Abstract
Spiny icefish (Chaenodraco wilsoni) and ocellated icefish (Chionodraco rastrospinosus) are two abundant channichthyids in the larval fish assemblages of the Ross Sea. However, little information is available about the early-life history traits of the two icefishes, which have limited the understanding of the biology and ecology of icefishes. The present study aims to investigate the early-life history traits of C. wilsoni and C. rastrospinosus in the Ross Sea. Through the analysis of sagittal otolith microstructure, the size and timing of hatching, onset of exogenous feeding, and growth rate were assessed in the two icefishes. In C. wilsoni, individuals with a standard length (SL) of 14–43 mm were estimated ages of 28–100 days. An exponential model was well fitted to the age–length dataset, indicating a mean growth rate of 0.34 mm day−1. Larval hatching occurred at a size of 9.28 mm and was distributed over a relatively long period, lasting from October to December. The onset of exogenous feeding took place between 16 and 29 days after hatching. In C. rastrospinosus, individuals with SL of 14–48 mm were estimated ages of 29–126 days and mean growth rate of 0.38 mm day−1. The larval size at hatching was estimated as 10.90 mm and hatching dates extended over a long period from September to December. The yolk-sac duration estimated from hatching to the onset of the exogenous feeding check was 21–41 days, which was longer than in other channichthyids. This study provides new information on the biological features and life history strategies of C. wilsoni and C. rastrospinosus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Ross Sea is a large embayment of the Antarctic continental shelf and is one of the most biologically productive and dynamic environmental regions within the Southern Ocean (Smith et al. 2018). Because of its high primary productivity, the Ross Sea plays an important role as a spawning and nursing ground for many fish species (Granata et al. 2002), especially for Antarctic silverfish (Pleuragramma antarctica) (La Mesa and EaStman 2012), Chionodraco humatus (Vacchi et al. 1996) and Antarctic toothfish (Dissostichus mawsoni) (Collins et al. 2010; Hanchet et al. 2015). According to a survey on the abundance and distribution of ichthyoplankton in the Ross Sea, larvae and juveniles of Nototheniidae and secondarily of Channichthyidae have been the most common species in the continental shelf ichthyoplankton community (Granata et al. 2002). In the 1970s and 1980s, some icefishes were abundant enough to be exploited by commercial fisheries as either the main target species (Champsocephalus gunnari, Chaenodraco wilsoni) or bycatch (Chaenocephalus aceratus, Pseudochaenichthys georgianus, and Chionodraco rastrospinosus) (Kock 1992). With intensive exploitation of Antarctic fishery resources, some icefishes experienced strong fishing pressure, resulting in stock declines and changes in population structure and life history traits (Papetti et al. 2010; CCAMLR 2012). Therefore, the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) established a marine protected area (MPA) in the Ross Sea to protect important areas related to the life history of Antarctic toothfish and the core distributions of trophically dominant pelagic prey species, including Antarctic silverfish and icefishes (Parker et al. 2019).
Icefishes are unique among vertebrates in that their members lack erythrocytes and respiratory pigment hemoglobin, thus they are commonly referred to as “white-blooded” fishes (Kock 2005). Icefish exhibits typical life history traits, including low reproductive effort associated with the production of large eggs, a long pelagic larval and juvenile phase lasting several months to 2–4 years in some species, and slow somatic growth after sexual maturity (Kock and Kellermann 1991; Kock 2005). It is important to understand the early-life history traits of fish, such as larval duration and incubation time, because these characteristics strongly influence the dispersal rate of larvae and the connectivity between populations (Galarza et al. 2009). Early-life history traits have been studied in some channichthyid larvae, especially in mackerel icefish (Champsocephalus gunnari) (Morley et al. 2005), blackfin icefish (Chaenocephalus aceratus) (La Mesa and Ashford 2008a), Dacodraco hunteri (La Mesa et al. 2012), and ocellated icefish (Chionodraco rastrospinosus) (La Mesa et al. 2013a). However, the current knowledge of the early ontogeny of channichthyids is incomplete and is restricted to a few species and/or sites (North 2010). The early-life history traits of spiny icefish (Chaenodraco wilsoni) and C. rastrospinosus inhabiting the Ross Sea remain unknown. Due to a lack of knowledge on the age, growth, and exact timing of the reproductive and life history traits of icefish (Traczyk and Meyer-Rochow 2019), stock assessment and resource management data are inaccurate. Furthermore, knowledge of early-life history events in these fish are essential for monitoring the population dynamics of fish stocks and effective management of fishery resources.
Otolith microstructural analysis techniques can provide accurate estimates of the hatching period and variability of individual growth rates and can allow accurate determination of the timing of significant events during the early ontogeny of Antarctic fishes (Kellermann et al. 2002; Morley et al. 2005). In the last two decades, the analysis of microincrement patterns of sagittal otoliths has been widely applied in the study of early-life history traits of Antarctic fishes (Radtke and Kellermann 1991; Llompart et al. 2020). Chaenodraco wilsoni and C. rastrospinosus larvae are the most common channichthyids within the pelagic communities in the Ross Sea and have often been found to co-occur with Antarctic silverfish (Granata et al. 2002; La Mesa et al. 2011). To date, La Mesa et al. (2013a) have provided the only report of the early-life history of C. rastrospinosus off the Antarctic Peninsula based on otolith microstructure and stomach content analyses. The timing of key events occurring in the larval stage in these two species is largely unknown, especially in C. wilsoni. In the present study, we analyzed the otolith microstructure of C. rastrospinosus and C. wilsoni larvae collected in the Ross Sea. Our aims are to provide insights into the timing and duration of key events in early ontogeny, such as larval hatching and the onset of exogenous feeding, and to estimate growth rates. Our results may be regarded as the basis for the future monitoring and conservation of fish stock recruitment and reproduction in the region.
Materials and methods
Fish sampling
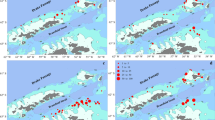
Fifty-seven icefishes (14–48 mm in standard length, SL) were collected with a rectangular midwater trawl (RMT, with a mouth of 4 m × 2 m) in the Ross Sea during 4–11 January 2020 (Fig. 1, Table 1) aboard the research vessel Xuelong, during 36th Chinese Antarctic Research Expeditions. The RMT was towed for 60 min at depths between 0 m and 800 m with 3 knots. Larvae and juveniles were collected from 3 survey stations and then stored in 95% ethanol solution.
The specimens were identified as Chaenodraco wilsoni and Chionodraco rastrospinosus according to the descriptions of Efremenko (1985) and Kellermann (1990). The stage of development was assigned to each specimen according to North and Kellermann (1990). The SL of each larva and juvenile was measured to the nearest 0.1 mm. Body shrinkage due to preservation was considered to be negligible (Fey 1999).
Otolith preparation
Sagittal otoliths were extracted with fine needles under a stereomicroscope (JSZ5A), embedded in epoxy resin and mounted on glass slides. The otolith surface was manually polished with 2500 grit waterproof and then polished with 0.05 μm alumina powder until the daily growth increments could be observed clearly under a light microscope. Finally, the otolith microstructure could be observed under 1000 × magnification using a microscope system (OLYMPUS BX53 and DP74).
Age determination
Each microstructure showed a common bipartite structure consisting of a discontinuous zone (D-zone) and an incremental zone (L-zone), which appear as dark and light regions under the transmitted light microscope (La Mesa et al. 2013a, b). The daily periodicity of growth increments has been confirmed in C. rastrospinosus (La Mesa and Ashford 2008b; La Mesa et al. 2013a) but has not been reported in C. wilsoni. We assumed the daily periodicity of increment deposition in the otoliths of C. wilsoni based on previous results because our samples showed similar deposition patterns and increment widths to those validated in Champsocephalus gunnari, Chaenocephalus aceratus, and Pseudochaenichthys georgianus (Morley et al. 2005; La Mesa and Ashford 2008a; Traczyk et al. 2021). The age of each specimen was estimated by counting all increments recorded from the primordium to the otolith margin along the same count path. Each otolith increment was read twice with ImageJ software and otoliths in which the counts differed by more than 5% were read again.
Data analyses
To estimate the reproducibility of aging precision (Campana 2001), the index of the average percent error (APE) and the mean coefficient of variation (CV) were calculated by comparing each reading. Linear, exponential, logistic, and Gompertz growth models were used to fit the age–length relationships (La Mesa et al. 2013a, b, 2017) and the best-fitting function was selected based on the correlation coefficient (R2) and Akaike’s information criterion (AIC) (Akaike 1973). The exponential model was fitted by linear regression performed on loge-transformed data to stabilize the variance, which increased considerably with fish size in the untransformed data.
The applied growth models were as follows:
where SL is the standard length (SL, mm) at age t, α is the initial SL (or incubation size), g is the instantaneous growth rate, t is the age of fish, L∞ is the theoretical asymptotic length, t0 is the theoretical age when fish length is equal to 0, and k is the growth rate at which SL approaches L∞.
Finally, the daily increase in size at the mean SL of the fish was calculated. The hatch date distribution was back calculated by subtracting the age estimates from the date of capture. The otolith radius (OR) was determined using ImageJ software along the axis with the longest distance from the nucleus to the outer edge. Least squares linear regression was used to estimate the relationship between the OR and the fish length (standard length, SL).
Mann–Whitney U test was used to compare the length–frequency distributions and exogenous feeding time among C. wilsoni and C. rastrospinosus. Analysis of covariance (ANCOVA) was used to compare otolith radius between species, with larval size as the covariate. A multiple slope test between linear models was used to compare the growth rate between species. Significant level was termed as p < 0.05. The statistical analysis and graph plotting were performed using SPSS 25.0 and R (version 4.0.3).
Results
Length–frequency distribution
A total of 57 larvae and juvenile icefish were caught in the uppermost layer of the water column, from 0 m to 400 m depth. The length–frequency distributions of C. wilsoni SL ranged from 14 mm to 43 mm (mean ± SD = 18.21 ± 5.28; n = 34), with a main peak at 16–20 mm SL. The length–frequency distributions of C. rastrospinosus SL ranged from 14 to 48 mm (mean ± SD = 22.13 ± 7.78; n = 23), with a single mode at 16–20 mm (Fig. 2). Significant differences were found in length–frequency distributions between C. wilsoni and C. rastrospinosus (Mann–Whitney U test, Z = −2.625, p = 0.009).
Morphometry and microstructure of sagittal otoliths
The sagittal otoliths of larvae and juveniles of C. wilsoni and C. rastrospinosus had a discoid shape. Within the core area, the primordium was an optically dense and irregular discoidal area containing multiple nuclei (Fig. 3). Away from the core, a hatch check (H) could be clearly observed on all otoliths and it was assumed that the first growth ring was laid down at hatching. A second marked check was located at a distance of 16–29 and 21–41 microincrements from the core in C. wilsoni and C. rastrospinosus, respectively. This check was associated with the beginning of exogenous feeding. Significant differences were found in exogenous feeding time between the both icefishes (Mann–Whitney U test, Z = −4.546, p < 0.0001). The dates of the first exogenous feeding check ranged from October to January in C. wilsoni and from October to December in C. rastrospinosus, with both showing a pronounced peak in December (Fig. 4).
In C. wilsoni, the otolith radius varied from 58.47 μm to 190.53 μm (mean ± SD = 80.11 ± 27.76; n = 34) in fishes of 14 mm to 43 mm SL and showed a linear increase at a rate of 4.99 μm with each mm increase in SL. The otolith radius varied from 64.98 μm to 256.88 μm (mean ± SD = 103.88 ± 44.22; n = 23) in C. rastrospinosus individuals of 14 mm to 48 mm SL and showed a linear increase at a rate of 5.51 μm with each mm increase in SL. Otolith radius did not differ between species after larval size was taken into account (ANCOVA, F1,54 = 1.076, p = 0.304). The relationship between SL and OR was well fitted by a linear equation (Fig. 5).
Age and growth
Overall, 34 individuals of C. wilsoni and 23 individuals of C. rastrospinosus were successfully aged through microincrement counts. The estimated age ranged from 28 to 100 days for C. wilsoni and 29–126 days for C. rastrospinosus. Counting variability between readings was generally low, with differences of less than 5% of the relevant mean. The APE and CV indices were 2.8% and 1.98%, respectively, indicating good consistency between readings.
The exponential model best described the age (t, days) and standard length (SL, mm) of C. wilsoni (Table 2, Fig. 6a). The growth function was determined as follows:
Based on this relationship, the size at hatching in C. wilsoni was estimated to be approximately 9.28 mm SL. The percentage of the daily increase in size (G = g × 100, % SL day−1) was 1.6% SL day−1 and the daily growth rate at the mean size was 0.34 mm day−1.
In addition, the exponential model fits the age–length data of C. rastrospinosus well (Table 2, Fig. 6b). The growth function was as follows:
The model estimated a hatch size of 10.90 mm SL, the percent daily increase in size (G) was 1.2% SL day−1, and the daily growth rate at the mean size was 0.38 mm day−1.
Back-calculated hatch days
Based on the estimated age and date of capture, C. wilsoni hatching occurred from October to December, with a pronounced peak in November (Fig. 7a). In C. rastrospinosus, the hatch dates were spread over a relatively long period, from September to December, with an apparent peak in November (Fig. 7b).
Discussion
Icefishes are characterized by a long pelagic larval phase, and the duration of and growth rate during this phase strongly influence the dispersal range and connectivity between populations and determine the year-class strength (La Mesa et al. 2013b; Riginella et al. 2016). This study investigated the early-life history traits of C. wilsoni and C. rastrospinosus in the Ross Sea for the first step. The results fill a knowledge gap and support the small amount of biological data available in the literature. Based on four surveys of ichthyoplankton in the Ross Sea region from 1987 to 1996, Granata et al. (2002) reported that the length range of C. rastrospinosus larvae and juveniles was 15–55 mm SL in January to February. Our results based on samples collected in January fell within the same range (14–48 mm SL). Larvae and juveniles of C. wilsoni and C. rastrospinosus occurred in the upper 0 m to 400 m of the water column in this survey, which was consistent with previous research records. Post-larvae and juveniles of icefish commonly prefer subsurface waters and inhabit the upper 100 m of the water column (Loeb et al. 1993; Kellermann 1996).
In the Ross Sea, C. rastrospinosus spawns from January to March and has a long-hatching period, lasting four to six months (Prutko 2004). We conclude that the hatching period of this species lasts from September to December, which is in line with the previously reported spawning time in the Ross Sea (Prutko 2004). This is also consistent with the observation of early larvae of 15–36 mm SL captured in offshore waters outside Terra Nova Bay in the Ross Sea from November to January (Granata et al. 2002). Furthermore, La Mesa et al. (2011) speculated that the hatching of Chionodraco hamatus took place in spring (September to November) and early larvae of 14–20 mm SL were collected in January. This suggests that the hatching time of members of the genus Chionodraco is the same in the Ross Sea (Vacchi et al. 2003). However, the hatching date of C. rastrospinosus in the Ross Sea is slightly different from that reported around the Antarctic Peninsula (August to November) (La Mesa et al. 2013a). The spawning of C. wilsoni likely occurs from October to December at the tip of the Antarctic Peninsula and from November to December in the Cosmonauts Sea (Pshenichnov 1988; Kock et al. 2008). After a relatively long-incubation period (8–9 months), C. wilsoni hatches beginning in the late austral winter (August to September) (Kock et al. 2008). Our results indicate that the hatching period of C. wilsoni is from October to December in the Ross Sea, which is different from the findings of a previous study (Kock et al. 2008). Kamler (2002) suggested that the incubation time of eggs is determined by extrinsic and intrinsic factors affecting the ontogenetic rate, among which the most important are water temperature and the maternal contribution to offspring eggs size. Therefore, it is reasonable for the incubation period of a single species to vary in different geographical regions as well as interannually, according to the environmental conditions of the sea water. Moreover, previous studies reported that the egg diameter of C. rastrospinosus ranges from 4.8 mm to 5.0 mm, which is among the maximum values reported for notothenioids, while the egg diameter of C. wilsoni ranges from 4.3 mm to 4.8 mm (Kock 2005; Kock et al. 2008). It is a rather common feature among channichthyids for larger egg to take a longer time to hatch (La Mesa et al. 2013a). In this study, C. rastrospinosus showed a relatively long-incubation time compared to C. wilsoni, which confirmed the general rule that the incubation time is directly related to egg size under similar environmental conditions (Kock 1989).
An exogenous feeding check is a rather common feature among channichthyids, which begin to feed exogenously at least one to two months after hatching (La Mesa and Ashford 2008a). The present study clearly documented the second check located outward from the hatching check as the exogenous feeding check (Fig. 3). The exogenous feeding period lasted from 16 to 29 days in C. wilsoni and 21–41 days in C. rastrospinosus. The timing and duration of the yolk-sac larval stage of icefish (i.e., from hatching to complete yolk-sac absorption) differed somewhat among individuals (Kock 2005). It has been reported that the timing of exogenous feeding increases with increasing egg size and larval size at hatching (La Mesa and Ashford 2008a). Accordingly, the onset of exogenous feeding was found to be later in C. rastrospinosus than in C. wilsoni in our study because these species produced large eggs and abundant yolk reserves. In contrast, our results are slightly different from La Mesa et al. (2013a), who reported that the exogenous feeding period (or yolk-feeding period) of C. rastrospinosus around the Antarctic Peninsula lasted from 23 to 52 days. Larvae and post-larvae of channichthyids prey heavily on Antarctic krill (Euphausia superba), ice krill (E. crystallorophias), and larval notothenioids (La Mesa et al. 2009, 2011). Larvae and juveniles of Antarctic silverfish are the dominant and most abundant species in the Ross Sea, representing approximately 98% of the total ichthyoplankton (Guglielmo et al. 1997). In particular, Dacodraco hunteri (La Mesa et al. 2012) and Chionodraco hamatus larvae smaller than 40 mm SL (La Mesa et al. 2011) feed almost exclusively on Antarctic silverfish larvae in the Ross Sea. The analysis of stomach contents showed that C. rastrospinosus larvae mainly fed on furcilia, adult euphausiids, and Antarctic silverfish larvae (La Mesa et al. 2013a). In contrast to the relatively long-hatching period, our results showed that the exogenous feeding time of C. wilsoni and C. rastrospinosus was concentrated in a short period, mainly in December. Vacchi et al. (2003) reported that Antarctic silverfish eggs occupied a layer of platelet ice under fast ice and hatched in the Ross Sea from mid-November to December. It is reasonable to speculate that the onset of exogenous feeding in C. wilsoni and C. rastrospinosus is consistent with the hatching time of Antarctic silverfish larvae. This phenomenon is in line with the “match-mismatch” hypothesis (Cushing 1990), which suggests that the availability of sufficient food during the timing of exogenous feeding determines the survival and recruitment success of larvae. Interestingly, the both species hatched at similar times in the Ross Sea and showed overlapping timing of the onset of exogenous feeding, suggesting that C. wilsoni and C. rastrospinosus are likely to compete for food during their early stages.
The larval size at hatching was 9.28 mm in C. wilsoni and 10.90 mm in C. rastrospinosus according to otolith microstructure analysis, which is below the lower range of hatching size reported for channichthyids (13–17 mm) (Kock 2005). However, the hatching size of C. rastrospinosus around the Antarctic Peninsula was reported to be 17.2 mm (La Mesa et al. 2013a). The timing of and size at complete yolk resorption are likely variable and related to local environmental conditions (La Mesa et al. 2012). Notably, the sizes at hatching of 9.28 mm and 10.90 mm reported here for C. wilsoni and C. rastrospinosus have to be considered theoretical, because they were estimated by fitting an exponential model. Given that smaller C. wilsoni larvae (7 mm SL) are found around the Antarctic Peninsula (Kock et al. 2008) and considering the size of the smallest larva of C. wilsoni and C. rastrospinosus (14 mm SL) collected in the present study, this estimate seems to be quite feasible. In addition, the hatching size of C. rastrospinosus larvae is larger than that of C. wilsoni larvae, in accordance with the production of large eggs by C. rastrospinosus (Kock 2005).
The larvae and post-larvae of a few high-Antarctic species, such as C. rastrospinosus and Chaenocephalus aceratus, grow comparatively fast and appear to achieve lengths of up to 60–80 mm in their first year (Kellermann 1990). Previous studies based on otolith microstructure reported that the daily growth rate of Champsocephalus gunnari was 0.31 mm around the South Shetland Islands and the instantaneous growth rate of Chaenocephalus aceratus was estimated to be 0.23 mm day−1 (La Mesa and Ashford 2008a; La Mesa et al. 2013b). La Mesa et al. (2013a) reported that the daily growth rate of C. rastrospinosus collected around the Antarctic Peninsula with the SL of 39 mm to 69 mm was 0.25 mm day−1. The growth rate reported by La Mesa et al. (2013a) is slightly lower than our estimate of a daily growth rate of 0.38 mm day−1 for C. rastrospinosus collected with SL of 14 mm to 48 mm in the Ross Sea. The lack of synchrony of early ontogenetic steps among individuals and the variation among the cohorts in terms of size and the timing of hatching may explain the variability observed in the larval growth rate of this species (North 1998; Kamler 2002). Different environmental conditions in terms of water temperature and food species also greatly affect the larval growth rate; for example, the Ross Sea is characterized by high phytoplankton productivity, which is beneficial to larval growth (Davis et al. 2017). Because of their different hatching periods and the interannual changes in environmental conditions, such as prey availability, the both species grew at different rates during their early-life period (F = 5.048, p = 0.024). Moreover, endogenous factors can cause substantial variation in larval growth, which is also associated with high heritability of growth rates (Purdom 1993). In future studies, we should focus on the influence of food or environmental factors on the early-life history traits of icefish in this sensitive area of the Ross Sea and conduct long-term monitoring of the early-life history strategies of icefish.
Conclusion
In conclusion, microstructure analysis of sagittal otoliths in C. wilsoni and C. rastrospinosus provides novel information on the hatching periods and growth rates of these two icefishes in the Ross Sea. Our study indicates that C. wilsoni and C. rastrospinosus exhibit the main biological features found in many icefish species: they grow comparatively fast (0.34 mm day−1 and 0.38 mm day−1) when they are immature and have a long-incubation period. The newly hatched C. rastrospinosus have a considerable yolk supply, enabling them to start exogenous feeding later than C. wilsoni. In addition, the incubation time of C. wilsoni was shorter than C. rastrospinosus and the newly hatched larvae of C. wilsoni were also smaller. Despite the differences in hatching time and larval size at hatching, C. wilsoni and C. rastrospinosus share some common life history features. The duration period of hatching was relatively long, with the peak time of exogenous feeding overlapped and concentrated in December.
References
Akaike H (1973) Information theory and an extension of the maximum likelihood principle. In: Petran BN, F Csaaki (eds) Second international symposium on information theory. Akademia Kiado, Budepest, pp 267–281
Campana SE (2001) Accuracy, precision, and quality control in age determination, including a review of the use and abuse of age validation methods. J Fish Biol 59:197–242. https://doi.org/10.1006/jfbi.2001.1668
CCAMLR (2012) Report of the Working Group on Fish Stock Assessment. In: Report of the Thirty-first Meeting of the Scientific Committee (SC-CAMLR-XXXI), Annex 7, Appendix G. Hobart, Australia
Collins MA, Brickle P, Brown J, Belchier M (2010) The Patagonian toothfish: biology, ecology and fishery. Adv Mar Biol 58:227–300. https://doi.org/10.1016/S0065.2881(10)58004.0
Cushing DH (1990) Plankton production and year-class strength in fish populations - an update of the match mismatch hypothesis. Adv Mar Biol 26:249–293. https://doi.org/10.1016/S0065-2881(08)60202-3
Davis LB, Hofmann EE, Klinck JM, Pinones A, Dinniman MS (2017) Distributions of krill and Antarctic silverfish and correlations with environmental variables in the western Ross Sea, Antarctica. Mar Ecol Prog Ser 584:45–65. https://doi.org/10.3354/meps12347
Efremenko VN (1985) Illustrated guide to fish larvae of the Southern Ocean. Biomass Handbook 22:74
Fey DP (1999) Effects of preservation technique on the length of larval fish: Methods of correcting estimates and their implication for studying growth rates. Arch Fish Mar Res 47:17–29
Galarza JA, Carreras-Carbonell J, Macpherson E, Pascual M, Roques S, Turner GF, Rico C (2009) The influence of oceanographic fronts and early-life-history traits on connectivity among littoral fish species. Proc Natl Acad Sci USA 106:1473–1478. https://doi.org/10.1073/pnas.0806804106
Granata A, Cubeta A, Guglielmo L, Sidoti O, Greco S, Vacchi M, La Mesa M (2002) Ichthyoplankton abundance and distribution in the Ross Sea during 1987–1996. Polar Biol 25:187–202. https://doi.org/10.1007/s00300-001-0326-y
Guglielmo L, Granata A, Greco S (1997) Distribution and abundance of postlarval and juvenile Pleuragramma antarcticum (Pisces, Nototheniidae) off Terra Nova Bay (Ross Sea, Antarctica). Polar Biol 19:37–51. https://doi.org/10.1007/s003000050214
Hanchet S, Dunn A, Parker S, Horn P, Stevens D, Mormede S (2015) The Antarctic toothfish (Dissostichus mawsoni): biology, ecology, and life history in the Ross Sea region. Hydrobiologia 761:397–414. https://doi.org/10.1007/s10750-015-2435-6
Kamler E (2002) Ontogeny of yolk-feeding fish: an ecological perspective. Rev Fish Biol Fisher 12:79–103. https://doi.org/10.1023/A:1022603204337
Kellermann A (1990) Catalogue of early life stages of Antarctic notothenioid fish. Ber Polarforsch 67:45–136. https://doi.org/10.2312/BzP_0067_1990
Kellermann AK, Gauldie RW, Ruzicka JJ (2002) Otolith microincrements in the Antarctic fishes Notothenia coriiceps and Pseudochaenichthys georgianus. Polar Biol 25:799–807. https://doi.org/10.1007/s00300-002-0432-5
Kellermann A (1996) Midwater fish ecology. In: Ross RM, Hofmann E, Quetin LB (eds) Foundations for Ecological Research West of the Antarctic Peninsula. Antarctic Research Series, American, pp 231–256
Kock KH (1989) Reproduction in Antarctic fish around Elephant Island. Arch Fisch Wiss 39:171–210
Kock KH (1992) Antarctic fish and fisheries. Cambridge University Publishing, Britain
Kock KH (2005) Antarctic icefishes (Channichthyidae): a unique family of fishes. A review. Part II Polar Biol 28:897–909. https://doi.org/10.1007/s00300-005-0019-z
Kock KH, Kellermann A (1991) Reproduction in Antarctic notothenioid fish. Antarct Sci 3:125–150
Kock KH, Pshenichnov L, Jones CD, Grorger J, Riehl R (2008) The biology of the spiny icefish Chaenodraco wilsoni Regan, 1914. Polar Biol 31:381–393. https://doi.org/10.1007/s00300-007-0366-z
La Mesa M, Ashford J (2008a) Age and early life history of juvenile Scotia Sea icefish, Chaenocephalus aceratus, from Elephant and the South Shetland Islands. Polar Biol 31:221–228. https://doi.org/10.1007/s00300-007-0351-6
La Mesa M, Ashford J (2008b) Age and growth of ocellated icefish, Chionodraco rastrospinosus DeWitt and Hureau, 1979, from the South Shetland Islands. Polar Biol 31:1333–1342. https://doi.org/10.1007/s00300-008-0471-7
La Mesa M, EaStman JT (2012) Antarctic silverfish: life strategies of a key species in the high-Antarctic ecosystem. Fish and Fish 13:241–266. https://doi.org/10.1111/j.1467-2979.2011.00427.x
La Mesa M, Felice AD, Jones CD, Koch KH (2009) Age and growth of spiny icefish (Chaenodraco wilsoni regan, 1914) off Joinville-D’Urville Islands (Antarctic Peninsula). CCAMLR Sci 16:115–130
La Mesa M, Catalano B, Greco S (2011) Larval feeding of Chionodraco hamatus (Pisces, Channichthyidae) in the Ross Sea and its relation to environmental conditions. Polar Biol 34:127–137. https://doi.org/10.1007/s00300-010-0866-0
La Mesa M, Catalano B, Greco S (2012) Some biological characteristics of early larvae Dacodraco hunteri (Notothenioidei: Channichthyidae) in the western Ross Sea. Polar Biol 35:655–660. https://doi.org/10.1007/s00300-011-1111-1
La Mesa M, Catalano B, Jones CD (2013a) Early life history of the ocellated icefish, Chionodraco rastrospinosus, off the Antarctic Peninsula. Antarctic Sci 25:373–380. https://doi.org/10.1017/S0954102012001095
La Mesa M, Catalano B, Koubbi P, Jones CD (2013b) Early ontogeny of the mackerel icefish, Champsocephalus gunnari, in the southern Scotia Arc. Polar Biol 36:797–805. https://doi.org/10.1007/s00300-013-1304-x
La Mesa M, Riginella E, Catalano B, Jones CD, Mazzoldi C (2017) Maternal contribution to spawning and early life-history strategies of the genus Lepidonotothen (Nototheniidae, Perciformes) along the southern Scotia Arc. Polar Biol 40:1441–1450. https://doi.org/10.1007/s00300-016-2068-x
Llompart FM, Fernandez DA, Aureliano D, La Mesa L (2020) Life-history traits of the Magellan plunderfish Harpagifer bispinis (Forster, 1801) in the Beagle Channel (Tierra del Fuego, South America). Polar Biol 43:1643–1654. https://doi.org/10.1007/s00300-020-02735-x
Loeb VJ, Kellermann A, Koubbi P, North AW, White MG (1993) Antarctic larval fish assemblages: a review. B Mar Sci 53:416–449
Morley SA, Belchier M, Dickson J, Mulvey T (2005) Daily otolith increment validation in larval mackerel icefish, Champsocephalus gunnari. Fish Res 75:200–203. https://doi.org/10.1016/j.fishres.2005.04.008
North AW (1998) Growth of young fish during winter and summer at South Georgia, Antarctica. Polar Biol 19:198–205. https://doi.org/10.1007/s003000050235
North AW (2010) Early life history strategies of notothenioids at South Georgia. J Fish Biol 58:496–505. https://doi.org/10.1006/jfbi.2000.1469
North AW, Kellermann A (1990) Key to the early stages of Antarctic fish. Ber Polarforsch 67:1–44
Papetti C, Zane L, Patarnello T (2010) Isolation and characterization of microsatellite loci in the icefish Chionodraco rastrospinosus (Perciformes, Notothenioidea, Channichthyidae). Mol Ecol Notes 6:207–209. https://doi.org/10.1111/j.1471-8286.2005.01194.x
Parker SJ, Mormede S, Hanchet SM, Devries A, Canese S, Ghigliotti L (2019) Monitoring Antarctic toothfish in McMurdo Sound to evaluate the Ross Sea region Marine Protected Area. Antarctic Sci 31:195–207. https://doi.org/10.1017/S0954102019000245
Prutko VG (2004) Observer notes (Subarea 88.1). WG-FSA-04/89, CCAMLR, Hobart, Australia
Pshenichnov LK (1988) Structure of the populations of Wilson’s ice fish on the marginal shelf rises of the Cosmonauts Sea. Biological resources of the Thalassobathyal zone of the World Ocean. VNIRO, Moscow, pp 58–59
Purdom CE (1993) Genetics and fish breeding. J Genet 72:105–106. https://doi.org/10.1007/BF02927927
Radtke RL, Kellermann A (1991) Microstructural analysis of growth patterns in the early life history of Antarctic fishes. In: Di Prisco G, Maresca B, Tota B (eds) Biology of Antarctic fish. Springer, Berlin, pp 101–115
Riginella E, Mazzoldi C, Ashford J, Jones CD, Morgan C, La Nesa M (2016) Life history strategies of the Scotia Sea icefish, Chaenocephalus aceratus, along the Southern Scotia Ridge. Polar Biol 39:497–509. https://doi.org/10.1007/s00300-015-1802-0
Smith WO, Ainley DG, Cattaneo-Vietti R (2018) Trophic interactions within the Ross Sea continental shelf ecosystem. Philos T R Soc B 362:95–111. https://doi.org/10.1098/rstb.2006.1956
Traczyk R, Meyer-Rochow VB, Hughes RM (2021) Age determination in the icefish Pseudochaenichthys georgianus (Channichthyidae) based on multiple methods using otoliths Aquat Biol 30:1–18. https://doi.org/10.3354/ab00736
Traczyk R, Meyer-Rochow VB (2019) Age Structure and Biomass of the Icefish Pseudochaenichthys georgianus Norman (Channichthyidae) Between 1976 and 2009: a Possible Link to Climate Change. Ocean Polar Res 41:233–250. https://doi.org/10.4217/OPR.2019.41.4.233
Vacchi M, Williams R, La Mesa M (1996) Reproduction in three species of fish from the Ross Sea and Mawson Sea. Antarctic Sci 8:185–192. https://doi.org/10.1017/S0954102096000259
Vacchi M, La Mesa M, Dalu M, MacDonald J (2003) Early life stages of the Antarctic Silverfish, Pleuragramma antarcticum in Terra Nova Bay, Ross Sea. Antarctic Sci 16:299–305. https://doi.org/10.1017/S0954102004002135
Acknowledgements
We are grateful to the scientific staff and the crew aboard the 36th Chinese National Antarctica Research Expeditions for their support in sampling processing and data collection. We are grateful to Dr. Dieter Piepenburg the editor of PB, Dr. Mauricio F. Landaeta and to one anonymous reviewer, whose suggestions greatly improved the early draft of the manuscript. Finally, we thank Nature Research Editing Service for revising language. This study was financially supported by the research project “Impact and Response of Antarctic Seas to Climate Change, IRASCC2020-2022” (Grant No. IRASCC 01-02-05C) from Chinese Arctic and Antarctic Administration (CAA), Ministry of Natural Resources of the People’s Republic of China.
Author information
Authors and Affiliations
Contributions
MD performed the experiments, analyzed the data, and wrote the draft of the manuscript. YT and CZ conceived and designed of the study and helped to revise the final manuscript. YL, ZY, and CZ collected the fish samples. CZ provides technical assistance. CL assisted sample processing. YT and JH provided funding for the study. All authors read and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest and that all applicable institutional, national, or international guidelines for the use and care of animals were strictly followed in the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duan, M., Zhang, C., Liu, Y. et al. Early-life history traits of two icefishes, spiny icefish Chaenodraco wilsoni and ocellated icefish Chionodraco rastrospinosus, in the Ross Sea revealed by otolith microstructure. Polar Biol 45, 427–436 (2022). https://doi.org/10.1007/s00300-021-03002-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-021-03002-3