver agreement was almost perfect (0.99; 95% CI: 0.99–0.99). Rchange (DN:pons) was associated with gadodiamide (p = 0.006) and gadopentetate dimeglumine (p < 0.001), but not with other GBCAs. Rchange (GP:TH) was not associated with GBCA administration.
Conclusions
Previous administration of linear agents gadoiamide and gadopentetate dimeglumine is associated with increased T1 signal intensity in the DN, whereas macrocyclic GBCAs do not show an association.
Key points
• Certain linear GBCAs are associated with T1 signal change in the dentate nucleus.
• The signal change is related to the administration number of certain linear GBCAs.
• Difference in signal change may reflect differences in stability of agents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gadolinium-based contrast agents (GBCAs) have been widely used, with more than 200 million doses administered worldwide for more than a quarter of a century [1]. Because free Gd3+ ion is toxic, it is chelated with a suitable ligand molecule. The biochemical properties of various GBCAs are determined by the chemical structure of the chelator, which can be linear or macrocyclic and ionic or nonionic [2]. Chelated GBCAs show fast clearance in vivo, with 98% clearance within 24 h via renal excretion [3], and were regarded as safe and stable until 2006.
In 2006, it was first suggested that GBCAs might be the cause of nephrogenic systemic fibrosis (NSF) [4], a devastating systemic disease in patients with renal insufficiency. The widely accepted mechanism of NSF is ‘transmetallation theory’. Endogeneous cations (e.g. Zn2+, Cu2+, Ca2+ ions) can compete with Gd3+ ions for the ligand. The released free Gd3+ ions can deposit in the tissues when retained in vivo by decreased renal function. This theory explained why NSF developed only in patients with significant renal disease and most commonly when patients were administered nonionic linear GBCAs, which are theoretically the most vulnerable chemical structure to dechelation [5].
The possibility of gadolinium deposition in the brain of patients with normal renal function was first proposed in 2014 [6]. Increased signal intensity in the globus pallidus (GP) and dentate nucleus (DN) on T1-weighted magnetic resonance imaging (MRI) was observed in patients with multiple previous GBCA exposures. Subsequent postmortem studies [7, 8] confirmed gadolinium deposition in these areas of T1 shortening using inductively coupled plasma mass spectrometry. To date, multiple studies regarding increased T1 hyperintensity in the GP and/or DN associated with linear agents have been published [6–17]. For macrocyclic agents, the controversy still remains. Most studies have shown no significant correlation between the T1 signal intensity change and exposure to macrocyclic agents [10, 12–14]. However, a previous study reported increased T1 signal intensity after multiple administrations of gadobutrol, a macrocyclic agent [18]. In addition, a recent study using inductively coupled plasma mass spectrometry detected gadolinium deposition in the brain with macrocyclic agents [19], which supports deposition of macrocyclic agents.
There have been several reports comparing the effect of two agents [6, 10, 12, 20]. However, as far we know, there have been no reports comparing the effect of more than two types of GBCA in one institute, although there have been studies in autopsy [8, 19] and an animal model [21].
In our institution we have administered double-dose GBCAs in patients with cancer to increase the detection sensitivity of brain metastasis since 2005 [22], and have reported the diagnostic yield of the double-dose enhanced examinations in relation to the size of brain lesions, or type of administered contrast agents [23, 24]. The purpose of our study is to determine the relationship between the number of administrations of various GBCAs and increased T1 signal intensity in the GP and DN in patients exposed to high doses of gadolinium to possibly reveal differences in gadolinium deposition between different agents based on the 10 years’ experience with double-dose enhanced MRI.
Materials and methods
This single-centre retrospective study was approved by our institutional review board. The requirement for informed consent was waived.
Patients
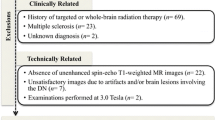
We extracted data for 179 consecutive patients who underwent double-dose MRI at least ten times. For imaging analysis, we included unenhanced T1-weighted imaging only performed with fast spin echo with inversion recovery examined in 3 T MRI and excluded examinations performed with different sequences or in 1.5 T MRI. We excluded examinations without documented information about the type and volume of administered GBCAs in the electronic medical record. The baseline MRI examination for evaluation was the first contrast-enhanced brain MRI study satisfying these conditions, whereas the final MRI examination represented the last one. We excluded patients who underwent contrast-enhanced MRI prior to the baseline examination (n = 36) and who were administered gadoxetate disodium at least once (n = 14). We also excluded patients with fewer than six administrations of GBCAs between baseline and final examinations (n = 3) based on a previous study reporting an increase in T1 signal intensity of DN in patients with six or more enhanced MRI scans [9]. Images of unsatisfactory quality due to MRI artifacts or brain lesions involving both sides of the GP, DN, thalamus (TH) or pons were excluded (n = 4). Finally, 122 patients were included in this study (Fig. 1).
Data analysis
From patient medical records, we extracted sex, age, interval between baseline and final MRI examinations, history of brain surgery, chemotherapy and radiation therapy, diagnosis of present illness, renal function, liver function, and number and type of all GBCAs administered. Radiation was defined as whole-brain radiation or tumour-selective radiation therapy.
Renal function was assessed by calculating estimated glomerular infiltration rate (eGFR) from blood samples taken at the time of the final MRI exam. Abnormal renal function was defined as eGFR less than 60 ml/min per 1.73 m2. Abnormal liver function was defined as abnormal serum concentrations of aspartate aminotransferase or alanine aminotransferase. The number and type of GBCAs administered were obtained for all types of enhanced MRI performed between baseline and final MRI examinations, including spine MRI, abdomen MRI or bone MRI. The GBCAs used at our institution during this time period included gadodiamide (Omniscan; GE Healthcare, Princeton, NJ, USA), gadopentetate dimeglumine (Magnevist; Bayer Healthcare Pharmaceuticals, Whippany, NJ, USA), gadobutrol (Gadovist; Bayer Healthcare Pharmaceuticals), and gadoterate meglumine (Dotarem; Guerbet, Bloomington, IN, USA).
Imaging protocols
MRI was performed with eight different 3 T MRI units (Achieva, Philips Medical System, The Netherlands; Ingenia, Philips Medical System; Discovery MR750, GE Medical Systems, Milwaukee, WI, USA,; and Triotim, Siemens, Erlangen, Germany). Axial unenhanced T1-weighted MRI was obtained with fast spin-echo with inversion recovery with the following parameters: repetition time ms/echo time ms, 2,000/10; inversion time, 1,000 ms; section thickness, 5 mm; spacing, 2 mm; matrix size, 256 x 256; echo train length, eight. Double-dose (0.2 mmol/kg) GBCA-enhanced brain MRI was performed, corresponding to 0.2 ml/kg of gadobutrol and 0.4 ml/kg of gadodiamide, gadopentate dimeglumine and gadoterate meglumine.
Imaging analysis
Quantitative analysis was conducted independently by two radiologists (S.K. and S.B., with 30 and 5 years of experience, respectively), who were blinded to patient information. A region of interest (ROI) was drawn in the bilateral GP, TH, DN and pons of both baseline and final unenhanced T1-weighted MRI. If the anatomical boundary was unclear on the T1-weighted images, T2-weighted images were additionally used for guidance. The measured values of right and left sides were averaged for all possible structures. The GP:TH signal intensity ratio was defined as the mean signal intensity of the GP divided by that of the TH, and DN:pons was defined as the mean signal intensity of the DN divided by that of the TH. GP:TH and DN:pons were calculated for the baseline and final MRI of all patients. The relative change (Rchange) of GP:TH and DN:pons was determined by the following formulas: Rchange (GP:TH) = (GP:THx-GP:TH0)/GP:TH0 and Rchange (DN:pons) = (DN:ponsx-DN:pons0)/DN:pons0, where x refers to the final MRI and 0 refers to the baseline MRI in the same patient.
Statistical analysis
Interobserver agreement between the ROI measurements for each structure for two readers was evaluated with Lin concordance correlation [25]. Lin concordance correlation coefficients less than 0.9 indicate poor agreement; 0.90–0.95, moderate agreement; >0.95–0.99, substantial agreement; and >0.99, almost perfect agreement.
Rchange (GP:TH) and Rchange (DN:pons) were evaluated with a generalized additive model (GAM) [26], a method of non-parametric regression analysis. A generalized cross-validation criterion was used as a smoothing parameter estimation method. The model was defined as follows: Rchange = sex + s(age) + s(interval) + neurosurgery + chemotherapy + radiation therapy + renal function + s(administered number of gadodiamide) + s(administered number of gadopentate dimeglumine) + s(administered number gadobutrol) + s(administered number of gadoterate meglumine). The function ‘s’ was defined as the smoothing function with penalized regression splines.
Statistical analyses were conducted using statistical software (R, Statistical Package version 3.3.0; R Foundation for Statistical Computing, Vienna, Austria; www.R-project.org). The mgcv package was used to apply the GAM function. Statistical significance was defined as a P value less than 0.05.
Results
Of the 244 evaluated MRI examinations from 122 patients, left GP was excluded in one patient (in final), left TH was excluded in one patient (in final), right DN was excluded in two patients (two in baseline and final), and left DN was excluded in three patients (one in final, two in baseline and final) due to the presence of metastatic lesions involving these structures. Right DN was excluded in one patient (in baseline) and left DN was excluded in one patient (in baseline and final) due to the presence of an artifact. In these patients, the ratios were calculated based on the values of the contralateral side alone. GPs and DNs of 41 patients who underwent whole brain radiation therapy were included for image analysis, whereas GPs and DNs with metastatic lesions with/without tumour selective radiotherapy were excluded from the image analysis.
A summary of patient data is shown in Table 1. No patients were diagnosed with NSF. Twenty-three patients (18.9%) underwent brain surgery and 121 (99.2%) underwent chemotherapy. 111 patients (91.0%) had a history of targeted or whole-brain radiation therapy. 120 patients (98.4%) developed brain metastasis finally. 117 patients (96.0%) had normal renal function and all patients had normal liver function.
Between baseline and final MRI examinations, six patients (4.9%) received only linear GBCAs (mean, 20.8 number of administration; range, 15–30), 44 patients (36.1%) received only macrocyclic GBCAs (mean, 26.1; range, 14–51), and 72 patients (59.0%) received both types of GBCAs (mean 31.5; range, 12–65) (Fig. 2).
Interobserver agreement was almost perfect for ROI measurement of all eight evaluated structures (0.99; 95% confidence interval: 0.99–0.99). Rchange (DN:pons) was significantly associated with number of administrations of gadodiamide (p = 0.006) and gadopentetate dimeglumine (p < 0.001). Sex, age, interval, neurosurgery, chemotherapy, radiation therapy, renal function and number of administrations of gadobutrol and gadoteratate meglumine were not related to Rchange (DN:pons) (Table 2). Figure 3 shows the relationship between Rchange (DN:pons) and the number of administrations of various GBCAs, using the GAM function to smooth the curve. Rchange (GP:TH) was not significantly associated with the number of exposures to all types of GBCAs and other variables (Table 3). Figure 4 shows the relationship between Rchange (GP:TH) and the number of administrations of various GBCAs.
Graphs of Rchange (DN:pons) according to various GBCAs. Graphs of Rchange for DN:pons between the baseline and final MRI according to the number of administrations of (a) gadodiamide, (b) gadopentetate dimeglumine, (c) gadobutrol and (d) gadoterate meglumine. R change relative change, DN:pons dentate nucleus-to-pons ratio, GBCA gadolinium-based contrast agent
Graphs of Rchange (GP:TH) according to various GBCAs. Graphs of Rchange for GP:TH between the baseline and final MRI according to the number of administrations of (a) gadodiamide, (b) gadopentetate dimeglumine, (c) gadobutrol and (d) gadoterate meglumine. R change relative change, GP:TH globus pallidus-to-thalamus ratio, GBCA gadolinium-based contrast agent
Discussion
In this study, we showed the different effects of various GBCAs on T1 signal intensity in the DN and GP. Macrocyclic agents were not associated with signal change in the DN or GP even at high-dose accumulation conditions. In contrast, two linear agents – gadodiamide and gadopentetate dimeglumine – were associated with an increased signal in the DN.
The different effects of linear and macrocyclic GBCAs can be attributed to differences in complex stabilities. Macrocyclic GBCAs are more stable than linear agents because Gd3+ is caged in the rigid macrocyclic ring system and more energy is needed to dissociate gadolinium from the macrocycle leading to a lower tendency for gadolinium dissociation or transmetallation, as has been proved in previous studies [27, 28]. This favours the hypothesis that dissociation of Gd3+ from its chelating ligand molecule is part of the mechanism of gadolinium deposition in the brain. However, the chemical form of gadolinium deposited in the neuronal tissues has not been fully investigated and it remains unclear whether it is intact GBCA, free Gd3+ ion or other chemical species generated by transmetallation. Phosphate- and carbonate-bound gadolinium is thought not to have a T1 shortening effect [29]. This means that the increased T1 signal intensity visible on MRI does not reflect the actual amount of retained gadolinium in human tissue [5]. If we determine the chemical speciation of retained gadolinium in brain, we can understand the pathophysiology of gadolinium deposition and its clinical significance.
In our study, Rchange (DN:pons) appears to be similar or slightly more prominent for gadopentetate dimeglumine than for gadodiamide in plotted graphs (Fig. 3). This is not consistent with a previous animal study in rats, which reported a higher T1 signal intensity change of deep cerebellar nuclei after injection of gadodiamide compared with gadopentetate dimeglumine [21]. Also, gadodiamide has been reported as the most commonly implicated agent of NSF, with approximately 1.5 times more cases of NSF compared to gadopentetate dimeglumine [30]. Gadopentetate dimeglumine, the linear ionic agent, is known to be more resistant to dechelation than gadodiamide, the linear nonionic form [31]. The slightly longer elimination half-life of gadopentetate dimeglumine (94 ± 11 min [standard deviation]) than gadodiamide (77.8 ± 16 min) might have contributed to this conflicting result [32, 33]; however, this small difference with overlapping confidence intervals is insufficient to explain the result. Difference in saturation effect after high-dose administration, which was recently reported by Robert et al. [34], could be another reason. Further comparison studies between groups exclusively administered high doses of each agent would reveal the different deposition rate between the two linear agents.
Rchange (GP:TH) did not show a significant correlation with exposure to any GBCA in our study. Several previous studies reported increased signal intensity in the GP, but to a lesser degree than the DN [6, 12, 16, 20]. Postmortem studies reported that GP contained a lower concentration of gadolinium than DN [7, 19], and in an animal study with rats no elevated signal intensities were observed in GP for any GBCA [21]. Reduced deposition of gadolinium in GP might result in the statistically insignificant signal change observed in our study. Further study with a larger sample size and higher doses of exposure might reveal different regional vulnerabilities to gadolinium deposition in the brain.
In our study, many patients had metastatic lesions in the brain (98.4%), a history of radiotherapy (91.0%), chemotherapy (99.2%) and surgery (18.9%). A previous study reported increased blood-brain barrier (BBB) permeability in the tumour area by about 20% [35]. Also it has been reported that ionizing radiation can disrupt the BBB and may enhance the delivery of the drugs to the brain [36, 37]. The association between the permeability status of the BBB and the deposition of gadolinium has not been revealed yet; however, postmortem brain specimen showed 18–42% of deposited gadolinium crossed the BBB [7]. Compromised BBBs in our patients might influence the delivery and deposition of gadolinium in the neuronal tissue. Chemotherapy can also interact with other drugs by physiochemical interactions or by competing for binding sites [38, 39]. Surgery and postoperative status affect perfusion, blood volume, drug metabolism and renal or biliary drug excretion [40]. The large proportion of patients with underlying brain lesions and treatment history in our study might have affected the distribution and deposition of gadolinium, even though these factors were not statistically significant.
Our study has several limitations. First, it is retrospective study from patients who were administered different types of GBCAs. The ideal study design would be to randomly assign patients to the different agents to exclude the effect of other confounding variables, though this would be unethical and impractical. Also it would have been better to select and compare patients who were exclusively administered one agent multiple times; however, only a small number of patients received one type of agent in our institution. Instead, we tried to reveal the impact of each GBCA by statistical analysis. However, the possibility of an interaction between effects of various GBCAs remains [41]. Second, MRI was performed with eight different 3 T MRI units and we did not consider the different MRI vendors, models and coils, which could affect the measured signal intensity; however, a study reported that measured signal-to-noise ratio and contrast-to-noise ratio were fairly uniform across scanners at the same field strength [42]. We hypothesized that the measured signal intensity ratio of two structures would be comparable between different vendors of same strength. Third, we calculated the DN:pons and GP:TH to indirectly reflect gadolinium deposition in the brain. However, the pons and TH are also sites of gadolinium deposition, as reported in an autopsy study [7], and therefore may be inappropriate as a reference. However, even the cerebrospinal fluid (CSF) space showed an increase in signal intensity after gadolinium injection in an animal model [21]. We could not find a better candidate as a reference. Further study using absolute T1 value or R1 relaxivity would be useful to assess absolute signal change in the brain. Last, the clinical significance of gadolinium deposition in the brain cannot be evaluated in this study. Many patients in our study showed neurological symptoms such as cognitive impairment, focal neurological deficit, delirium or ultimately seizure, but it was impossible to establish one cause in patients with underlying malignancy, brain metastatic lesions and poor general condition. Further studies without confounding factors are required to reveal the clinical significance of brain deposition of gadolinium.
In conclusion, our study suggests that previous administration of the linear GBCAs gadodiamide and gadopentetate dimeglumine may be associated with increased T1 signal intensity in the DN under high-dose accumulation conditions. Conversely, no association was noted in our study with the macrocyclic GBCAs, gadobutrol and gadoterate meglumine.
Abbreviations
- BBB:
-
Blood-brain barrier
- CSF:
-
Cerebrospinal fluid
- DN:
-
Dentate nucleus
- eGFR:
-
Estimated glomerular infiltration rate
- GAM:
-
Generalized additive model
- GBCA:
-
Gadolinium-based contrast agent
- GP:
-
Globus pallidus
- MRI:
-
Magnetic resonance imaging
- NSF:
-
Nephrogenic systemic fibrosis
- Rchange :
-
Relative change
- ROI:
-
Region of interest
- TH:
-
Thalamus
References
Hao D, Ai T, Goerner F, Hu X, Runge VM, Tweedle M (2012) MRI contrast agents: basic chemistry and safety. J Magn Reson Imaging 36:1060–1071
Ersoy H, Rybicki FJ (2007) Biochemical safety profiles of gadolinium-based extracellular contrast agents and nephrogenic systemic fibrosis. J Magn Reson Imaging 26:1190–1197
Oksendal AN, Hals PA (1993) Biodistribution and toxicity of MR imaging contrast media. J Magn Reson Imaging 3:157–165
Grobner T (2006) Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 21:1104–1108
Kanal E, Tweedle MF (2015) Residual or retained gadolinium: practical implications for radiologists and our patients. Radiology 275:630–634
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841
McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR et al (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275:772–782
Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J et al (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276:228–232
Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC (2014) Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Investig Radiol 49:685–690
Kanda T, Osawa M, Oba H, Toyoda K, Kotoku J, Haruyama T et al (2015) High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: association with linear versus macrocyclic gadolinium chelate administration. Radiology 275:803–809
Quattrocchi CC, Mallio CA, Errante Y, Cirimele V, Carideo L, Ax A et al (2015) Gadodiamide and dentate nucleus T1 hyperintensity in patients with meningioma evaluated by multiple follow-up contrast-enhanced magnetic resonance examinations with no systemic interval therapy. Investig Radiol 50:470–472
Radbruch A, Weberling LD, Kieslich PJ, Eidel O, Burth S, Kickingereder P et al (2015) Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 275:783–791
Cao Y, Huang DQ, Shih G, Prince MR (2016) Signal change in the dentate nucleus on T1-weighted MR images after multiple administrations of gadopentetate dimeglumine versus gadobutrol. AJR Am J Roentgenol 206:414–419
Radbruch A, Weberling LD, Kieslich PJ, Hepp J, Kickingereder P, Wick W et al (2015) High-signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evaluation of the macrocyclic gadolinium-based contrast agent gadobutrol. Investig Radiol 50:805–810
Adin ME, Kleinberg L, Vaidya D, Zan E, Mirbagheri S, Yousem DM (2015) Hyperintense dentate nuclei on T1-weighted MRI: relation to repeat gadolinium administration. AJNR Am J Neuroradiol 36:1859–1865
Roberts DR, Holden KR (2016) Progressive increase of T1 signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain Dev 38:331–336
Weberling LD, Kieslich PJ, Kickingereder P, Wick W, Bendszus M, Schlemmer HP et al (2015) Increased signal intensity in the dentate nucleus on unenhanced T1-weighted images after gadobenate dimeglumine administration. Investig Radiol 50:743–748
Stojanov DA, Aracki-Trenkic A, Vojinovic S, Benedeto-Stojanov D, Ljubisavljevic S (2016) Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol 26:807–815
Murata N, Gonzalez-Cuyar LF, Murata K, Fligner C, Dills R, Hippe D et al (2016) Macrocyclic and other non–group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Investig Radiol 51:447–453
Ramalho J, Castillo M, AlObaidy M, Nunes RH, Ramalho M, Dale BM et al (2015) High signal intensity in globus pallidus and dentate nucleus on unenhanced T1-weighted MR images: evaluation of two linear gadolinium-based contrast agents. Radiology 276:836–844
Jost G, Lenhard DC, Sieber MA, Lohrke J, Frenzel T, Pietsch H (2016) Signal increase on unenhanced T1-weighted images in the rat brain after repeated, extended doses of gadolinium-based contrast agents: comparison of linear and macrocyclic agents. Investig Radiol 51:83–89
Subedi KS, Takahashi T, Yamano T, Saitoh J, Nishimura K, Suzuki Y et al (2013) Usefulness of double dose contrast-enhanced magnetic resonance imaging for clear delineation of gross tumor volume in stereotactic radiotherapy treatment planning of metastatic brain tumors: a dose comparison study. J Radiat Res 54:135–139
Kim ES, Chang JH, Choi HS, Kim J, Lee SK (2010) Diagnostic yield of double-dose gadobutrol in the detection of brain metastasis: intraindividual comparison with double-dose gadopentetate dimeglumine. AJNR Am J Neuroradiol 31:1055–1058
Ahn SJ, Chung T-S, Chang J-H, Lee S-K (2014) The added value of double dose gadolinium enhanced 3D T2 fluid-attenuated inversion recovery for evaluating small brain metastases. Yonsei Med J 55:1231–1237
Lin LI (1989) A concordance correlation coefficient to evaluate reproducibility. Biometrics 45:255–268
Hastie T, Tibshirani R (1995) Generalized additive models for medical research. Stat Methods Med Res 4:187–196
Schmitt-Willich H (2007) Stability of linear and macrocyclic gadolinium based contrast agents. Br J Radiol 80:581–582, author reply 584-585
Idee JM, Port M, Raynal I, Schaefer M, Le Greneur S, Corot C (2006) Clinical and biological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: a review. Fundam Clin Pharmacol 20:563–576
Fretellier N, Idee JM, Dencausse A, Karroum O, Guerret S, Poveda N et al (2011) Comparative in vivo dissociation of gadolinium chelates in renally impaired rats: a relaxometry study. Investig Radiol 46:292–300
Edwards BJ, Laumann AE, Nardone B, Miller FH, Restaino J, Raisch DW et al (2014) Advancing pharmacovigilance through academic-legal collaboration: the case of gadolinium-based contrast agents and nephrogenic systemic fibrosis-a Research on Adverse Drug Events and Reports (RADAR) report. Br J Radiol 87:20140307
Frenzel T, Lengsfeld P, Schirmer H, Hutter J, Weinmann HJ (2008) Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 degrees C. Investig Radiol 43:817–828
Cacheris WP, Quay SC, Rocklage SM (1990) The relationship between thermodynamics and the toxicity of gadolinium complexes. Magn Reson Imaging 8:467–481
Chang CA (1993) Magnetic resonance imaging contrast agents. Design and physicochemical properties of gadodiamide. Investig Radiol 28:S21–S27
Robert P, Lehericy S, Grand S, Violas X, Fretellier N, Idee JM et al (2015) T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats: difference between linear and macrocyclic agents. Investig Radiol 50:473–480
Qin DX, Zheng R, Tang J, Li JX, Hu YH (1990) Influence of radiation on the blood-brain barrier and optimum time of chemotherapy. Int J Radiat Oncol Biol Phys 19:1507–1510
van Vulpen M, Kal HB, Taphoorn MJ, El-Sharouni SY (2002) Changes in blood-brain barrier permeability induced by radiotherapy: implications for timing of chemotherapy? (Review). Oncol Rep 9:683–688
Yuan H, Gaber MW, Boyd K, Wilson CM, Kiani MF, Merchant TE (2006) Effects of fractionated radiation on the brain vasculature in a murine model: blood-brain barrier permeability, astrocyte proliferation, and ultrastructural changes. Int J Radiat Oncol Biol Phys 66:860–866
Mattos DM, Gomes ML, Freitas RS, Boasquevisque EM, Cardoso VN, Paula EF et al (2000) The effect of vincristine on the biodistribution of technetium-99m DTPA, GHA, and DMSA in Balb/c female mice. J Nucl Med Technol 28:271–274
Frey KW, Hoer G (1965) Effect of thyreovalun, an antithyroid drug of plant origin, on the thyroid radioiodine (I-131) incorporation test. Arztl Forsch 19:155–157
Kennedy JM, Van Riji AM (1998) Effects of surgery on the pharmacokinetic parameters of drugs. Clin Pharmacokinet 35:293–312
Ramalho J, Semelka RC, AlObaidy M, Ramalho M, Nunes RH, Castillo M (2016) Signal intensity change on unenhanced T1-weighted images in dentate nucleus following gadobenate dimeglumine in patients with and without previous multiple administrations of gadodiamide. Eur Radiol 26:4080–4088
Magnotta VA, Friedman L, First B (2006) Measurement of signal-to-noise and contrast-to-noise in the fBIRN multicenter imaging study. J Digit Imaging 19:140–147
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Seung-Koo Lee.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Funding
The authors state that this work has not received any funding.
Statistics and biometry
One of the authors has significant statistical expertise.
Ethical approval
Institutional Review Board approval was obtained.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Methodology
Retrospective, diagnostic or prognostic study, performed at one institution.
Rights and permissions
About this article
Cite this article
Bae, S., Lee, HJ., Han, K. et al. Gadolinium deposition in the brain: association with various GBCAs using a generalized additive model. Eur Radiol 27, 3353–3361 (2017). https://doi.org/10.1007/s00330-016-4724-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4724-5