Abstract
Objectives
To assess the image quality and diagnostic accuracy of 320-row area detector CT (320-ADCT) coronary angiography using 40 mL of contrast material in comparison with 60-mL protocol.
Methods
This retrospective study included 183 patients who underwent 320-ADCT coronary angiography using 40 mL of contrast and additional 183 sex- and body mass index-matched patients using 60 mL of contrast constituting the control group. Both groups used the same 5-mL/sec injection rate. Quantitative image quality measurements and diagnostic accuracies were calculated and compared.
Results
Mean attenuation and contrast-to-noise ratio (CNR) at the aorta and all coronary arteries were lower in the 40-mL group than in the 60-mL group (all, p < 0.05), except for the CNR at proximal coronary arteries at 100 kVp (p = 0.073). However, the proportion of coronary segments with vessel attenuation >250 HU was not different between groups (all, p > 0.05), except for distal coronary arteries at 80 kVp (p = 0.001). Furthermore, there were no differences in per-patient and per-segment diagnostic accuracies between the groups (all, p > 0.05).
Conclusions
320-ADCT coronary angiography using 40 mL of contrast showed image quality and diagnostic accuracy comparable to the 60-mL protocol, demonstrating the clinical feasibility of lowering the risk of contrast-induced nephropathy through contrast volume reduction.
Key points
• 320-ADCT might enable reduction of contrast material volume.
• A 40-mL contrast protocol for 320-ADCT provided acceptable image quality.
• A 40-mL contrast protocol for 320-ADCT demonstrated sufficient diagnostic accuracy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Contrast-induced nephropathy (CIN) is the third most common cause of hospital-acquired renal failure, accounting for approximately 11 % of all such cases [1]. For patients with cardiovascular pathology such as diabetes mellitus and acute myocardial infarction, the incidence of CIN has been reported to be 14.5–50 %, which is much higher than the general population [2–6]. CIN has also been associated with an extended hospital stay, increased morbidity, as well as increased medical expenses [4, 7]. Although there remain some controversies on the causal relationship between contrast material and CIN, many previous studies have shown that the total iodine load and contrast material volume correlate relatively well with the risk of CIN development regardless of the patients’ underlying renal function [8–12]. Moreover, patients with inconclusive or positive results at coronary computed tomography (CT) angiography would likely undergo subsequent invasive coronary angiograms and intervention, for whom additional use of contrast material would be inevitable. Therefore, the administration of a minimum possible amount of contrast material to every patient is strongly warranted [13, 14].
Owing to recent technical developments, coronary CT angiography now enables not only accurate detection of coronary artery stenosis but also plaque characterization, ultimately leading to the detection of vulnerable plaques. For this purpose, optimization of the contrast material protocol and proper opacification of the vessel lumen is essential. Recently, an increase in the number of detector rows has reduced the image acquisition time by increasing the scan coverage per rotation. This reduction in image acquisition time, in turn, has shortened the injection duration of contrast material, subsequently increasing the injection rate: i.e., the contrast volume for 4-channel coronary CT angiography is approximately 120 mL at 3.5 mL/sec; for 8- to 16-channel CT, the contrast volume is decreased to 100 mL at 4 mL/sec; and for 64-channel CT, it is decreased to 80 mL at 5 mL/sec [15]. With the recent introduction of the 320-row area detector CT (320-ADCT) system, we can now perform a single heartbeat scan by providing 16-cm coverage during a single axial rotation, potentially leading to even further reduction in contrast material volume. Until now, however, only a few sporadic studies have been reported regarding 320-ADCT coronary angiography utilizing smaller contrast volume [16, 17]. Furthermore, they were based on a small study population and the diagnostic accuracy of this new system has not been validated yet. Thus, the purpose of our study is to assess the image quality and diagnostic accuracy of 320-ADCT coronary angiography using 40 mL of contrast material in comparison with that using 60 mL of contrast material.
Materials and methods
Patients
From November 2013 to April 2014, a total of 183 consecutive patients (mean age, 61.9 years; age range, 29–85 years) who underwent 320-ADCT coronary angiography using 40 mL of contrast material for the clinical evaluation of coronary artery stenosis were prospectively included, comprising our study group. For comparison, additional 183 sex- and body mass index (BMI)-matched patients (mean age, 63.2 years; age range, 22–87 years) who had also undergone 320-ADCT coronary angiography with the administration of 60 mL of contrast volume from May 2011 to October 2013 were retrospectively selected as the control group in inverse chronologic order. Patients with previous history of coronary stent insertion, coronary artery bypass surgery or structural anomalies such as Marfan disease were excluded from the study population. This retrospective study was approved by our institutional review board, and the requirement for informed consent was waived because all CT examinations were clinically indicated and the study patients had direct benefit from the smaller contrast material volume.
Image acquisition
All CT examinations were performed using a 320-ADCT scanner (Aquilion ONE; Toshiba Medical Systems, Otawara, Japan). For patients whose pre-scan heart rate (HR) was 65 beats per minute (bpm) or higher, 50–100 mg of oral metoprolol (Betaloc; AstraZeneca, Södertälje, Sweden) was administered 45–60 minutes prior to the CT examination unless the patient was contraindicated to beta blockers. Then, after calcium score scanning, 0.4 mg of sublingual nitroglycerin (Nitroquick; Ethex, St. Louis, MO, USA) was administered to all patients without contraindications. The only difference between two groups was the total volume of contrast material administered, i.e., 40 mL in the study group and 60 ml in the control group. To all patients, non-ionic contrast material (Iomeron 400; 400 mgI/mL; Bracco Diagnostics, Milan, Italy) was injected into an antecubital vein at a rate of 5 mL/sec, followed by 40 mL of normal saline flush with the same flow rate using a dual power injector (Stellant; Medrad, Indianola, PA, USA). The CT scan start button was pressed manually by an experienced radiologic technician at the moment when he/she observed that the attenuation of the right ventricle became lower than that of the left ventricle. Timely observation of that moment was guaranteed through visual monitoring of the four chambers at the mid-heart level that had been scanned and reconstructed at the interval of the scanner’s fastest rotation time, 350 millisecond (msec), using a real-time bolus-tracking system (SUREStart; Toshiba Medical Systems, Japan). Actual volume scanning of the coronary arteries was performed when the time period (T1) required for heart rate check-up (normally two to three heartbeats) had elapsed after the scan start button was pushed. This empirical method for selection of the optimal scan delay is based on our experiences that the T1 time period approximately corresponds to the time for coronary arteries to show peak enhancement. In other words, this individually tailored manual CT scan initiation method could determine maximum enhancement of the coronary arteries for every patient.
All CT scan and reconstruction parameters were the same as described in a previous study [18]. In brief, the gantry rotation time was 350 msec with the best temporal resolution of 175 msec. Data acquisition was performed using a mid-diastolic prospective scanning with an electrocardiogram (ECG)-gated window of 70–80 % of the R–R interval in patients with an HR of 65 bpm or less, and 30–80 % in patients with an HR higher than 65 bpm. Cross-sectional images were reconstructed using adaptive iterative dose reduction (AIDR; Toshiba Medical Systems), 3D (standard; 50 % AIDR) and the normal soft tissue reconstruction kernel (FC43). The image noise-targeted automatic exposure control (AEC) system (SUREExposure 3D; Toshiba Medical Systems, Japan) automatically decided the optimal tube current to match the target standard deviation value of 33 as the image quality index using the lowest possible tube voltage [19]. The effective dose was derived from the dose length product and a conversion coefficient for the chest as the investigated anatomic region. This conversion coefficient (k = 0.017 mSv * mGy-1 * cm -1) is averaged between male and female models.
Image analysis
All CT images and invasive coronary angiograms were retrospectively reviewed in consensus by two experienced cardiovascular radiologists (EA Park and W Lee with 9 and 13 years of clinical experience, respectively) who were blinded to the information of contrast material volume on a picture archiving and communication system workstation (Marosis; Marotech, Seoul, Korea).
Assessment of image quality
Image noise, vessel attenuation, and contrast-to-noise ratio (CNR) were compared between the study and control groups for the evaluation of image quality. Vessel attenuations at the mid-ascending aorta and coronary arteries were measured by drawing regions of interest as large as possible inside the vessel lumen with attention to avoid arterial walls and artefacts. The mean vessel attenuation was obtained after two measurements at each vessel including the mid-ascending aorta, left main coronary artery (LM), proximal left anterior descending artery (pLAD), proximal left circumflex artery (pLCx), proximal right coronary artery (pRCA), distal left anterior descending artery (dLAD), distal left circumflex artery (dLCx) and the distal right coronary artery (dRCA). The mean attenuation of the proximal coronary arteries was calculated as the average attenuation at the pLAD, pLCx, and pRCA in each patient. The mean vessel attenuation at the distal coronary arteries was acquired as the average attenuation at the dLAD, dLCx, and dRCA in each patient. Image noise was defined as the standard deviation of vessel attenuation at the mid-ascending aorta. After measuring the attenuation of the epicardial fat immediately adjacent to the coronary artery, the CNR was calculated at each artery, as follows: CNR = (vessel attenuation – epicardial fat attenuation) / image noise.
Assessment of diagnostic accuracy
Among the study population, 21 patients from each group underwent invasive coronary angiograms, since they were clinically indicated for further evaluation. Invasive coronary angiograms were performed using the standard protocol of our hospital technique by an experienced cardiologist within 2 months after coronary CT angiography. Coronary arteries were divided as suggested by the modified American Heart Association 15 coronary segment model and the presence of significant stenosis was defined as 50 % or greater stenosis [20]. As for the reference, the results of invasive coronary angiograms were analyzed. The presence of significant stenosis was documented after interpretation of two projections for each coronary vessel using quantitative coronary analysis (QCA, version 3.3; Philips, Eindhoven, The Netherlands).
Statistical analysis
Differences in patient demographics as well as image quality between the study and control groups were analyzed using the independent t test for the 80-kVp and 100-kVp protocol, and the Mann–Whitney test for the 120 kVp protocol. Comparison of the proportion of coronary artery segments at which the vessel attenuation was greater than 250 Hounsfield units (HU; the lower cut-off value for optimal coronary enhancement [21, 22]) was obtained using the chi-square test and Fisher’s exact test, as appropriate. Per-patient and per-segment diagnostic accuracies, including sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy, were initially analyzed for only “evaluable” patients and coronary segments, excluding “indeterminate” lesions with dense calcifications or motion artefacts. Thereafter, the analysis was repeated for all patients and coronary arteries by regarding “indeterminate” lesions as significant stenosis. Thereafter, the chi-square test and Fisher’s exact test were carried out for comparison of per-patient diagnostic accuracy as well as the disease prevalence between two groups. In terms of per-segment analysis, the results were compared by using generalized estimating equations, with the assumption of a binomial distribution of the dependent variable, a logit link function, the patient as a cluster, an independent correlation model within each cluster, and the robust sandwich estimator of the variance [23]. A two-sided significance level of 5 % was considered to indicate a statistically significant difference for all analyses. Statistical analyses were performed using the SPSS 19.0 software package (SPSS, Chicago, IL, USA).
Results
Patient demographics and CT parameters
Details regarding the patient demographics and CT parameters are described in Table 1. There were no significant differences in demographic features, including the gender proportion and the mean values of age, body weight, and BMI (all, p > 0.05). In addition, although the distribution of kVp in the study and control groups was not even, the difference was not statistically significant (p = 0.068). Furthermore, the mean BMI of the two groups was not different in each of the 80-kVp, 100-kVp, or 120-kVp protocols (all, p > 0.05). The effective radiation dose was comparable in both groups; 3.05 ± 1.99 mSv for the study group and 2.88 ± 1.96 mSv for the control group (p = 0.430). For each kVp subgroup, still no difference was found in the effective dose between the study and the control group; at 80 kVp, 1.95 ± 0.90 mSv vs. 2.03 ± 1.19 mSv (p = 0.611), at 100 kVp, 3.86 ± 2.16 mSv vs. 3.95 ± 2.15 mSv (p = 0.789), and at 120 kVp, 5.19 ± 2.29 mSv vs. 4.68 ± 3.28 mSv (p = 0.773).
Comparison of image quality
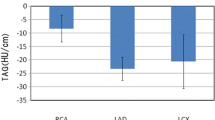
Image noise, mean vessel attenuations and the mean CNRs of the study and control groups according to tube voltage are provided in Table 2. The mean vessel attenuation at each coronary segment in the 40-mL and 60-mL groups is presented in Fig 1. In a subgroup analysis of the 80-kVp protocol, the mean vessel attenuations at the ascending aorta and all coronary vessels were significantly lower in the 40-mL group than the 60-mL group (all, p < 0.05); the mean differences of vessel attenuations were 148.3 ± 25.6 HU at the aorta, 130.8 ± 25.0 HU at the LM, 108.3 ± 22.8 HU at the proximal coronary arteries, and 132.3 ± 19.2 HU at the distal coronary arteries. A similar tendency was found in the 100-kVp protocol (all, p < 0.05), although the mean differences became much smaller; the mean differences of vessel attenuations were 107.6 ± 21.3 HU at the aorta, 74.0 ± 18.8 HU at the LM, 50.3 ± 16.6 HU at the proximal coronary arteries, and 56.9 ± 12.4 HU at the distal coronary arteries. Figures 2 and 3 provide representative CT images and reconstructions of each group. Finally, in the analysis of the 120-kVp protocol, the mean attenuations at all vessels were also lower in the 40-mL group, however, a statistical significance was not found.
Bar graphs illustrating the mean vessel attenuation (HU) at each coronary segment in the 40-mL and 60-mL groups. The error bar represents standard deviation (SD). (a) 80 kVp group (n = 82, 101 for the study and control group, respectively), (b) 100 kVp group (n = 93, 79 for the study and control group, respectively), and (c) 120 kVp group (n = 8, 3 for the study and control group, respectively). LM = left main coronary artery, pLAD = proximal left anterior descending artery, dLAD = distal left anterior descending artery, pLCx = proximal left circumflex artery, dLCx = distal left circumflex artery, pRCA = proximal right coronary artery, and dRCA = distal right coronary artery
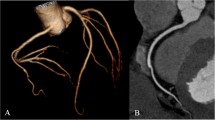
Three-dimensional volume rendering (a) and curved multiplanar reconstruction (b) images of a 63-year-old man weighing 74 kg (body mass index, 25.6 kg/m2). The CT scan was performed with 40 mL of contrast material and 100 kVp. The images display 70 % stenosis from mixed plaque (arrowheads) at the mid-segment of left anterior descending artery (LAD) with sufficient luminal attenuation of 487.6 HU and contrast-to-noise ratio of 17.71. Another mild luminal stenosis with mixed plaque (arrow) was noted at the proximal segment of the LAD. The window level/width was adjusted to 256/128 for the volume rendering image and 1231/363 for the curved multiplanar reconstruction image
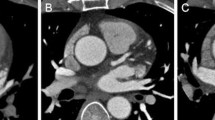
Three-dimensional volume rendering (a) and curved multiplanar reconstruction (b) images of a 65-year-old man weighing 65 kg (body mass index, 23.3 kg/m2). For this CT scan, 60 mL of contrast material was administered at 100 kVp. The images demonstrate 80 % luminal stenosis from non-calcified plaque (arrowheads) at the proximal segment of left anterior descending artery (LAD) well. The mean luminal attenuation of the proximal segment of LAD was 577.8 HU with the contrast-to-noise ratio of 29.3. The window width and level of each image was kept constant with Fig. 2
In terms of CNR, the mean value at the ascending aorta and all coronary vessels were also significantly lower in the 40-mL group in the 80-kVp and 100-kVp settings (all, p < 0.05), except for the CNR at proximal coronary segments under the 100-kVp protocol where statistical significance was not found (p = 0.073). At the 120-kVp setting, the CNR was rather higher in the 40-mL group than the 60-mL group, although it did not reach statistical significance. The coronary segments in which the attenuation was greater than 250 HU had a tendency to be more frequently found in the 60-mL group, however, no significant difference was found (all, p > 0.05) except for distal coronary segments using the 80 kVp (p = 0.001).
Comparison of diagnostic accuracy
Per-patient prevalence of significant stenosis was 78.9 % (15/19) in the 40-mL group and 100 % (18/18) in the 60-mL group (p = 0.106). Per-segment prevalences were 10.5 % (30/286) and 18.3 % (51/279) in the 40-mL group and the 60-mL group, respectively (p = 0.008). Tables 3 and 4 provide the comparison of per-patient and per-segment diagnostic accuracies between the study and control groups. Two patients and their eight coronary segments from the study group and three patients and their eleven coronary segments from the control group were considered to be “indeterminate” lesions. There were no significant differences in per-patient diagnostic accuracy between two groups, whether excluding the “indeterminate” lesions or including the “indeterminate” lesions by counting them as significant stenosis (all, p > 0.05). The results of per-segment analysis revealed equivalent diagnostic accuracy when "indeterminate" lesions were excluded (all, p > 0.05). If we assess all of the coronary segments, the PPV of the 40-mL group showed lower value compared to that of the 60-mL group (p = 0.016), but all of the other diagnostic performances were comparable between the 40-mL and 60-mL group (all, p > 0.05).
Discussion
Our study demonstrated that 320-ADCT coronary angiography using a small volume of 40 mL of contrast material is feasible by showing diagnostic accuracy comparable to that using 60 mL of contrast material. Even though the mean vessel attenuation and CNR at all coronary arteries were lower in the 40-mL group than the 60-mL group, the proportion of coronary segments which met the minimum optimal luminal attenuation of 250 HU was not different except at the distal coronary arteries using the 80-kVp protocol, suggesting an equivalent diagnostic range of image quality. Therefore, our study results demonstrated the potential of reducing the risk of contrast-induced nephropathy through contrast volume reduction in 320-ADCT coronary angiography.
Recently, several researchers have investigated the validation of coronary CT angiography using a small volume of contrast material at 256- or 320-ADCT and dual-source CT, all of which can acquire a full cardiac CT dataset within one or a few heart beats [16, 17, 24–26]. Indeed, a few studies among them have proposed that the contrast material could be reduced down to 30–40 mL at a rate of 5 mL/sec with selection of patients weighing less than 85 kg [16, 24]. However, most of these studies were based on small populations and its diagnostic accuracy has not been validated yet. Our study approached diagnostic accuracy in a relatively large number of patients and showed the potential for contrast material volume reduction without significant deterioration of image quality and diagnostic accuracy.
Coronary artery enhancement is affected by various factors: (a) patient-related factors including body weight, BMI, or haemodynamics, (b) contrast protocols such as the contrast material concentration, injection rate, or injection duration, (c) scan parameters such as the tube voltage or scan timing, and (d) the diameter and location of the coronary artery itself.
The iodine concentration and the volume of contrast material determine the total iodine load delivered to the patients. In our study, we utilized a smaller volume of relatively higher concentrated contrast material. A conversion into total iodine load revealed that our study group was administered with 256 mgI/kg on average. Even though high iodine-concentrated contrast material was used in our study, total iodine load per weight in our study group was similar to those of two Asian study groups having shown the feasibility of a small amount of contrast material: 315 mgI/kg [27] and 245 mgI/kg [28].
The scan timing is commonly determined using either the test bolus or the bolus tracking method. The former may serve as a pretest opportunity to check the overall system and the latter may be better in terms of the efficiency of contrast material usage [29, 30]. The additional contrast material used in the test bolus method may reduce the merit of small contrast volume coronary CT angiography [31]. On the other hand, it is often challenging to select the peak enhancement using the bolus tracking method as the time window of optimal coronary enhancement is relatively short when a smaller volume of contrast material is administered. Another difficulty is the scan delay is dependent on the scanner itself at hand and requires deliberate adjustment. In our study, for the individually tailored selection of peak coronary enhancement, the scan starting time was manually determined by an experienced radiologic technician at the moment when the attenuation of the right ventricle was observed to be lower than that of the left ventricle via visual monitoring of the four chambers at the mid-heart level. We could speculate that an empirically determined scan delay of T1 in our study is similar to the time window of the contrast material in the left ventricle filling the ascending aorta for a sufficient time resulting in the maximum enhancement of the coronary arteries, thus congruent with actual haemodynamics. On the ground of that, when adequately trained, radiologic technicians did not fail to determine the peak enhancement in any case, even though this method may have an inherent demerit of subjectivity. Since we could perform CT scans at the moment of possible peak coronary enhancement for each patient, the mean coronary attenuation was higher in the control group than the study group, despite the same injection rate in both groups [15, 32, 33].
Many researchers have studied the optimal CT attenuation of coronary arteries for the accurate evaluation of stenosis degree and plaque characterization [21, 22, 34]. The proposed optimal enhancement, thus far, can be summarized to be 250–450 HU; poor enhancement of less than 250 HU may reduce the detectability of coronary artery stenosis, on the other hand, too strong enhancement of coronary arteries greater than 450 HU may obscure the presence of calcified plaques resulting in underestimation of the coronary artery stenosis. Our study found that the proportion of coronary arteries which met the minimum optimal attenuation of 250 HU in the study group was comparable to that of the control group, except at distal coronary segments using the 80-kVp protocol. This result is consistent with that of a previous study which compared 40-mL and 70-mL contrast volumes in 320-ADCT coronary angiography [16]. Moreover, the mean attenuation of the proximal coronary artery using the 80-kVp protocol in our study group was 604 HU, which is even higher than the suggested optimal coronary attenuation. This result suggests the possibility of even further reduction in contrast material volume for this specific group.
Lower CT tube voltage could contribute to reduction of the contrast material volume, as suggested by previous studies [35, 36], since it allows stronger enhancement of contrast material per unit of iodine concentration as the output energy is much closer to the K edge of iodine, 33 keV. In our study, the tube voltage of each patient was determined using the guidance of an image noise-targeted AEC system; the mean BMI of the study and control groups using the 80-kVp protocol were 22.8 kg/m2 and 23.4 kg/m2, respectively. As the attenuation and body habitus in the thorax are often discordant with the body weight or BMI, individual selection of the optimal tube voltage by an image noise-targeted AEC system may be more beneficial than the determination of tube voltage according to body weight or BMI. Lim et al. also demonstrated that an image noise-targeted AEC system could safely select patients for the use of a low tube voltage with additional advantage of radiation dose reduction [19].
There are several limitations in our study. The first is its retrospective and non-randomized design. The second is the fixed contrast material volume and injection rate we applied to all patients regardless of their body weight or BMI. Previous studies had shown that an individualized contrast protocol, such as a body weight-adjusted contrast volume and injection rate, may be more effective and ideal in achieving the optimal coronary attenuation for each patient [30, 37]. However, we simplified the protocol by using two kinds of fixed contrast material volumes, leaving all other scan parameters that could affect the coronary artery attenuation identical, so as to find out the sole contribution of contrast volume to the image quality and diagnostic accuracy. In a sense, our protocol may be easier to perform in clinical practice. The third limitation is the relatively low body weight and BMI of our study population. Though being typical for an East Asian population, it could impose a limitation in the extrapolation of our results to heavy or obese patients. Lastly, the prevalence of significant stenosis was different between the study and control group in our study, thus, the direct comparison of PPV and NPV between these groups has inherent limitation.
In conclusion, the diagnostic accuracy of 40 mL of contrast material in 320-ADCT coronary angiography was comparable to that of the 60-mL contrast material protocol. Even though the mean vessel attenuation and CNR at all coronary vessels were lower in the 40-mL protocol, the proportion of coronary vessels which met the minimum optimal coronary enhancement of 250 HU was not significantly different between the study and control groups, except at distal coronary segments using the 80-kVp protocol, suggesting an equivalent diagnostic range of image quality. Our results suggest that 320-ADCT coronary angiography using 40 mL of contrast material would be clinically feasible and may contribute to the reduction in the risk of CIN.
Abbreviations
- 320-ADCT:
-
320-row area detector computed tomography
- BMI:
-
Body mass index
- CIN:
-
Contrast-induced nephropathy
- CNR:
-
contrast-to-noise ratio
References
McCullough PA, Adam A, Becker CR et al (2006) Epidemiology and prognostic implications of contrast-induced nephropathy. Am J Cardiol 98:5–13
Parfrey P (2005) The clinical epidemiology of contrast-induced nephropathy. Cardiovasc Intervent Radiol 28:S3–S11
Berg KJ (2000) Nephrotoxicity related to contrast media. Scand J Urol Nephrol 34:317–322
McCullough PA, Wolyn R, Rocher LL, Levin RN, O'Neill WW (1997) Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med 103:368–375
Manske CL, Sprafka JM, Strony JT, Wang Y (1990) Contrast nephropathy in azotemic diabetic patients undergoing coronary angiography. Am J Med 89:615–620
Maeder M, Klein M, Fehr T, Rickli H (2004) Contrast nephropathy: review focusing on prevention. J Am Coll Cardiol 44:1763–1771
McCullough PA, Soman SS (2005) Contrast-induced nephropathy. Crit Care Clin 21:261–280
Morcos S, Thomsen H, Webb J (1999) Contrast-media-induced nephrotoxicity: a consensus report. Eur Radiol 9:1602–1613
Rihal CS, Textor SC, Grill DE et al (2002) Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 105:2259–2264
Freeman RV, O’Donnell M, Share D et al (2002) Nephropathy requiring dialysis after percutaneous coronary intervention and the critical role of an adjusted contrast dose. Am J Cardiol 90:1068–1073
McDonald RJ, McDonald JS, Bida JP et al (2013) Intravenous contrast material-induced nephropathy: causal or coincident phenomenon? Radiology 267:106–118
McDonald RJ, McDonald JS, Carter RE et al (2014) Risk of intravenous contrast material-mediated acute kidney injury: a propensity score-matched study stratified by baseline-estimated glomerular filtration rate. Radiology 271:65–73
Stacul F, van der Molen AJ, Reimer P et al (2011) Contrast induced nephropathy: updated ESUR contrast media safety committee guidelines. Eur Radiol 21:2527–2541
Goldenberg I, Matetzky S (2005) Nephropathy induced by contrast media: pathogenesis, risk factors and preventive strategies. CMAJ 172:1461–1471
Wintersperger BJ, Nikolaou K (2005) Basics of cardiac MDCT: techniques and contrast application. Eur Radiol 15:B2–B9
Hein PA, May J, Rogalla P, Butler C, Hamm B, Lembcke A (2010) Feasibility of contrast material volume reduction in coronary artery imaging using 320-slice volume CT. Eur Radiol 20:1337–1343
Kumamaru KK, Steigner ML, Soga S et al (2011) Coronary enhancement for prospective ECG-gated single RR axial 320-MDCT angiography: comparison of 60-and 80-mL iopamidol 370 injection. Am J Roentgenol 197:844–850
Yoo R-E, Park E-A, Lee W et al (2013) Image quality of adaptive iterative dose reduction 3D of coronary CT angiography of 640-slice CT: comparison with filtered back-projection. Int J Cardiovasc Imaging 29:669–676
Lim J, Park E-A, Lee W, Shim H, Chung JW (2015) Image quality and radiation reduction of 320-row area detector CT coronary angiography with optimal tube voltage selection and an automatic exposure control system: comparison with body mass index-adapted protocol. Int J Cardiovasc Imaging 31:23–30
Cerqueira MD, Weissman NJ, Dilsizian V et al (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart a statement for healthcare professionals from the cardiac imaging committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105:539–542
Becker CR, Hong C, Knez A et al (2003) Optimal contrast application for cardiac 4-detector-row computed tomography. Invest Radiol 38:690–694
Johnson PT, Pannu HK, Fishman EK (2009) IV contrast infusion for coronary artery CT angiography: literature review and results of a nationwide survey. Am J Roentgenol 192:W214–W221
Liang KY, Zeger SL (1986) Longitudinal data analysis using generalized linear models. Biometrika 73:13–22
Lembcke A, Schwenke C, Hein PA et al (2014) High-pitch dual-source CT coronary angiography with low volumes of contrast medium. Eur Radiol 24:120–127
Wang Z, Chen Y, Wang Y et al (2014) Feasibility of low-dose contrast medium high pitch CT angiography for the combined evaluation of coronary, head and neck arteries. PLoS One 9, e90268
Weigold WG, Olszewski ME, Walker MJ (2009) Low-dose prospectively gated 256-slice coronary computed tomographic angiography. Int J Cardiovasc Imaging 25:217–230
Yamamuro M, Tadamura E, Kanao S et al (2007) Coronary angiography by 64-detector row computed tomography using low dose of contrast material with saline chaser: influence of total injection volume on vessel attenuation. J Comput Assist Tomogr 31:272–280
Tatsugami F, Matsuki M, Inada Y et al (2010) Feasibility of low-volume injections of contrast material with a body weight–adapted iodine-dose protocol in 320-detector row coronary CT angiography. Acad Radiol 17:207–211
Mahnken AH, Rauscher A, Klotz E et al (2007) Quantitative prediction of contrast enhancement from test bolus data in cardiac MSCT. Eur Radiol 17:1310–1319
Silverman PM, Roberts S, Tefft MC et al (1995) Helical CT of the liver: clinical application of an automated computer technique, SmartPrep, for obtaining images with optimal contrast enhancement. Am J Roentgenol 165:73–78
Bae KT, Seeck BA, Hildebolt CF et al (2008) Contrast enhancement in cardiovascular MDCT: effect of body weight, height, body surface area, body mass index, and obesity. Am J Roentgenol 190:777–784
Fleischmann D (2005) How to design injection protocols for multiple detector-row CT angiography (MDCTA). Eur Radiol 15:E60–E65
Cademartiri F, Mollet NR, Runza G et al (2005) Influence of intracoronary attenuation on coronary plaque measurements using multislice computed tomography: observations in an ex vivo model of coronary computed tomography angiography. Eur Radiol 15:1426–1431
Malayeri AA, Zimmerman SL, Lake ST, Fishman EK, Johnson PT (2014) 128-Slice dual source coronary CTA: defining optimal arterial enhancement levels. Emerg Radiol 21:499–504
Zhang LJ, Wang Y, Schoepf UJ et al (2015) Image quality, radiation dose, and diagnostic accuracy of prospectively ECG-triggered high-pitch coronary CT angiography at 70 kVp in a clinical setting: comparison with invasive coronary angiography. Eur Radiol. doi:10.1007/s00330-015-3868-z
Kok M, Turek J, Mihl C et al (2015) Low contrast media volume in pre-TAVI CT examinations. Eur Radiol. doi:10.1007/s00330-015-4080-x
Zhu X, Zhu Y, Xu H, Tang L, Xu Y (2012) The influence of body mass index and gender on coronary arterial attenuation with fixed iodine load per body weight at dual-source CT coronary angiography. Acta Radiol 53:637–642
Acknowledgments
We would like to acknowledge Ms. Hyunsook Hong from the medical research collaborating center of our institution for her assistance with the statistical analysis. The scientific guarantor of this publication is Jin Wook Chung.
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. Institutional review board approval was obtained. Written informed consent was waived by the institutional review board. Methodology: retrospective, case-control study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, R., Park, EA., Lee, W. et al. Feasibility of 320-row area detector CT coronary angiography using 40 mL of contrast material: assessment of image quality and diagnostic accuracy. Eur Radiol 26, 3802–3810 (2016). https://doi.org/10.1007/s00330-016-4275-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4275-9