Abstract
Life history characteristics of Watases lanternfish Diaphus watasei from a deep sea fishing ground of the East China Sea were studied. Overall, 452 adult individuals with standard length ranging from 91 to 147 mm were collected in the continental slope during 3 years. The growth pattern and hatching date were inferred from otolith microstructure analysis, and the stomach contents were examined to clarify feeding ecology. Three distinct zones of the otolith microstructure were depicted, the numbers of increments in the central and middle zones of the D. watasei otolith are in the lowest level in myctophid species studied. The maximum number of growth increments indicated a short lifespan of 2 years. The spawning time almost lasted throughout the year, and then two cohorts were separated according to the estimated hatching time. The length–weight relationship revealed a negative allometric pattern and significant difference between the two cohorts, and a significant seasonal difference was also detected in somatic growth pattern. A piscivorous habit dominated by Maurolicus muelleri was revealed across sampling seasons and standard length groups. Overall, D. watasei show distinct life history characteristics compared to coinhabiting myctophids, and such characteristics could reduce interspecific competition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mesopelagic fishes in the world's oceans are featured by their enormous biomass, which is estimated to be in the order of 10 billion tons (Irigoien et al. 2014). Of these, myctophid fishes are dominant and abundant components, comprising at least 20% of the oceanic ichthyofauna; they are distributed throughout the oceans from subarctic and antarctic to tropical waters (Catul et al. 2011). In each ecosystem, myctophids occupy an intermediate trophic position in the pelagic food web and link the energy transfer from zooplankton to higher trophic levels (Beamish et al. 1999; Olivar et al. 2015). The roles that myctophids play in the food web and substance transport strongly depend on their life history traits (Caiger et al. 2021). Therefore, to understand the mechanism of the high population productivity, studies on the life history characteristics of myctophids have been conducted in the world’s oceans, including age, growth, life span, feeding ecology and spawning strategies (Gartner 1993; Suntsov and Brodeur 2008; Sassa et al. 2014; Sarmiento-Lezcano et al. 2018; Wang et al. 2019; Silva et al. 2022).
The East China Sea (ECS) is one of the largest marginal seas of the western Pacific. Huge quantities of nutrient input from the Changjiang River and Kuroshio current, contributing the high biological production, support important fishing grounds for various pelagic and demersal fishes (Hama et al. 1997; Tang et al. 2000; Yamada et al. 2007). Pseudoceanic myctophids, such as Diaphus garmani, D. watasei, D. chrysorhynchus, and Benthosema pterotum, that adapt to certain habitats of continental slopes, such as slopes of islands and sea mounts, occur dominantly on the continental shelf and continental slope of the ECS (Sassa et al. 2003, 2010; Tanaka et al. 2013). These myctophids are ecologically important as prey or as competitors for commercial species (Mio et al. 1984). Therefore, ecology information on these myctophids is essential to better understand the ECS ecosystem and will also provide insights into population dynamics of commercially important species. Furthermore, with the decline of the coastal fisheries resources in the ECS and the establishment of marine protected areas (Zhang et al. 2023), new fishing ground in the deeper continental slope is explored in recent years. The Diaphus species no longer is an incidental catch (Kawaguchi and Shimizu 1978) but become one of the secondary target catches in the deep sea scampi trawler and are utilized as fish meal because of its high abundance and lipid content. Information inferred from age and growth and reproductive strategy are fundamental for understanding the population dynamics of fishes and ensuring rational development. To date, there have been several studies on age, growth, reproductive biology, and feeding ecology of Diaphus species in the ECS (Tanaka et al. 2013; Sassa et al. 2016; Cao 2017). However, for D. watasei, the dominant Diaphus species in the deep sea trawler, ecology information is quite limited (Sassa et al. 2016; Huang 2019), especially age and growth information. D. watasei mainly distribute in the depth range of 200–700 m and have a large body size (maximum 170 mm) compared with coinhabiting myctophids in the East China Sea (Sassa et al. 2016). The species is featured by a complex life history: the egg is laid in the mesopelagic layer, and juveniles (< 100 mm) undergo diel vertical migration, while adults settle down in bottom habitat with no more diurnal migration (Sassa et al. 2016; Huang 2019). Life history information is essential for fishery management and better understanding on the ecological role of D. watasei.
In this study, we collected D. watasei from 3 years during fishing season through commercial bottom trawler. The main goals were to estimate the age-based life-history parameters, such as growth pattern and hatching time, but stomach content was also analyzed to clarify feeding habit. The result could fill gap in knowledge on the life history characteristics and feeding ecology of D. watasei and provide theoretical support for future fishery management.
Materials and methods
Sampling
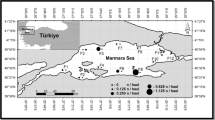
Samples were randomly collected through commercial deep sea trawler (net length 40 m, mesh size of cod end 20 mm) in the offshore of the East China Sea from two fishing seasons (from December 2021 to April 2022 and from November 2022 to February 2023) (125°36′ E–128°36′ E, 27°27′ N–29°56′ N) (Fig. 1, Table 1). The specimens were preserved by freezing (−20 °C) soon after capture. In the laboratory, fish specimens were measured to the nearest 1 mm (standard length, SL) and weighed to the nearest 0.1 g [body weight (W)]. Sagittal otoliths were extracted, cleaned of adhering soft tissue, air dried in room temperature, and then stored in small, coded tubes for microstructure analysis.
The stomachs were dissected and the contents removed and preserved in 70% alcohol. Prey items were observed and enumerated under a binocular microscope. Contents were identified to the lowest taxonomic level that the digestive state would allow. All individual prey items within stomach contents were weighed to the nearest 0.01 mg.
Ageing
For microstructure analysis, right intact otolith was embedded with sulcus side down in epoxy resin (EpoThin, Buehler). Then, a frontal section (thickness of 0.8 mm) along the anterior–posterior axis, including the core area, was cut with a low speed saw (Buehler) (Fig. 2). The section was mounted on a glass slide using thermal plastic glue (Crystalbond 509) and double sides ground by a MetaServ 250 grinding machine (Buehler) with alternating waterproof sandpaper (240–4000 grit) until clear appearance of microincrements. The ground surface of otolith was further polished with 0.3 μm aluminium powder to enhance the clarity of microincrements. The polished otoliths were photographed under an optical microscope (×200 magnification) (OLYMPUS BX53 and DP74).
Otolith sections without unreadable patterns were used for ageing. Increments were counted from the core to the otolith edge, starting from the first increment (assuming as hatching ring) surrounding the primordium (Figs. 3, 4). Along the ageing axis, different zones that link to life history traits can be identified according to particular features (darker increments and accessory primordium) (Takakazu and Grace 1990; Linkowski 1997; Hosseini-Shekarabi et al. 2015). Generally, the central zone (CZ), surrounding the primordium, indicates the larval stage duration. Outside of central zone, the middle zone (MZ) presents the corresponding duration of metamorphosis. The external zone is at the outermost zone of the otolith, and its increment characterizes the active, free swimming stage (Hosseini-Shekarabi et al. 2015).
Increments in each otolith zone were counted three times. When the differences among these three counts exceeded 5%, the otolith was discarded. The readings with standard error < 5% were considered valid, and the mean value was used for subsequent analysis.
Daily rhythm in otolith increment formation has been confirmed for Diaphus spp. in the East China Sea (Moku et al. 2005), but it was not fully proved in D. watasei. Therefore, daily increment was assumed, and the number of increments was estimated as daily age. Hatching dates were further estimated from the sampling dates and number of increments, namely: hatching date = sampling date − number of increments (estimated daily age).
Data analysis
The contribution and relative importance of prey item to stomach contents were quantified using four commonly used dietary composition indicators: (1) the percentage frequency of occurrence (%F) of each prey item in nonempty stomachs, (2) the percentage weight of individual prey item to the total weight of prey items (%W), (3) the percentage of each prey item by number (%N) to the total number, and (4) the index of relative importance (IRI = (%N + %M)%F). Then, the percent composition of IRI (%IRI) was calculated for major prey categories to readily allow comparison among categories.
The %IRI was calculated as:
where i is prey item.
To compare potential season/size related diet shift, individuals were assigned to two seasons (spring and winter) and three standard length groups (≤ 110 mm, 110–125 mm, and > 125 mm).
The length–weight relationship was described using the power function:
where W is body weight, SL is standard length, and a and b are regression coefficients. When b = 3 the growth pattern is isometric, whereas when b < 3 and b > 3 the growth is negative and positive, respectively.
A Von Bertalanffy growth model was fitted to estimate ‘length at age’ followed the function:
where \({L}_{t}\) is the standard length at age t, \({L}_{\infty }\) is the theoretical asymptotic length, k is the growth coefficient, t is number of increments, and \({t}_{0}\) is theoretical age when L = 0.
Seasonal differences in life history characteristics, such as length–weight relationship and growth rate, between the identified cohorts were tested by the ANCOVA and F-test, respectively. Statistical significance was set at the 5% level for the tests.
All data analysis and graph plots were performed using the program R 3.6.3.
Results
During the three years, 452 individuals of D. watasei were collected. Standard length of the individuals ranged between 91 and 147 mm with body weight between 9.2 and 38.5 g (Table 1).
Otoliths have oval shape, with crenate margins. The distal face is relatively flat. The anterior region presents peaked, small rostrum and antirostrum. The posterior region is round (Fig. 2).
The microstructure analysis indicated the presence of three main growth regions comparable with the central (CZ), middle (MZ), and external zones (EZ) (Fig. 3). A primordium located in the center. The central zone (larval stage), characterized by fine increments, extended from the primordium outwards to the middle zone. The outermost of central zone was marked by a well-defined darker ring (check 1, Fig. 4). The middle zone (metamorphosis stage) formed dark and wide rings, covered by an opaque area. The boundary of the opaque area, marked by an accessory primordium (check 2, Fig. 4), indicated the transition to the external zone that extend from the outer boundary of the middle zone to the otolith edge. Increment near the edge appeared fine and less distinct.
Valid results about the number of increments were obtained from 335 individuals. The microincrement ranged from 16 to 23 in the otolith central zone and from 7 to 12 in the middle zone. Overall, total micro-increments ranged between 362 and 608.
The estimated hatching time of D. watasei spread crossed a year lasting from April to February of the next year (Fig. 5). Two cohorts were separated by hatching time: summer cohort lasted from April to September occupying the majority (the known spawning season) and winter cohort lasted from October to February (Discussion).
The length–weight relationships by cohorts were as follows:
The estimated b values for the two cohorts were both smaller than the theoretical value of isometric growth (P < 0.05), indicating a negative allometric growth pattern (Fig. 6). Body weight of summer cohort increased faster with standard length compared with the winter cohort (P < 0.05).
The von Bertalanffy growth curves by cohorts were expressed as (Fig. 7):
Summer cohort showed higher growth rate to smaller \({L}_{\infty }\) (P < 0.05).
227 stomachs with contents were analyzed, and the identified diet consisted of eight prey taxa. Stomach contents of D. watasei were composed mainly of fishes and crustaceans and squid (Tables 2, 3), and fishes occupied a great majority, indicating a completely piscivorous habit. During spring, D. watasei consumed more prey species compared with winter, especially Abralia multihamata. The differing prey items contributed low %IRI, and Maurolicus muelleri overwhelmingly dominated (Table 2). Similarly, prey items with low %IRI differed among the three standard length groups (Table 3). Diaphus garmani, Abralia multihamata and Maurolicus muelleri composed the main prey species, of which Maurolicus muelleri was overwhelming.
Discussion
This study is the first report on the diet composition of adult D. watasei in the ECS. A piscivore habit was revealed through stomach content analysis, which differs with coinhabiting Diaphus garmani, Diaphus chrysorhynchus, and Benthosema pterotum that prey on crustacean zooplankton, such as copepods, euphausiids, and decapod larvae (Tanaka et al. 2013; Xiao 2016). Diet of lanternfishes could reflect their structural morphology, differing energy requirements, and general life strategies (Suntsov and Brodeur 2008; Battaglia et al. 2014). Benefiting from strong swimming ability resulted by large body size, D. watasei show an intermediate ecomorphological type between common active and inactive myctophids (Barham 1971). Unlike common size-related diet shift in myctophid (Battaglia et al. 2014), active feeding activity is kept across juvenile and adult life stages of D. watasei, and then, no distinct size/season related diet shift occurred (Huang 2019). In contrast, the major diet shift occurred with settlement, juvenile D. watasei with vertical migration mainly prey on Benthosema pterotum and Sergestidae spp. (Huang 2019), and nonmigratory larger ones in this study transfer to Maurolicus muelleri that occupied the majority prey. Such highly piscivore habit is also recorded in Electrona risso, which feeds mainly on the small mesopelagic fish Cyclothone braueri (Battaglia et al. 2016). Further, Maurolicus muelleri have an unique vertical migration. The population migrates to the surface after sunset, during midnight actively swim with a step-wise pattern between surface and 100 m depth and then return to bottom habitat during the daytime (Christiansen et al. 2019). Thus, adult D. watasei could feed more in the daytime, nonmigrating adult individuals have a chance to prey on Maurolicus muelleri during this period. Such feeding periodicity also differs with Diaphus garmani (active at night) and Diaphus chrysorhynchus (both day and night) (Tanaka et al. 2013). Therefore, D. watasei show a specialized predator with positive selectivity toward prey items, such selectivity could avoid competition with coinhabiting myctophids and also suggest less food abundance in the East China Sea (Suntsov and Brodeur 2008).
Three well-defined growth zones were observed for D. watasei otolith, corresponding to observations in other studies on myctophid otoliths (Takakazu and Grace 1990; Linkowski 1996; Hosseini-Shekarabi et al. 2015; Zhang et al. 2021). The numbers of increments in the central zone of D. watasei otolith are relatively low and concentrated among the subtropical and tropical myctophid species, i.e., Benthosema suborbitale (30–50) (Gartner 1991), B. pterotum (22–32) (Hosseini-Shekarabi et al. 2015), Ceratoscopelus warmingii (22–52) (Linkowski 1997), and Symbolophorus californiensis (30–64) (Takagi et al. 2006). The numbers of increments in the middle zone of D. watasei otolith are also lower than B. suborbitale (13–43) (Gartner 1991), C. warmingii (20–65) (Linkowski 1997; Takagi et al. 2006), S. californiensis (23–61) (Takagi et al. 2006), Tarletonbeani acrenularis (51–102) and B. pterotum (8–22) but similar to Diaphus kapalae (10–12) (Suthers 1996) and Lampanycto deshectoris (5–9) (Young et al. 1988). By comparison, D. watasei show far one of the lowest increments for the central and middle zones in myctophid species studied. Generally, a short metamorphosis period indicates a larvae morphology that does not differ greatly from that of the adults; thus, the metamorphosis does not take much time because of the absence of an abrupt change in overall morphology (Hosseini-Shekarabi et al. 2015). D. watasei keep stubby type since larval stage (Sassa et al. 2004), which provides evidence for the common rule. In addition, increment near the edge appeared less distinct compared with others in the external zone, which could be resulted by stable environmental condition lacking diurnal rhythm after settlement.
The microstructure analysis revealed a life span of about 2 years of D. watasei in the East China Sea, longer than coinhabiting B. pterotum (~205 days) and D. garmani (~343 days) (Cao 2017; Feng 2017). In contrast, Sebastine et al. (2013) reported a life span of 4 years in the Arabian Sea through monthly length frequency method. Such regional difference requires further study. The estimated hatching time could provide a better understanding on spawning season of D. watasei. For now, the known spawning season was obtained from gonadosomatic index analysis, Sassa et al. (2016) inferred that the period from June to September formed the spawning season combining larval survey result. The present study investigates less sampled area and months in the study of Sassa et al. (2016). Similar to the result of Sassa et al. (2016), from June to September was identified as the main season through otolith microstructure. Considering the gap in January and February, March and April were incorporated into the main season and regarded as summer cohort. The estimated hatching time further identified a minor season during winter, which is consistent with the result of larvae surveys that few larvae were recorded in January and February (Sassa and Konishi 2015; Wan and Zhang 2016). Thus, from October to February was regarded as winter cohort. Such division could be biased due to the limitation of sampling effort and require further studies. Overall, the revealed year-round spawning season of D. watasei in the East China Sea is similar to the population in the Arabian Sea (Sebastine et al. 2013), while peak season differed. Such differences could be caused by temperature, similar discrepancy in spawning seasons among latitudes and areas are reported in several broadly distributed myctophid species (Gartner 1993; Sassa et al. 2014; Hosseini-Shekarabi et al. 2015).
A negative allometric growth was detected in D. watasei, while generally mesopelagic fishes, especially myctophids, demonstrate isometric growth (López-Pérez et al. 2020). In addition, population of D. watasei in the Arabian Sea demonstrated an isometric pattern (Sebastine et al. 2013). Generally, the allometric pattern does not show significant regional difference, although slight variation can be resulted by exogenous and endogenous factors (Battaglia et al. 2010; Lopez-Perez et al. 2020). Therefore, such discrepancy suggests ontogenetic variation in growth pattern. With changes in feeding ecology and physiology that affect body proportions at particular life stages, species could actively transfer growth pattern to increase competitive advantage (Czudaj et al. 2022).The negative pattern is mostly observed in species without extensive vertical migration, as these species live in deeper waters and have higher water content (Olivar et al. 2017; López-Pérez et al. 2020). The ontogenetic change also often occurs with maturity of mesopelagic fishes, such as Electrona risso, Lampanyctus lineatus and L. nobilis (Czudaj et al. 2022). Since D. watasei mature at standard length of 120 mm (Sassa et al. 2016), the observed negative growth could be resulted by energy tradeoff between somatic growth and reproductive investment. Furthermore, food availability and environmental factors influence condition factors as well. Diet and experienced temperature of D. watasei change with settlement. To adapt these external and internal changes, non-migrating adult D. watasei adjust its body composition (muscle mass and water content) and metabolism, and then cause ontogenetic variation in growth pattern.
In comparison, summer cohort showed better condition and could invest more energy in reproduction, which corresponds to the estimated main spawning season. Consistent with the significant difference in length–weight relationship between the two cohorts, growth model also demonstrated significant seasonal difference. Summer cohort demonstrated faster rate to smaller \({L}_{\infty }\). As common environmental factors influences fish somatic growth, response to temperature and prey availability varies among mesopelagic species. Vinciguerria mabahiss grew faster than the population in the warmer waters (Aldanondo et al. 2022), while a nonsignificant relationship between growth and temperature was detected for Maurolicus australis (Molina-Valdivia et al. 2021). In addition, otolith growth trajectories of Vinciguerria mabahiss were similar for individuals born throughout the season, while the earliest cohorts of Maurolicus mucronatus demonstrated the highest maximum growth rates (Aldanondo et al. 2022). As for D. watasei in the study area, temperature in the mesopelagic layer between 300 and 500 m depth is almost a constant value of < 12 °C all year round (Seikai National Fisheries Research Institute, http://snf.fra.affrc.go.jp/suion/index.html). Therefore, the detected growth difference could be resulted by the environmental differences experienced by migrating juveniles that carry over to adult stage (Wang et al. 2016).
The study provides a better understanding on the life history characteristics of D. watasei in the East China Sea. The result reveals that D. watasei is a short lived, medium growing species with a long spawning season. The piscivore habit reduces the competition between D. watasei and coinhabiting myctophids and also make D. watasei occupy an important role, which transfer energy from the euphotic zone to deep waters in the food web of the East China Sea.
Data availability
Data are available upon request from the corresponding author.
References
Aldanondo N, Kaartvedt S, Irigoien X (2022) Growth patterns of two Red Sea mesopelagic fishes. Mar Biol 170:8. https://doi.org/10.1007/s00227-022-04144-6
Barham EG (1971) Deep-sea fishes: lethargy and vertical orientation. Maury Center for Ocean Science, Washington DC
Battaglia P, Malara D, Romeo T, Andaloro F (2010) Relationships between otolith size and fish size in some mesopelagic and bathypelagic species from the Mediterranean Sea (Strait of Messina, Italy). Sci Mar 74:605–612. https://doi.org/10.3989/scimar.2010.74n3605
Battaglia P, Esposito V, Malara D, Falautano M, Castriota L, Andaloro F (2014) Diet of the spothead lanternfish Diaphus metopoclampus (Cocco, 1829) (Pisces: Myctophidae) in the central Mediterranean Sea. Ital J Zool 81:530–543. https://doi.org/10.1080/11250003.2014.948500
Battaglia P, Andaloro F, Esposito V, Granata A, Guglielmo L, Guglielmo R, Musolino S, Romeo T, Zagami G (2016) Diet and trophic ecology of the lanternfish Electrona risso (Cocco 1829) in the Strait of Messina (central Mediterranean Sea) and potential resource utilization from the Deep Scattering Layer (DSL). J Mar Syst 159:100–108. https://doi.org/10.1016/j.jmarsys.2016.03.011
Beamish R, Leask K, Ivanov O, Balanov A, Orlov A, Sinclair B (1999) The ecology, distribution, and abundance of midwater fishes of the Subarctic Pacific gyres. Prog Oceanogr 43:399–442. https://doi.org/10.1016/S0079-6611(99)00017-8
Caiger PE, Lefebve LS, Llopiz JK (2021) Growth and reproduction in mesopelagic fishes: a literature synthesis. ICES J Mar Sci 78:765–781. https://doi.org/10.1093/icesjms/fsaa247
Cao Y (2017) Study on the age and growth of Diaphus garmani by otolith in I-lan Bay. Master thesis, National Taiwan Ocean University. Available from: https://hdl.handle.net/11296/nxaf9f. Accessed 15 Jun 2018
Catul V, Gauns M, Karuppasamy P (2011) A review on mesopelagic fishes belonging to family Myctophidae. Rev Fish Biol Fisher 21:339–354. https://doi.org/10.1007/s11160-010-9176-4
Christiansen S, Titelman J, Kaartvedt S (2019) Nighttime swimming behavior of a mesopelagic fish. Front Mar Sci 6:787. https://doi.org/10.3389/fmars.2019.00787
Czudaj S, Mllmann C, Fock HO (2022) Length-weight relationships of 55 mesopelagic fishes from the eastern tropical North Atlantic: across- and within-species variation (body shape, growth stanza, condition factor). J Fish Biol 101:26–41. https://doi.org/10.1111/jfb.15068
Feng Y (2017) Study on the age and growth of Benthosema pterotum by otolith in I-Lan Bay. Master thesis, National Taiwan Ocean University. Available from: https://hdl.handle.net/11296/d8b5y7. Accessed 15 June 2018
Gartner J (1991) Life histories of three species of lanternfishes (Pisces: Myctophidae) from the eastern Gulf of Mexico: I. Morphological and microstructural analysis of sagittal otoliths. Mar Biol 111:11–20. https://doi.org/10.1007/BF01986339
Gartner JV (1993) Patterns of reproduction in the dominant lanternfish species (Pisces: Myctophidae) of the eastern Gulf of Mexico, with a review of reproduction among tropical-subtropical Myctophidae. Bull Mar Sci 52:721–750
Hama T, Shin K, Handa N (1997) Spatial variability in the primary productivity in the East China Sea and its adjacent waters. J Oceanogr 53:41–51. https://doi.org/10.1007/BF02700748
Hosseini-Shekarabi S, Valinassab T, Bystydzieńska Z, Linkowski T (2015) Age and growth of Benthosema pterotum (Alcock, 1890) (Myctophidae) in the Oman Sea. J Appl Ichthyol 31:51–56. https://doi.org/10.1111/jai.12620
Huang D (2019) Feeding habits of Diaphus watasei juvenile fish in offshore waters of southwestern Taiwan. Master thesis, National Taiwan Ocean University. Available from: https://hdl.handle.net/11296/dthbya. Accessed 22 Jul 2020
Irigoien X, Klevjer TA, Røstad A, Martinez U, Boyra G, Acuña JL, Bode A, Echevarria F, Gonzalez-Gordillo JI, Hernandez-Leon S (2014) Large mesopelagic fishes biomass and trophic efficiency in the open ocean. Nat Commun 5:3271. https://doi.org/10.1038/ncomms4271
Kawaguchi K, Shimizu H (1978) Taxonomy and distribution of the lanternfishes, genus Diaphus (Pisces, Myctophidae) in the western Pacific, eastern Indian Oceans and the southeast Asian Seas. Bulletin of the Ocean Research Institute, University of Tokyo, 10, 1-145
Linkowski T (1996) Lunar rhythms of vertical migrations coded in otolith microstructure of North Atlantic lanternfishes, genus Hygophum (Myctophidae). Mar Biol 124:495–508. https://doi.org/10.1007/BF00351031
Linkowski T (1997) Morphological variation, systematics and speciation of the Ceratoscopelus townsendi-C. warmingii complex (Osteichthyes: Myctophidae) based on the studies on the morphology and microstructure of otoliths. Morski Instytut Rybacki, Gdynia
López-Pérez C, Olivar MP, Hulley PA, Tuset VM (2020) Length–weight relationships of mesopelagic fishes from the equatorial and tropical Atlantic waters: influence of environment and body shape. J Fish Biol 96:1388–1398. https://doi.org/10.1111/jfb.14307
Mio S, Tagawa M, Shinohara F, Yamada U (1984) Ecological study on the demersal fish associations in the East China Sea and the Yellow Sea, with reference to food relationships. Bulletin of the Seikai Regional Fisheries Research Laboratory (Japan)
Moku M, Hayashi A, Mori K, Watanabe Y (2005) Validation of daily otolith increment formation in the larval myctophid fish Diaphus slender-type spp. J Fish Biol 67:1481–1485. https://doi.org/10.1111/j.0022-1112.2005.00824.x
Molina-Valdivia V, Bustos CA, Castillo MI, Search FV, Landaeta MF (2021) Oceanographic influences on the early life stages of a mesopelagic fish across the Chilean Patagonia. Prog Oceanogr 195:102572. https://doi.org/10.1016/j.pocean.2021.102572
Olivar MP, González-Gordillo JI, Salat J, Chust G, Cózar A, Hernández-León S, Puelles M, Irigoien X (2015) The contribution of migratory mesopelagic fishes to neuston fish assemblages across the Atlantic, Indian and Pacific Oceans. Mar Freshw Res 67:1114–1127. https://doi.org/10.1071/MF14391
Olivar MP, Hulley PA, Castellón A, Emelianov M, López C, Tuset VM, Contreras T, Molí B (2017) Mesopelagic fishes across the tropical and equatorial Atlantic: biogeographical and vertical patterns. Prog Oceanogr 151:116–137. https://doi.org/10.1016/j.pocean.2016.12.001
Sarmiento-Lezcano AN, Triay-Portella R, Castro JJ, Rubio-Rodríguez U, Pajuelo JG (2018) Age-based life-history parameters of the mesopelagic fish Notoscopelus resplendens (Richardson, 1845) in the Central Eastern Atlantic. Fish Res 204:412–423. https://doi.org/10.1016/j.fishres.2018.03.016
Sassa C, Konishi Y (2015) Late winter larval fish assemblage in the southern East China Sea, with emphasis on spatial relations between mesopelagic and commercial pelagic fish larvae. Cont Shelf Res 108:97–111. https://doi.org/10.1016/j.csr.2015.08.021
Sassa C, Kawaguchi K, Loeb VJ (2003) Early development of Diaphus garmani (Myctophidae) in the transition region of the western North Pacific. Ichthyol Res 50:0094–0097. https://doi.org/10.1007/s102280300015
Sassa C, Kawaguchi K, Mori K (2004) Late winter larval mesopelagic fish assemblage in the Kuroshio waters of the western North Pacific. Fish Oceanogr 13:121–133. https://doi.org/10.1046/j.1365-2419.2003.00275.x
Sassa C, Tsukamoto Y, Yamamoto K, Tokimura M (2010) Spatio-temporal distribution and biomass of Benthosema pterotum (Pisces: Myctophidae) in the shelf region of the East China Sea. Mar Ecol Prog Ser 407:227–241. https://doi.org/10.3354/meps08526
Sassa C, Ohshimo S, Tanaka H, Tsukamoto Y (2014) Reproductive biology of Benthosema pterotum (Teleostei: Myctophidae) in the shelf region of the East China Sea. J Mar Biol Assoc UK 94:423–433. https://doi.org/10.1017/S0025315413001318
Sassa C, Tanaka H, Ohshimo S (2016) Comparative reproductive biology of three dominant myctophids of the genus Diaphus on the slope region of the East China Sea. Deep-Sea Res Pt I 115:145–158. https://doi.org/10.1016/j.dsr.2016.06.005
Sebastine M, Bineesh K, Abdussamad E, Pillai N (2013) Myctophid fishery along the Kerala coast with emphasis on population characteristics and biology of the headlight fish, Diaphus watasei Jordan & Starks, 1904. Indian J Fish 60:7–11
Silva MA, Fonseca CT, Olivar MP, Bernal A, Spitz J, Chouvelon T, Jonasdottir S, Colaço A, Carmo V, Sutton T (2022) MesopTroph, a database of trophic parameters to study interactions in mesopelagic food webs. Sci Data 9:716. https://doi.org/10.1038/s41597-022-01831-3
Suntsov AV, Brodeur RD (2008) Trophic ecology of three dominant myctophid species in the northern California Current region. Mar Ecol Prog Ser 373:81–96. https://doi.org/10.3354/meps07678
Suthers IM (1996) Spatial variability of recent otolith growth and RNA indices in pelagic juvenile Diaphus kapalae (Myctophidae): an effect of flow disturbance near an island? Mar Freshw Res 47:273–282. https://doi.org/10.1071/mf9960273
Takagi K, Yatsu A, Moku M, Sassa C (2006) Age and growth of lanternfishes, Symbolophorus californiensis and Ceratoscopelus warmingii (Myctophidae), in the Kuroshio-Oyashio Transition Zone. Ichthyol Res 53:281–289. https://doi.org/10.1007/s10228-006-0346-2
Takakazu O, Grace C (1990) Otolith microstructure and early ontogeny of a myctophid species Benthosema pterotum. Nippon Suisan Gakk 56:1987–1995. https://doi.org/10.2331/suisan.56.1987
Tanaka H, Sassa C, Ohshimo S, Aoki I (2013) Feeding ecology of two lanternfishes Diaphus garmani and Diaphus chrysorhynchus. J Fish Biol 82:1011–1031. https://doi.org/10.1111/jfb.12051
Tang T, Tai J, Yang Y (2000) The flow pattern north of Taiwan and the migration of the Kuroshio. Cont Shelf Res 20:349–371. https://doi.org/10.1016/S0278-4343(99)00076-X
Wan R, Zhang R (2016) Fish egg, larvae and juveniles in the offshore waters of China and their adjacent waters. Shanghai Scientific & Technical Publishers, Shanghai
Wang L, Li J, Zhao J, Wei H, Hu B, Dou Y, Sun Z, Zou L, Bai F (2016) Solar-, monsoon- and Kuroshio-influenced thermocline depth and sea surface salinity in the southern Okinawa Trough during the past 17,300 years. Geo-Mar Lett 36:281–291. https://doi.org/10.1007/s00367-016-0448-4
Wang Y, Zhang J, Chen Z, Jiang Y, Xu S, Li Z, Wang X, Ying Y, Zhao X, Zhou M (2019) Age and growth of Myctophum asperum in the South China Sea based on otolith microstructure analysis. Deep -Sea Res Pt II 167:121–127. https://doi.org/10.1016/j.dsr2.2018.07.004
Xiao Y (2016) Feeding habits of Benthosema pterotum (Posces: myctophids) in the coastal waters off southwestern Taiwan. Master thesis, National Kaohsiung University of Science and Technique. Available from: https://hdl.handle.net/11296/4h7353. Accessed 7 Sep 2017
Yamada U, Tokimura M, Horikawa H, Nakabo T (2007) Fishes and fisheries of the East China and Yellow Seas. Tokai University Press, Tokyo
Young J, Bulman C, Blaber S, Wayte S (1988) Age and growth of the lanternfish Lampanyctodes hectoris (Myctophidae) from eastern Tasmania, Australia. Mar Biol 99:569–576. https://doi.org/10.1007/BF00392564
Zhang J, Wang Y, Chen Z, Jiang Y, Xu S (2021) Age and growth of Ceratoscopelus warmingii (Myctophidae) in the South China Sea based on sagittal otolith microstructure. Mar Biol Res 17:733–743. https://doi.org/10.1080/17451000.2021.2015390
Zhang C, Guo H, Chen W (2023) Comparative early growth patterns across four dominant fish species in a marine protected area in the East China Sea. Reg Stud Mar Sci 61:102862. https://doi.org/10.1016/j.rsma.2023.102862
Acknowledgements
We express our sincere thanks to Captain Huisen Li and Mr. Bingxuan Chen for their great assistance in specimen collection.
Funding
This work was financially supported by the National Nature Science Foundation of China (no. 41976091).
Author information
Authors and Affiliations
Contributions
C.Z.: data curation, formal analysis, methodology, writing—original draft and review and editing. H.G.: data curation and visualization.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This research was carried out in accordance with all applicable institutional guidelines at the time that the study was conducted.
Consent for publication
All authors reviewed, edited, and approved the manuscript for submission.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, C., Guo, H. Age, growth and feeding habit of Watases lanternfish Diaphus watasei (Pisces: Myctophidae) in the East China Sea. Fish Sci 90, 555–564 (2024). https://doi.org/10.1007/s12562-024-01796-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-024-01796-9