Abstract
Benthic communities are responding to rapid environmental change in the Arctic, yet the ecologically important meiofauna remain poorly studied, leaving little baseline information for evaluating such changes. We investigated the community structure of meiofaunal nematodes (> 63 µm) on the Northeast Chukchi Sea (NEC) continental shelf (< 50 m), and along a broad longitudinal and bathymetric gradient in the Beaufort Sea (BEAU; 20–1200 m). In total, 139 nematode genera representing 32 families were identified. Nematode communities differed between the NEC and BEAU, but the opportunistic genera Sabatieria (Comesomatidae) and Daptonema (Xyalidae) were abundant in both seas. Despite the relatively small sampling area of similar depth across the NEC, spatial variation was reflected by the high abundance of Sabatieria at a subset of sites with higher proportion of fine sediment. Nematode communities in the western BEAU offshore of the Colville River showed little difference among depths, in contrast to areas further east where shelf and upper slope communities were more distinct. Diversity indices were higher in the BEAU than in the NEC, with maximum values in the easternmost area (Banks Island); lower evenness was recorded in the western BEAU (Colville Plume), which was dominated by Sabatieria at all depths. Organic matter quality and quantity influenced community structure in the BEAU. This study provides the first genus-level characterization of nematode communities across this environmentally heterogeneous region. With increased exploration for natural resources and reduced ice coverage, the baseline community structure information provided here supports evaluation of ecosystem change in the Arctic.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rapid environmental change is occurring in the Arctic, with effects observed across all marine trophic levels (Wassmann and Reigstad 2011; Grebmeier 2012). In the Pacific-Arctic region, strong benthic–pelagic coupling supports high benthic biomass such that benthic organisms play an important role in ecosystem functioning (e.g., Grebmeier et al. 1988; Whitehouse et al. 2014). Macrofaunal and epibenthic communities are now reasonably well described (e.g., Bluhm et al. 2009; Blanchard et al. 2013a, b; Grebmeier et al. 2015b), forming persistent biomass “hotspots” in some areas of the northern Bering and southern Chukchi Sea (Grebmeier et al. 2015a, b). These communities have not been immune to environmental change (Grebmeier 2012), with evidence of shifts in relative abundance of the dominant benthic taxa, and in the magnitude and locations of phytodetritus deposition (e.g., Lovvorn et al. 2016). However, despite the intensive study of macrofaunal and epibenthic communities, particularly in the Chukchi Sea, relatively little is known about the benthic meiofauna in this region and throughout the Arctic.
Metazoan meiofaunal communities (eukaryotic organisms 45 µm–1 mm, typically dominated by Nematoda) have long been viewed as useful bioindicators because they are relatively non-motile, have low dispersal capability, reproduce rapidly, and can respond very quickly to disturbance (Platt et al. 1984; Bongers et al. 1991; Schratzberger et al. 2000; Zeppilli et al. 2015). The limited data available from the Arctic suggest that meiofaunal abundance reflects regional, temporal, and depth-dependent patterns in primary production, with abundances ranging from 10 to 1000 s of individuals 10 cm−2 (Piepenburg et al. 1997; Vanreusel et al. 2000; Bessière et al. 2007; Fonseca and Soltwedel 2007; Giere 2009; Lin et al. 2014). Meiofaunal organisms can contribute considerably to total benthic biomass (McLachlan and Brown 2006), and play important roles in trophic interactions, bioturbation, and remineralization of organic matter, particularly in deeper waters (Piepenburg et al. 1995; Grzelak and Kotwicki 2012; reviewed by Schratzberger and Ingels 2018).
Free-living marine nematodes are particularly abundant and diverse, accounting for the majority of benthic meiofaunal abundance in most ocean regions (Heip et al. 1985; Moens et al. 2013). For example, nematodes represent 50–95% of the metazoan meiofauna in the Northeast Chukchi and Canadian Beaufort Seas (Nelson et al. 2014; Hajduk 2015). Given the wide range of ecological roles reflected by the diverse body types, life histories, and feeding strategies of nematodes (Moens et al. 2013; Schratzberger and Ingels 2018), characterizing nematode communities at higher taxonomic resolution (genus and/or morphospecies level) yields valuable insights into benthic ecosystem functioning. This information supports classification based on ecological function including feeding mode (Wieser 1953; Moens and Vincx 1997), susceptibility to disturbance (Bongers 1990), and association with environmental parameters such as pore-water oxygen concentration (Soetaert et al. 2002).
A handful of Arctic studies, mostly targeting bathyal and abyssal areas in the eastern region, have examined nematode community structure and/or functional groups (Vanaverbeke et al. 1997, 2004; Vanreusel et al. 2000; Fonseca and Soltwedel 2007, 2009; Hoste et al. 2007; Gallucci et al. 2008, 2009). A few studies of coastal shelf nematodes have also been conducted in the White Sea and Kara Sea in the eastern Arctic (Miljutin et al. 2014; Portnova et al. 2017). These studies demonstrate a decline in abundance of nematodes and total meiofauna with depth in Arctic shelf-slope environments (Vanaverbeke et al. 1997; Bessière et al. 2007; Lin et al. 2014), which is consistent with global patterns in benthic communities (Rex et al. 2006; Wei et al. 2010). Moreover, spatial and temporal variation in nematode abundance and biomass appears to be affected by organic matter input and availability (Vanreusel et al. 2000; Bessière et al. 2007; Hoste et al. 2007; Fonseca and Soltwedel 2009).
The continental shelf and slope of the Chukchi and Beaufort Seas, located in the Alaskan (USA) and Canadian sectors of the Pacific-Arctic, have been the focus of much research in recent years, partly fueled by interest in mineral resource exploration and extraction (e.g., Rand and Logerwell 2011; Blanchard et al. 2013a; Day et al. 2013; Dunton et al. 2014). Although meiofaunal benthic communities were rarely considered, multiple interdisciplinary field programs conducted over the last two decades have generated baseline information for use in monitoring human impacts and environmental change. These studies have highlighted the influence of water-mass structure on composition of pelagic and benthic communities. Northward-flowing water masses of Pacific origin transit the broad, shallow (~ 50 m) Chukchi Sea shelf, and enter the Arctic Ocean in the northeast via Barrow Canyon (Weingartner et al. 2005, 2013). The composition of zooplankton communities reflects water-mass distribution in the Chukchi Sea (Ershova et al. 2015), whereas benthic communities exhibit patchiness related to effects of seafloor topography on circulation, and deposition of organic matter (Blanchard et al. 2013a, b; Blanchard et al. 2017). Pacific water masses flowing eastward into the Beaufort Sea (BEAU) interact with Atlantic-origin water masses at about 250 m depth. The narrower BEAU shelf also receives inputs of freshwater and terrestrial organic matter from multiple large rivers including the Colville and Mackenzie Rivers. The vertical layering of water masses in the BEAU influences biogeographic patterns and species composition of zooplankton, fish, and benthic invertebrate communities (Conlan et al. 2008; Logerwell et al. 2011; Nephin et al. 2014; Majewski et al. 2017; Smoot and Hopcroft 2017b; Ravelo et al. 2020). Bathymetric trends in the quantity and quality of sinking organic matter also influence benthic communities and food-web structure on the outer BEAU shelf and slope (Nephin et al. 2014; Roy et al. 2014, 2015; Stasko et al. 2018b).
In this study, we explore spatial patterns in nematode community structure across the NEC and BEAU shelf and upper slope, and compare these patterns with well-established trends observed in other faunal groups. Specifically, we examined nematode specimens from samples collected opportunistically by three interdisciplinary field programs, which were primarily designed to assess habitat characteristics and community structure of fishes and benthic macroinvertebrates in areas of interest for mineral resource extraction. Our primary goal was to characterize nematode communities across this large, environmentally heterogeneous area, drawing comparisons between the NEC and BEAU and along the broad longitudinal and bathymetric gradients within the BEAU. In addition, we evaluated the potential environmental factors affecting spatial patterns in nematode communities, with the caveat that logistical constraints and objectives of each field program yielded inconsistencies in sampling method and collection of environmental data. Relationships to environmental variables are thus explored using discrete datasets collected within the NEC and the BEAU. This study provides the first genus-level characterization of nematode communities in the Pacific-Arctic, constituting an important baseline for observing future trends in the Arctic.
Materials and methods
Samples were collected in the NEC in September 2012 as part of the Chukchi Sea Environmental Studies Program (CSESP), which was designed to establish a baseline of ecological conditions in petroleum lease-sale areas. We selected a subset of sampling locations distributed across the region for detailed taxonomic analysis of the meiofaunal nematodes, in an effort to generate a first description of nematode community structure for the NEC. A detailed description of the CSESP program, including sampling design and methods for collection of environmental data, is provided in Blanchard et al. (2017) and references therein, as well as in the CSESP reports available at www.ChukchiScience.com (cf., Blanchard and Knowlton 2013). BEAU samples were collected in 2012 (23–28 September) as part of the US-Canada Transboundary Fish and Lower Trophic Communities Project (USTB), and in 2013 (29 August–3 September) and 2014 (2–14 August) by the Beaufort Sea Regional Environmental Assessment Program (BREA) conducted by the Department of Fisheries and Oceans Canada. USTB and BREA sampled a broad area of the BEAU shelf and slope, including a series of transects extending along lines of longitude from 20 to 1200 m depth (Fig. 1; Online Resource 1). These studies assessed demersal fish communities and habitat characteristics to inform environmental monitoring and impact assessment in mineral resource exploration and extraction areas. The oceanographic setting for the USTB and BREA study areas, including bottom-water temperature and salinity data used here, was described elsewhere (Eert et al. 2015; Niemi et al. 2015; Smoot and Hopcroft 2017a, b). Given the opportunistic nature of sampling and the lack of nematode taxonomic records for this area, we focused on a subset of stations sampled in each respective field program, prioritizing maximum spatial coverage over within-site or local-scale replication. This morphological taxonomic work was conducted to ground-truth molecular analyses of community structure from paired samples, which will be presented in subsequent publications.
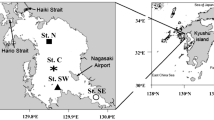
Map of study area. Sample locations are colored according to geographical areas including the Northeast Chukchi Sea (NEC; gray), Colville River plume (COP, teal), Mackenzie River plume (MAP, yellow), Eastern Beaufort Sea (EBS, white), and Banks Island (BNK, purple). Lower case letters next to NEC samples indicate the SIMPROF (a–e) groups for this region (Table 1, Fig. 3). Sampling depth ranges are represented by different symbols: shallow (square, < 100 m), mid (circle, 200–500 m), and deep (triangle, > 500 m). Teal and yellow stars (on coastline) indicate mouths of Colville and Mackenzie Rivers, respectively
Meiofauna samples were collected in the NEC using a 0.1-m2 Van Veen grab (n = 14), and in the BEAU (n = 26) using a 0.25-m2 box core (BX-650, Ocean Instruments, Inc., San Diego, CA). While grabs and box cores are not optimal for quantitative meiofaunal sampling, logistical constraints of the various field programs required that we rely on these approaches. All grabs and cores were visually inspected upon recovery, and only high-quality samples with clear top-water and undisturbed sediment–water interface were retained. The top 1 cm of sediment was sampled from the surfaces of grabs and box cores using a 7-cm diameter sub-core, and immediately preserved in 10% formalin. NEC samples were rinsed over a 63- and a 500-μm sieve, and meiofaunal organisms were isolated from the material retained on the finer sieve using a Lugol's extraction (Burgess 2001). For the BEAU, meiofauna were extracted from the sediment matrix by decanting (Creer et al. 2010), and washed through a 63-μm sieve. After repeating the decantation ten times, the material retained on the sieve was transferred to a petri dish to isolate nematodes. Given that our sampling area spans shelf and deep-sea locations (20–1200 m), as well as practical constraints on sample processing, we selected a sieve size at the upper end of the range typically used in meiofauna studies (cf., Somerfield and Warwick 2013). Some smaller genera may not be retained on the 63-μm sieve particularly in deep-sea settings (Leduc et al. 2010); however, in our experience (J. Sharma, pers. obs.) and also noted by Somerfield and Warwick (2013), it is primarily juveniles that are lost.
For each sample, the first 100 nematodes per core were hand-picked on a gridded petri dish under a Nikon SMZ-1B stereoscope and transferred to anhydrous glycerin (Seinhorst 1959). When fewer than 100 nematodes were present in a sample (n = 2 BEAU samples), all individuals were picked. This number of individuals has been shown to reflect the overall composition of the nematode community in a given sample (Soetaert and Heip 1990), such that the most common species can be assessed with this relatively small sample size. Although rarer taxa may be missed using this approach, these common species likely dominate in ecological importance, and in driving spatial patterns detected using multivariate statistics. This approach is thus commonly used to economize effort given the labor-intensive nature of nematode identification, while still capturing meaningful ecological patterns (cf., Urban-Malinga et al. 2006; Pusceddu et al. 2014).
Nematodes were mounted in glycerin on glass slides lined with paraffin (Hooper 1986) and identified to genus level under a Zeiss Axioskop microscope using identification keys for free-living marine nematodes (Platt and Warwick 1983; Schmidt-Rhaesa 2014; Guilini et al. 2017), based on the classification scheme of De Ley and Blaxter (2002). Genus-level identification appears to be as effective as species-level identification for detecting significant ecological patterns in nematode communities (Somerfield and Clarke 1995; Vanreusel et al. 2010). All nematodes examined have been vouchered and deposited at the Smithsonian National Museum of Natural History (NMNH accession number TM2080466).
Taxonomic identifications of nematode genera were linked to functional roles based on morphological and life-history attributes, including trophic diversity, tail shape, and adult body shape. Nematodes were classified into four feeding groups based on the morphology of their buccal cavity as defined by Wieser (1953): selective deposit feeders (1A), non-selective deposit feeders (1B), epigrowth feeders (2A), and predators and omnivores (2B). In addition, taxa were assigned a c–p value using a five-point scale based on life-history strategies. For example, enrichment opportunists (c–p = 1) are characterized by short generation times (days) and rapid reproductive rates, whereas extreme persisters (c–p = 5) have longer generation times (months) and slow reproductive rates (Bongers et al. 1991, 1995). The family c–p score was assigned when a genus value was not available. The maturity index (MI) was then calculated by using the weighted means (taxon c–p value multiplied by taxon frequency) for the c–p values of taxa in a given sample. We also evaluated differences in morphological features that have been shown to reflect community attributes and/or functional roles, including tail shape (conical, elongated/filiform, clavate, and short/round; Thistle et al. 1995) and body shape. Body shape categories are based on length-to-width ratio (stout, slender, long/thin; Soetaert et al. 2002), and have been used to distinguish among nematode communities associated with different environments.
Environmental data
A different suite of environmental variables was quantified for each sampling area based on the specific objectives of each field program (Table 1). For grain size analysis (CSESP and USTB samples only), subsamples were removed from the top 5-cm surface layers of box cores and grabs using a 60-cc syringe, and frozen at − 20 °C. In the laboratory, samples were thawed and homogenized, and transferred to a beaker with 20 ml of 2 g L−1 sodium hexametaphosphate (a dispersant) and 30 ml water. Samples were sieved on 2-mm and 63-μm nested sieves to separate gravel, sand, and mud (= silt + clay) fractions (Wentworth 1922). All three fractions were dried at 90 °C and weighed; weights were recorded as proportions of the total sample weight, and %sand and %mud were included as variables in statistical analyses. Porosity, calculated as the mass ratio of water to mineral material in a given volume of sediment, was also included.
For chlorophyll-a (chl-a) and phaeopigment analysis (BEAU only), subsamples were taken using a 60-cc syringe inserted to 1-cm sediment depth and stored in Whirl-pak® bags wrapped in aluminum foil at − 80 °C until processing. Samples were thawed, homogenized, and weighed prior to analysis. Each sample was suspended in 5 ml 100% acetone, mixed using a vortex mixer, and sonicated in an ice water bath for 10 min. Samples were extracted overnight at − 20 °C. Each sample was then centrifuged to remove sediment, and the supernatant was transferred to a clean test tube. Chlorophyll-a concentration of the supernatant was measured using a TD-700 fluorometer (Turner Designs, San Jose, CA, USA); samples were then acidified with HCl, and fluorescence readings were taken of the acidified samples to produce phaeopigment concentration values (Arar and Collins 1997). A standard curve produced using commercially available chl-a standard was used to convert fluorescence readings into concentrations.
For stable isotope and total organic carbon (TOC) analysis, subsamples were scraped from the upper 1-cm surface layer of box cores and grabs, and frozen at -20 °C. Prior to analysis, each sample was thawed and homogenized, and ~ 1-ml subsamples were suspended in 5 ml of 1 N HCl to remove inorganic carbonates (Iken et al. 2010; Goñi et al. 2013). Samples were loosely capped and allowed to sit overnight or until bubbling ceased, indicating complete dissolution of carbonate. After adding distilled water, samples were vortexed and centrifuged at 2500 rpm for 5 min, and the supernatant was discarded. This process was repeated until pH was close to neutral. Samples were then freeze-dried and submitted to the Alaska Stable Isotope Facility (ASIF) for analysis. Stable isotope values for carbon and nitrogen were determined using a Costech ESC4010 elemental analyzer interfaced with a continuous-flow isotope ratio mass spectrometer (CF-IRMS; Thermo Finnigan Delta VPlus). Results are expressed as conventional δ-notation in parts per thousand (‰) according to the equation δ(‰) = ([Rsample/Rstandard] − 1) × 1000, where R is the ratio of 13C:12C or 15N:14N. Standards were Vienna Pee Dee Belemnite (VPDB) for δ13C values, and atmospheric N2 for δ15N. Percent organic carbon and percent nitrogen were determined based on elemental analysis of the pre-weighed sample, and presented here as TOC (mg g−1 dry sediment) and mass ratio of carbon to nitrogen (C:N).
Statistical analyses
Nematode genera counts for each sample were converted to relative abundances (i.e., standardized to total number of individuals identified per sample) prior to statistical analyses. All multivariate analyses of community structure were implemented in the software package PRIMER v7 (Clarke and Gorley 2015). Relative abundance data were square-root transformed to give more weight to the rarer taxa, and analyses were performed on the Bray–Curtis similarity matrix constructed from the transformed data. No a priori structure was present in the NEC dataset, so a hierarchical cluster analysis (CLUSTER) with similarity profile (SIMPROF) test was used to identify groups of samples with similar nematode communities. For the BEAU, samples were grouped a priori by the geographical areas indicated in Fig. 1, so spatial patterns were visualized using nMDS to evaluate differences in community structure among these geographical sampling areas (COP-Colville River plume, MAP-Mackenzie River plume, EBS-Eastern Beaufort Sea, BNK-Banks Island). Examination of the nMDS ordination further suggested trends related to depth in three areas within the BEAU (MAP, EBS, BNK). Samples within these three areas were then further grouped by depth ranges, classified as shallow (-S; < 100 m), mid (-M; 200–500 m), and deep (-D; > 500 m) (Fig. 1; Online Resource 1). ANOSIM analyses were then conducted to test for significant differences in community structure between the NEC and BEAU, and among these geographical areas and depth ranges within the BEAU. The taxa that accounted for differences among geographical areas were identified using similarity percentages (SIMPER) analysis.
To examine variation in meiofaunal nematode communities with depth, a PERMANOVA analysis (Anderson et al. 2008; Anderson 2017) was conducted using only BEAU data (NEC stations were all roughly the same depth). Although ‘mid’ (200–500 m) and ‘deep’ (> 500 m) sites appeared to be distinct based on the nMDS ordination, there were too few ‘deep’ samples to support a robust statistical analysis, so samples were pooled into ‘shelf’ (≤ 100 m) and ‘slope’ (> 100 m) sites. The PERMANOVA model was constructed to test for effects of geographical area and shelf/slope on meiofaunal nematode communities (i.e., using factors ‘area’ and ‘shelf/slope’), using Type III sums of squares with unrestricted permutations.
Relationships between nematode community structure and continuous environmental variables were evaluated using distance-based linear models (DISTLM; Anderson et al. 2008) with stepwise selection procedure using an information criterion (AIC; Akaike 1974) for model selection. Different suites of environmental variables were available for testing in the NEC (porosity, % mud, salinity, temperature, δ13C, δ15N, C:N, TOC) and BEAU (porosity, depth, chl-a and phaeopigment concentrations, TOC, δ13C, δ15N, C:N, temperature, salinity). Draftsman plots were examined to check for collinearity between environmental variables, and it was not necessary to exclude any variables. One of the 50-m BNK sites was excluded from this analysis due to missing environmental data.
Univariate descriptors of meiofaunal nematode communities including total number of genera (S), genus richness (d), evenness (J′), Shannon–Wiener diversity index (log2, H′), and the expected number of genera (ES50) were compared between the NEC and BEAU, and among geographical areas within the BEAU using one-way ANOVA in STATISTICA v7.1. Cochran’s C was used to test for homogeneity of variance and, where necessary, data were log (x + 1)-transformed. Tukey’s multiple comparison tests were used when significant differences were detected (p < 0.05; Sokal and Rohlf 1995).
Results
Nematode community structure
A total of 4454 nematode specimens (NEC: 1499; BEAU: 2955) were morphologically identified. In the 26 samples examined from the BEAU, we recorded 116 nematode genera representing 30 families. In the NEC, we examined material from 14 stations and recovered 82 genera from 26 families. Although most of the nematode families were shared between both seas, two families (Fusivermidae and Scaptrellidae) were found exclusively in the NEC and six families (Desmoscolecidae, Leptolaimidae, Ceramonematidae, Ironidae, Neotonchidae, and Benthimermithidae) were found exclusively in the BEAU (Online Resource 2). Approximately 95% of the total relative abundance was attributed to 12 families in the NEC and to 16 families in the BEAU (Table 2, Online Resource 2). At the family level, Comesomatidae was the most abundant taxon in both the NEC (48.4%) and the BEAU (35.3%). Other abundant nematode families (relative abundance > 5%) included Chromadoridae (NEC: 5.9%, BEAU: 13.9%), Sphaerolaimidae (BEAU: 5.1%), Thoracostomopsidae (NEC: 5.7%), and Xyalidae (NEC: 16.0%, BEAU: 10.1%) (Table 2). The ten most abundant families accounted for 93.0% of the total individuals in the NEC, and from 80.8% (BNK-S) to 99.6% (MAP-D) in the BEAU (Table 2).
At the genus level, nematode assemblages were mostly dominated by Sabatieria (NEC: 37.6%, BEAU: 22.3%) followed by Daptonema (NEC: 10.7%), Cervonema (NEC: 4.8%, BEAU: 11.1%), Dichromadora (BEAU: 8.2%), and Halalaimus (BEAU: 5.7%) (Table 2). Nematode community structure differed significantly between the BEAU and NEC (Fig. 2; ANOSIM global R = 0.518, p = 0.001). According to the SIMPER analysis, the average dissimilarity in nematode genus composition between the NEC and BEAU was 72.7%. In addition to the most abundant genera (Sabatieria, Cervonema, Daptonema, Dichromadora, and Halalaimus), taxa contributing > 2% to this dissimilarity included Axonolaimus, Dorylaimopsis, Halichoanolaimus, and Sphaerolaimus. Together, these genera accounted for ~ 30% of the difference between the NEC and BEAU.
A SIMPROF analysis of community structure in the NEC indicated variations in nematode communities across the sampling area (Fig. 3). SIMPER analysis suggested that taxa contributing most to within-group similarity were Sabatieria, Daptonema, Oxyonchus, Oncholaimus, Viscosia, Anticoma, Paramonohystera, and Cervonema (Fig. 3). Sabatieria was highly abundant at most sites except for a few locations where Daptonema was more dominant (Fig. 3, SIMPER group a).
nMDS ordination showing variation in nematode community structure within the Northeast Chukchi Sea (NEC). Ovals indicate SIMPROF groups, which are labeled a–e for reference to station location and corresponding environmental data (Fig. 1, Table 1). Pie slices represent relative abundance of all genera (with feeding group) contributing > 10% to the within-group similarity for at least one SIMPROF group, based on SIMPER analysis. Ordination is based on the Bray–Curtis similarity matrix of relative abundance data
In the BEAU, Sabatieria was highly dominant at most sites, except at MAP-D and BNK-M where Dichromadora was most abundant, and at BNK-S and BNK-M which were dominated by Nudora and Dichromadora, respectively (Table 2). At COP in the western BEAU, community structure was similar across the wide depth range sampled (50–1000 m; Fig. 4). COP significantly differed from all other areas, and had the highest proportion of Sabatieria overall (41.6%). Further east, samples differed across depths. In the MAP and EBS areas, communities were more differentiated between shallow shelf (< 100 m), mid (200–500 m), and deep (> 500 m) sites (Fig. 4). The 20-m EBS-S samples formed a well-supported group. The two 75-m MAP-S samples also grouped together with COP. The 350-m MAP-M samples grouped with those at similar depth just to the east (EBS), whereas MAP-D samples (> 750 m) formed a separate group. BNK samples differed between shallow (50 m) and mid-depths (250–380 m). ANOSIM detected significant differences among all sample groups (global R = 0.296, p = 0.001). Pairwise testing showed no difference between NEC and COP (p = 0.211), whereas COP and BNK-M were significantly different from all other areas (Online Resource 3). ANOSIM results also indicated depth-related trends in some areas. In particular, shallower shelf sites showed relatively little differentiation across the study area, with no difference detected between NEC, EBS-S, MAP-S, and BNK-S. Further exploration of the effect of depth within the BEAU based on PERMANOVA analysis indicated a significant interaction effect for the factors area and shelf/slope (Table 3), supporting the observation that nematode communities were structured by depth in some areas of the BEAU but not in others (Fig. 4).
nMDS ordination showing variation in nematode community structure within the Beaufort Sea (BEAU). Ordination is based on the Bray–Curtis similarity matrix for relative abundance data. Points are labeled with approximate sampling depths, and coded by geographical sampling area: COP = Colville River plume; MAP = Mackenzie River plume; BNK = Banks Island; EBS = Eastern Beaufort Sea (Fig. 1)
All diversity indices were higher in the BEAU than in the NEC (Table 4). The genus Sabatieria was highly dominant in the NEC and in the western BEAU (COP and MAP-M). Consequently, both COP and MAP areas showed the lowest values for evenness. Additionally, MAP had the fewest genera and the lowest values of genus richness and Shannon–Wiener diversity (H′). Within the BEAU, all indices were significantly higher in the BNK area than in other sampling areas.
Functional-group composition of nematode communities
Non-selective deposit feeders (1B) dominated in both the NEC and BEAU, with slightly higher relative abundances in the NEC (62.9%) than in the BEAU (50.2%) (Table 5). Epistratum feeders (2A) were the second most abundant group in the BEAU (20.6%), in contrast to predators/omnivores (2B) in the NEC (18.4%). When summed across depth zones, feeding group 1B accounted for 46–62% of individuals at COP, MAP, and EBS, but was least abundant (35.0%) at BNK. In COP, relative proportions of each feeding group were similar across depth zones, dominated by group 1B (59.2%) with approximately equal numbers of other feeding types. Proportions of each feeding group differed with depth in MAP and EBS. In MAP, individual nematodes were distributed relatively evenly among all feeding groups in shallow water (MAP-S), whereas group 1B was most abundant at 350 m in MAP-M (62.7%), and group 2A was most abundant at depth (MAP-D, 48.2%). At the shallow EBS-S sites, 75.7% of individuals were classified as group 1B, compared to only 46.9% in EBS-M where relatively more (25.2%) epistratum feeders (2A) were found. Proportions of individuals in each feeding group were similar at BNK-S and BNK-M, with about one-third of individuals in group 1B and one-third in group 2A. Selective deposit feeders (group 1A) were most abundant in BNK (24.3%). Predators/omnivores (group 2B) were most abundant in NEC and COP (~ 18%), and accounted for some of the differentiation in community structure among sites within the NEC (Fig. 3).
Based on the c–p value scale, enrichment opportunists (c–p = 1) were not detected at any location (Table 5). However, general opportunists (c–p = 2) were dominant across both the NEC (76.2%) and BEAU (64.0%). Despite differences in feeding group composition across depths in MAP, c–p values of 2 were most common (55–67%) at all depths. EBS-S was also dominated by individuals with c–p = 2 (79.1%), whereas this proportion decreased to 56.2% at EBS-M where more individuals with c–p = 4 (31.4%) were found. A more even distribution of individuals across categories 2, 3, and 4 was found at BNK, although c–p scores of 2 were still most common at both BNK-S (41.3%) and BNK-M (56.9%). Values of the maturity index ranged from 2.33 (NEC) to 2.75 (EBS), which is consistent with the dominance of non-selective deposit feeders (feeding group 1B) at most sites (Bongers et al. 1991).
The slender body morphology, which characterizes several of the most abundant taxa such as Sabatieria, Cervonema, Dichromadora, and Daptonema, was by far the most abundant at all locations and depths (> 80%; Table 5). Long/thin body shapes (e.g., Halalaimus) were found in all sampling areas, but accounted for only 5–15% of individuals in each area, with the highest numbers found at COP (14.5%) and MAP (13.1%). Stout body types, as seen in Desmoscolex, were most abundant at BNK (6.1%) and accounted for < 0.4% in other areas.
Clavate tails were the most common shape in the NEC (55.6%), but in the BEAU individuals were roughly evenly proportioned among conical (37.3%), clavate (33.1%), and elongated/filiform (27.8%; Table 5). Clavate tail shapes, which characterize the highly abundant Sabatieria spp., were similarly abundant at COP (~ 50.1%), and declined in abundance further east (31.3% and 37.1% at MAP and EBS, respectively, 13.5% and BNK). Conical tail shapes, as seen in Chromadoridae, were most abundant at BNK (49.0%), followed by elongated/filiform (36.6%). Taxa with short/round tail shapes were rare (maximum 3.8% at COP). At MAP, similar proportions of each group were found at MAP-S and MAP-M, but at MAP-D conical tail shapes were more common (59.5%). At EBS-S, clavate shapes were most abundant (48.2%), whereas conical was more abundant at EBS-M (50.0%). Proportions were similar at BNK-S and BNK-M, with ~ 50% conical tail shapes and ~ 30% elongated/filiform tail shapes in both depth zones.
Environmental predictors of nematode community structure
Relationships between nematode community structure and environmental variables could not be evaluated for the dataset as a whole (i.e., NEC and BEAU combined) due to inconsistent measurements among field programs. In the NEC, sediment chl-a and phaeopigment concentrations were not available, but some grain size information could be included in the analysis. The DISTLM model selected based on AIC retained only % mud, which accounted for 18.8% of total variation in nematode communities within the NEC (AIC = 108.91, R2 = 0.188, pseudo-F = 2.783, p = 0.005). SIMPROF group a, for which the genus Sabatieria contributed little to within-group similarity (Fig. 3), included three stations with sandier substrate, lower TOC, and higher C:N indicative of more degraded organic matter (Table 1). The remaining stations all had higher %mud and similar C:N, but SIMPROF groups differed in terms of TOC content.
Four environmental predictors of nematode community structure within the BEAU were retained in the best-fit DISTLM model (AIC = 210.39, R2 = 0.322, dbRDA visualized in Fig. 5), explaining 32.3% of the total variance: TOC (8.6%), porosity (8.7%), phaeopigment concentration (9.2%), and δ13C (5.8%). COP was largely separated by phaeopigment concentrations, which were up to three times higher than in MAP or EBS, particularly at depths > 200 m (Table 1). Shallow EBS-S sites were characterized by high TOC concentrations, while deeper EBS-M slope sites clustered more closely with MAP based largely on porosity. Surface sediments were highly 13C-enriched (-11‰) at the 50-m BNK-S sites, thus explaining a large portion of the variance in nematode community structure. Depth and water-mass characteristics (temperature and salinity) did not account for significant portions of this variance. Overall, spatial patterns of nematode communities within the BEAU were best explained by the amount and quality of organic matter and by porosity (Fig. 5). However, the total amount of variation explained by the DISTLM model is fairly low, suggesting other environmental factors influencing nematode community composition were not accounted for. Grain-size characteristics, which were not available for BEAU, are likely among these factors as suggested by their importance in NEC.
Distance-based redundancy analysis (dbRDA) of nematode community structure, and correlations with environmental variables within the Beaufort Sea (BEAU). Points in the ordination are coded according to geographical area: COP = Colville River plume; MAP = Mackenzie River plume; BNK = Banks Island; EBS = Eastern Beaufort Sea (Fig. 1). In the inset table, multiple partial correlations are given for the four variables retained in the best-fit DISTLM model (AIC = 210.39, R2 = 0.322) including total organic carbon (TOC; F = 2.437, p = 0.002), porosity (F = 2.613, p = 0.004), phaeopigment concentration (phaeo; F = 2.989, p = 0.001), and δ13C (F = 1.960, p = 0.005)
Discussion
Nematode community structure varied spatially across the broad longitudinal and bathymetric gradient sampled here, with notable differences between the Northeast Chukchi (NEC) and Beaufort (BEAU) Seas, and among geographical areas within the BEAU. These patterns should be considered with some caution given the differences in extraction methods (e.g., Escobar-Briones et al. 2008) and in sampling gear types between these two regions, although methods were consistent within NEC and BEAU. Sampling gear has been shown to affect multivariate dispersion (i.e., variability) of nematode community composition, although no differences were found in univariate diversity indices or in overall patterns of community structure when comparing box cores and grabs (Somerfield and Clarke 1997; Somerfield et al. 2006). Reports of increased dispersion with grab samples relative to box cores may partially explain the spatial heterogeneity in community structure and lower diversity observed in the NEC. However, nematode community structure in the NEC was not significantly different from that in the Colville River plume (COP) area in the western BEAU where samples were collected with a box core, thus suggesting patterns are not strictly the result of sampling bias. Moreover, our sample collection and processing methods were consistent within the NEC and BEAU, and thus should not have affected patterns within each of these regions.
The effect of sampling year or month may have influenced patterns observed within the BEAU as geographical areas were sampled at different times. However, we expect the environmental heterogeneity encompassed by this broad longitudinal and bathymetric gradient likely overwhelms any temporal trend in the dataset. Benthic macrofaunal communities, particularly in high-latitude regions, have shown limited temporal variability relative to the water column such that dominant patterns in the benthos tend to reflect longer-term rather than seasonal or interannual trends (e.g., Mincks et al. 2005; Grebmeier et al. 2015a, b). Although meiofauna may be expected to react more rapidly to environmental variation on short (e.g., seasonal or interannual) time scales (Zeppilli et al. 2015), the spatial patterns we observed in nematode communities are consistent with biogeographic patterns also observed in other faunal groups (e.g., Conlan et al. 2008; Blanchard and Feder 2014; Ravelo et al. 2015).
Wide-spread dominance of Sabatieria in the Northeast Chukchi and Beaufort Seas
Sabatieria was the dominant nematode genus in both the NEC and BEAU. This genus, along with other abundant taxa including Daptonema, Cervonema, and Halalaimus, occurs in high numbers at shelf-break and slope locations globally (e.g., Vanreusel et al. 1992; Soetaert et al. 1995; Vanhove et al. 1999). To date, no other published data on nematode communities in the NEC or BEAU are available for comparison, but Sabatieria was also numerically dominant at shelf-break depths (~ 250 m) in the White Sea (Miljutin et al. 2014). However, Sabatieria and Daptonema were notably low in abundance at similar depths in the Arctic Laptev Sea and Eastern Greenland margin, attributed to low organic matter input in ice-covered waters (Vanaverbeke et al. 1997; Fonseca and Soltwedel 2007).
Sabatieria is a large genus containing over 100 accepted species with evidence of cryptic speciation (De Groote et al. 2017), so the broad distribution observed here may also be concealing intra-specific variation in relationship to environmental parameters. At least four morphospecies of Sabatieria were identified in our samples, three of which were only found in the NEC. One of these morphospecies exhibited a long/thin body morphology, as opposed to a slender morphology seen in the other taxa, further suggesting a different ecological role and/or association with different habitat characteristics in different species. Molecular analyses of individuals obtained from a paired set of samples collected alongside those analyzed here further identified seven putative species of Sabatieria as well as two species of Cervonema (Pereira et al. 2020).
Nematode communities were dominated by non-selective deposit feeders (group 1B) such as Sabatieria and Cervonema throughout the NEC and BEAU study areas, which also resulted in low values of the maturity index between 2 and 3. These values suggest the presence of disturbed or stressful environmental conditions that may favor taxa with more opportunistic life-history strategies. Alternatively, this index may simply reflect trophic conditions, because a maturity-index value of 2.1 typically corresponds to dominance of non-selective deposit feeders (Bongers et al. 1991). Feeding mode may be constrained by sediment type and/or the nature of the available food source, whereas the opportunistic life-history characteristics of group c–p 2 are favored in disturbed, stressful, or eutrophic areas with anoxic sediments (Bongers et al. 1991).
The overwhelming dominance of Sabatieria and its role in driving functional group patterns suggests that community structure, particularly in NEC and COP, was indeed influenced by a shallow anoxic or suboxic layer in sediments associated with high organic loading. Comesomatidae, including Sabatieria, Cervonema, and Dorylaimopsis, are often dominant in highly enriched sediments with low oxygen levels, likely due to efficiency in oxygen uptake conferred by the higher surface area-to-volume ratio of the long, slender body type (Soetaert and Heip 1995; Moens et al. 2013). These large-bodied, slender taxa are also more mobile, and are thus thought to be less affected by total organic matter input (e.g., chl-a concentration) or other prevailing environmental conditions at a given location (Grzelak et al. 2016). These lines of evidence suggest that low-oxygen conditions may be prevalent in sediments—a factor that has not been well documented or considered previously in this region. Interestingly, anaerobic bacteria were dominant in prokaryote communities collected from surface sediment (1 cm) at the same sites in the COP and MAP-M areas where we observed greatest dominance of Sabatieria, further suggesting a shallow anoxic sediment layer (Walker et al. submitted). Moreover, the dominance of Sabatieria down to depths of 1000 at COP rules out other potential sources of disturbance such as ice scour or wind-driven resuspension of sediments that may confer an advantage to taxa with opportunistic life strategies (c–p = 1 or 2). The prevalence of conical/clavate tails as seen in Sabatieria has been noted to be associated with high-energy environments, which is also consistent with potential disturbance via resuspension of sediments (Semprucci et al. 2018). Dominance of this genus in anthropogenically impacted sediments has been previously discussed (Schratzberger et al. 2009), and it is reportedly well adapted to extended periods of anoxia (Jensen 1984). Cervonema and Sabatieria were also noted as indicator species in continental shelf sediments impacted by the oxygen minimum zone off the coast of Chile (Neira et al. 2013).
Spatial patterns in nematode communities
Genus composition of nematode communities was relatively heterogeneous among sites within the NEC, despite the fact that all sediment samples were collected from a relatively localized area and at similar depths (< 50 m). Similar variation has been reported for macrofaunal communities in the same study area (Blanchard and Feder 2014), and attributed to complex interactions between topographic features and hydrography that cause spatial variation in grain size and organic matter deposition (Blanchard et al. 2013a; Weingartner et al. 2013). Similarly, fine sediment was the only environmental parameter explaining a significant proportion of the variance in nematode community structure in our study. Sabatieria was abundant at the muddy sites with higher TOC content and lower C:N, whereas Daptonema was more abundant at sandy sites with lower TOC and higher C:N. The sandiest nearshore sites were characterized by relatively higher proportions of scavenging taxa (feeding group 2B) including Oxyonchus and Viscosia. These larger-bodied taxa are typically more prominent in shallow coastal habitats, which has been attributed to their larger size and ability to adhere to sediment grains and/or hard substrate (reviewed by Moens et al. 2013). Key taxa at the sandier sites also exhibited the slender body morphology, but differed in terms of tail shape. This is somewhat surprising given that tail shape tends to be related to sediment grain size (e.g., Schratzberger et al. 2007), which was identified as the primary environmental factor driving spatial patterns in the NEC. On the other hand, tail shape also affects nematode behavior, including mobility. In predatory nematodes, different tail shapes may reflect feeding strategies, with long-tailed nematodes acting as lurking predators (i.e., hemisessile lifestyle) because rapid movement is hindered by the long tail, and short-tailed nematodes acting as vagile predators (i.e., mobile lifestyle) that actively search for prey (Riemann 1974; Bussau 1995; Riemann et al. 2003).
Nematode communities at COP in the western BEAU did not significantly differ from those in the NEC, but they were different from other areas further east in the BEAU. This similarity in community structure between NEC and COP may reflect northward transport of organisms from the Chukchi Sea into the BEAU and Arctic basin via Barrow Canyon, enhanced by the formation of eddies along the shelf-break in the western BEAU (Winsor and Chapman 2004; Pickart et al. 2005). Benthic biomass and sediment chl-a are also similarly high in the NEC and COP areas, potentially resulting from advective transport of organic matter from the productive Chukchi Sea shelf into the western BEAU (Dunton et al. 2005). Both areas are also characterized by muddy sediment. Interestingly, NEC and COP also displayed the highest percentage of omnivores/predatory nematodes (feeding group 2B, > 18%). According to (Soetaert and Heip 1995), a high-quality food supply can support increased trophic diversity in deep-sea communities. Thus, the high proportion of predatory nematodes in these areas could result from high inputs of organic matter indicated by elevated sediment chl-a concentration. The extremely high dominance of a single opportunistic taxon (Sabatieria) at COP is also consistent with the classic diversity patterns observed at organically enriched sites (Pearson and Rosenberg 1978). Moreover, COP had the highest pigment concentrations measured in any of the BEAU areas, which could lead to shallow anoxia in sediments favoring a genus like Sabatieria with tolerance for low-oxygen conditions.
Nematode community structure exhibited turnover along the longitudinal gradient sampled in the BEAU, but depth-related trends also varied among sampled areas. Nematode communities in COP showed no distinct trends related to depth, but differences between shelf and upper slope sites increased moving east along the BEAU shelf. In particular, communities deep in the Mackenzie plume (MAP) area (750 m) were distinct from shallower MAP sites and more similar to other deep sites further east near Banks Island (BNK). While other Arctic studies have suggested a latitudinal effect on nematode community structure (Vanreusel et al. 2000; Renaud et al. 2006), longitudinal gradients have been noted in the Mediterranean Sea and Gulf of Mexico in response to availability of organic matter (Danovaro et al. 2008; Sharma et al. 2012; Pape et al. 2013).
A biogeographic boundary at ~ 200 m depth related to vertical layering of major water masses occurs in zooplankton and in macro- and megafaunal benthos in the BEAU (e.g., Conlan et al. 2008; Ravelo et al. 2015; Smoot and Hopcroft 2017b). Organic matter quality at the seafloor is directly influenced by these water-mass boundaries, because sinking organic matter is entrained near the vertical transition from Pacific Halocline to Atlantic water at ~ 200 m depth (McLaughlin et al. 1996; Forest et al. 2007), and grazed by large aggregations of zooplankton (Smoot and Hopcroft 2017b) which impact the amount and quality of organic matter that reaches the seafloor (Stasko et al. 2018a). In our study, nematode communities at 350 m in the MAP-M area were similar to those in shallower water, potentially suggesting a deeper transition in community structure than reported for other groups. The deeper boundary may reflect a different response in meiofaunal nematodes to quality and/or quantity of organic matter relative to that of macrofauna (Campanyà-Llovet et al. 2017). In addition, riverine input of terrestrial organic matter also increases from west to east (Dunton et al. 2006; Divine et al. 2015; Bell et al. 2016), such that shallower areas of the Mackenzie shelf are more heavily influenced by terrestrial inputs from the Mackenzie River (Goñi et al. 2013; Bell et al. 2016). This large input of terrestrial-derived organic matter has been shown to dampen the change in organic matter quality that typically occurs with depth due to microbial processing of marine-derived particles through the water column (Stasko et al. 2018b).
The highest proportions of epistratum feeders (group 2A) were found at MAP-D and at BNK-S and BNK-M, where a more limited, refractory food source was also found. Epistratum feeding behavior can include piercing cells to suck out contents, which may allow feeding on dead cells deposited as phytodetritus, or scraping microbes off solid surfaces or mucus threads (Moens and Vincx 1997; Moens et al. 2013). This feeding strategy has been reported elsewhere in the polar deep sea where similarly low levels of refractory food may be expected (Vanhove et al. 1999), and may indicate a greater role of bacteria in nematode diets (Iken et al. 2001; Ingels et al. 2010). Bacterivorous deposit-feeding taxa also occur in greater proportions in the deep Arctic basin, and in slope areas where ice cover reduces input of phytodetritus (Vanaverbeke et al. 1997; Vanreusel et al. 2000). Low organic input may also enhance competition within nematode communities, or with macrofauna, leading to the elevated diversity observed in these areas. Seven of the eleven most abundant genera in MAP-D had the slender body morphology and conical tail shape, although the association of these morphologies with particular habitat types remains unclear.
A distinct nematode assemblage was evident in the Banks Island (BNK) area in the eastern BEAU, including the highest values of all univariate community descriptors. Although nematode communities were variable within BNK, shallower sites were still more similar to deep (> 750 m) BNK sites than to shelf and upper slope sites to the west. This overlap between BNK and the deeper slope sites may reflect lower inputs of organic matter and/or a more refractory carbon source in the BNK area, comparable to a food-poor deep-sea setting. Stable carbon isotope values were typical of ice algal input, particularly at the shallower shelf sites of BNK, and sediment pigment concentrations were much lower overall than in other areas. Stable isotope studies in the adjacent Amundsen Gulf also suggest a lower quality organic matter reaching the seafloor, and evidence of ice algal consumption associated with the marginal ice zone (Stasko et al. 2018a).
Stout body types were also found almost exclusively in BNK, as exemplified by Desmoscolex which occurred mainly in this area. Taxa with this body morphology are typically found in well-oxygenated sediments (Schratzberger et al. 2007; Grzelak et al. 2016) because the capacity of oxygen uptake is a function of the surface area-to-volume ratio (Braeckman et al. 2013). Sabatieria, often associated with disturbed, low-oxygen sediments as described above, was notably low in abundance in BNK, further suggesting the environment in this area differs from other locations. Greater width-to-length ratio in stout body morphologies is associated with a high-quality food source (Tita et al. 1999). The relatively high abundance of this morphology at BNK would thus seem inconsistent with the low chl-a and phaeopigment concentrations detected; however, TOC and C:N ratios were comparable to other areas suggesting comparable food availability.
Conclusions
Nematode community variations in the NEC and BEAU reflect broad spatial and bathymetric gradients in quantity and quality of organic matter, whereas grain size may play a greater role within particular geographical areas. The heterogeneity in community structure within the relatively localized sampling area of similar depth across the NEC suggests small-scale patchiness, perhaps related to complex hydrography and resuspension events as observed in the Hanna Shoal region of the Chukchi Sea (e.g., Blanchard and Feder 2014). The wide distribution of Sabatieria across the study area with declining abundance from west to east mirrors regional patterns in particulate patterns in organic matter, suggesting this taxon may be a useful indicator of the trophic conditions for benthos and/or prevalence of disturbed sediments. Moreover, the combination of functional traits exhibited by Sabatieria and other dominant taxa suggests that the spatial extent of a shallow anoxic layer may play a role in governing community structure and diversity patterns.
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723. https://doi.org/10.1109/TAC.1974.1100705
Anderson MJ (2017) Permutational multivariate analysis of variance (PERMANOVA). In: Balakrishnan N, Colton T, Everitt B, Piegorsch W, Ruggeri F, Teugels JL (eds) Wiley StatsRef: Statistics Reference Online. Wiley. https://doi.org/10.1002/9781118445112.stat07841
Anderson M, Gorley R, Clarke K (2008) PERMANOVA+ for PRIMER. Guide to software and statistical methods, Primer-E, Plymouth
Arar EJ, Collins GB (1997) In vitro determination of chlorophyll a and pheophytin a in marine and freshwater algae by fluorescence. United States Environmental Protection Agency, Office of Research and Development, National Exposure Research Laboratory, Cincinnati
Bell LE, Bluhm BA, Iken K (2016) Influence of terrestrial organic matter in marine food webs of the Beaufort Sea shelf and slope. Mar Ecol Prog Ser 550:1–24
Bessière A, Nozais C, Brugel S, Demers S, Desrosiers G (2007) Metazoan meiofauna dynamics and pelagic–benthic coupling in the Southeastern Beaufort Sea, Arctic Ocean. Polar Biol 30:1123–1135. https://doi.org/10.1007/s00300-007-0270-6
Blanchard AL, Feder HM (2014) Interactions of habitat complexity and environmental characteristics with macrobenthic community structure at multiple spatial scales in the northeastern Chukchi Sea. Deep-Sea Res II 102:132–143
Blanchard AL, Knowlton A (2013) Chukchi Sea Environmental Studies Program 2008–2012: benthic ecology of the Northeastern Chukchi Sea. https://chukchiscience.com/science/benthic-ecology
Blanchard AL, Parris CL, Knowlton AL, Wade NR (2013a) Benthic ecology of the northeastern Chukchi Sea. Part I. Environmental characteristics and macrofaunal community structure, 2008–2010. Cont Shelf Res 67:52–66. https://doi.org/10.1016/j.csr.2013.04.021
Blanchard AL, Parris CL, Knowlton AL, Wade NR (2013b) Benthic ecology of the northeastern Chukchi Sea. Part II. Spatial variation of megafaunal community structure, 2009–2010. Cont Shelf Res 67:67–76. https://doi.org/10.1016/j.csr.2013.04.021
Blanchard AL, Day RH, Gall AE, Aerts LAM, Delarue J, Dobbins EL, Hopcroft RR, Questel JM, Weingartner TJ, Wisdom SS (2017) Ecosystem variability in the offshore northeastern Chukchi Sea. Prog Oceanogr 159:130–153. https://doi.org/10.1016/j.pocean.2017.08.008
Bluhm BA, Iken K, Hardy SM, Sirenko BI, Holladay BA (2009) Community structure of epibenthic megafauna in the Chukchi Sea. Aquatic Biol 7:269–293
Bongers T (1990) The maturity index: an ecological measure of environmental disturbance based on nematode species composition. Oecologia 83:14–19. https://doi.org/10.1007/BF00324627
Bongers T, Alkemade R, Yeates GW (1991) Interpretation of disturbance-induced maturity decrease in marine nematode assemblages by means of the Maturity Index. Mar Ecol Prog Ser 76:135–142
Bongers T, De Goede R, Korthals G, Yeates G (1995) Proposed changes of cp classification for nematodes. Russ J Nematol 3:61–62
Braeckman U, Vanaverbeke J, Vincx M, van Oevelen D, Soetaert K (2013) Meiofauna metabolism in suboxic sediments: currently overestimated. PLoS ONE 8:e59289. https://doi.org/10.1371/journal.pone.0059289
Burgess R (2001) An improved protocol for separating meiofauna from sediments using colloidal silica sols. Mar Ecol Prog Ser 214:161–165
Bussau C (1995) New deep-sea Nematoda (Enoplida, Thoracostomopsidae, Oncholaimidae, Enchelidiidae) from a manganese nodule area of the eastern South Pacific. Zool Scr 24:1–12. https://doi.org/10.1111/j.1463-6409.1995.tb00470.x
Campanyà-Llovet N, Snelgrove PVR, Parrish CC (2017) Rethinking the importance of food quality in marine benthic food webs. Prog Oceanogr 156:240–251. https://doi.org/10.1016/j.pocean.2017.07.006
Clarke K, Gorley R (2015) PRIMER v7: User Manual/Tutorial. PRIMER-E, Plymouth, UK
Conlan K, Aitken A, Hendrycks E, McClelland C, Melling H (2008) Distribution patterns of Canadian Beaufort Shelf macrobenthos. J Mar Syst 74:864–886
Creer S, Fonseca VG, Porazinska DL, Giblin-Davis RM, Sung W, Power DM, Packer M, Carvalho GR, Blaxter ML, Lambshead PJD, Thomas WK (2010) Ultrasequencing of the meiofaunal biosphere: practice, pitfalls and promises. Mol Ecol 19:4–20
Danovaro R, Gambi C, Lampadariou N, Tselepides A (2008) Deep-sea nematode biodiversity in the Mediterranean basin: testing for longitudinal, bathymetric and energetic gradients. Ecography 31:231–244. https://doi.org/10.1111/j.0906-7590.2008.5484.x
Day RH, Weingartner TJ, Hopcroft RR, Aerts LAM, Blanchard AL, Gall AE, Gallaway BJ, Hannay DE, Holladay BA, Mathis JT, Norcross BL, Questel JM, Wisdom SS (2013) The offshore northeastern Chukchi Sea, Alaska: a complex high-latitude ecosystem. Cont Shelf Res 67:147–165. https://doi.org/10.1016/j.csr.2013.02.002
De Groote A, Hauquier F, Vanreusel A, Derycke S (2017) Population genetic structure in Sabatieria (Nematoda) reveals intermediary gene flow and admixture between distant cold seeps from the Mediterranean Sea. BMC Evol Biol 17:154. https://doi.org/10.1186/s12862-017-1003-2
De Ley P, Blaxter M (2002) Systematic position and phylogeny. In: Lee DL (ed) The biology of nematodes. Taylor & Francis, New York, pp 1–30
Divine LM, Iken K, Bluhm BA (2015) Regional benthic food web structure on the Alaska Beaufort Sea shelf. Mar Ecol Prog Ser 531:15–32
Dunton KH, Goodall JL, Schonberg SV, Grebmeier JM, Maidment DR (2005) Multi-decadal synthesis of benthic-pelagic coupling in the western Arctic: role of cross-shelf advective processes. Deep-Sea Res II 52:3462–3477
Dunton KH, Weingartner T, Carmack EC (2006) The nearshore western Beaufort Sea ecosystem: circulation and importance of terrestrial carbon in arctic coastal food webs. Prog Oceanogr 71:362–378. https://doi.org/10.1016/j.pocean.2006.09.011
Dunton KH, Grebmeier JM, Trefry JH (2014) The benthic ecosystem of the northeastern Chukchi Sea: an overview of its unique biogeochemical and biological characteristics. Deep-Sea Res II 102:1–8. https://doi.org/10.1016/j.dsr2.2014.01.001
Eert J, Meisterhans G, Michel C, Niemi A, Reist J, Williams W (2015) Physical, chemical and biological oceanographic data from the Beaufort Regional Environmental Assessment: Marine Fishes Project, August–September 2012. Canadian Data Report of Hydrogrography and Ocean Sciences 197 Fisheries and Oceans Canada, Ottawa
Ershova EA, Hopcroft RR, Kosobokova KN (2015) Inter-annual variability of summer mesozooplankton communities of the western Chukchi Sea: 2004–2012. Polar Biol 38:1461–1481. https://doi.org/10.1007/s00300-015-1709-9
Escobar-Briones EG, Díaz C, Legendre P (2008) Meiofaunal community structure of the deep-sea Gulf of Mexico: Variability due to the sorting methods. Deep-Sea Res II 55:2627–2633. https://doi.org/10.1016/j.dsr2.2008.07.012
Fonseca G, Soltwedel T (2007) Deep-sea meiobenthic communities underneath the marginal ice zone off Eastern Greenland. Polar Biol 30:607–618. https://doi.org/10.1007/s00300-006-0220-8
Fonseca G, Soltwedel T (2009) Regional patterns of nematode assemblages in the Arctic deep seas. Polar Biol 32:1345–1357. https://doi.org/10.1007/s00300-009-0631-4
Forest A, Sampei M, Hattori H, Makabe R, Sasaki H, Fukuchi M, Wassmann P, Fortier L (2007) Particulate organic carbon fluxes on the slope of the Mackenzie Shelf (Beaufort Sea): Physical and biological forcing of shelf-basin exchanges. J Mar Syst 68:39–54
Gallucci F, Fonseca G, Soltwedel T (2008) Effects of megafauna exclusion on nematode assemblages at a deep-sea site. Deep-Sea Res 55:332–349. https://doi.org/10.1016/j.dsr.2007.12.001
Gallucci F, Moens T, Fonseca G (2009) Small-scale spatial patterns of meiobenthos in the Arctic deep sea. Mar Biodivers 39:9–25. https://doi.org/10.1007/s12526-009-0003-x
Giere O (2009) Meiobenthology: the microscopic motile fauna of aquatic sediments. Springer, Berlin, p 527
Goñi MA, O’Connor AE, Kuzyk ZZ, Yunker MB, Gobeil C, Macdonald RW (2013) Distribution and sources of organic matter in surface marine sediments across the North American Arctic margin. J Geophys Res-Oceans 118:4017–4035. https://doi.org/10.1002/jgrc.20286
Grebmeier JM (2012) Shifting patterns of life in the Pacific Arctic and Sub-Arctic seas. Annu Rev Mar Sci 4:63–78. https://doi.org/10.1146/annurev-marine-120710-100926
Grebmeier JM, McRoy CP, Feder HM (1988) Pelagic-benthic coupling on the shelf of the northern Bering and Chukchi Seas. I. Food supply source and benthic biomass. Mar Ecol Prog Ser 48:57–67
Grebmeier JM, Bluhm BA, Cooper LW, Danielson SL, Arrigo KR, Blanchard AL, Clarke JT, Day RH, Frey KE, Gradinger RR, Kędra M, Konar B, Kuletz KJ, Lee SH, Lovvorn JR, Norcross BL, Okkonen SR (2015a) Ecosystem characteristics and processes facilitating persistent macrobenthic biomass hotspots and associated benthivory in the Pacific Arctic. Prog Oceanogr 136:92–114. https://doi.org/10.1016/j.pocean.2015.05.006
Grebmeier JM, Bluhm BA, Cooper LW, Denisenko SG, Iken K, Kędra M, Serratos C (2015b) Time-series benthic community composition and biomass and associated environmental characteristics in the Chukchi Sea during the RUSALCA 2004–2012 Program. Oceanography 28:116–133
Grzelak K, Kotwicki L (2012) Meiofaunal distribution in Hornsund fjord, Spitsbergen. Polar Biol 35:269–280. https://doi.org/10.1007/s00300-011-1071-5
Grzelak K, Gluchowska M, Gregorczyk K, Winogradow A, Weslawski JM (2016) Nematode biomass and morphometric attributes as biological indicators of local environmental conditions in Arctic fjords. Ecol Indic 69:368–380. https://doi.org/10.1016/j.ecolind.2016.04.036
Guilini K, Bezerra T, Eisendle-Flöckner U, Deprez T, Fonseca G, Holovachov O, Leduc D, Miljutin D, Moens T, Sharma J (2017) NeMys: World database of free-living marine nematodes. http://www.nemysugentbe
Hajduk M (2015) Density and distribution of meiofauna in the northeastern Chukchi Sea. MS Thesis, University of Alaska Fairbanks
Heip C, Vincx M, Vranken G (1985) The ecology of marine nematodes. Oceanogr Mar Biol Annu Rev 23:399–489
Hooper D (1986) Handling, fixing, staining and mounting nematodes. In: Southey JF (ed) Laboratory methods for work with plant and soil nematodes. Her Majesty’s Stationery Office, London, pp 58–80
Hoste E, Vanhove S, Schewe I, Soltwedel T, Vanreusel A (2007) Spatial and temporal variations in deep-sea meiofauna assemblages in the Marginal Ice Zone of the Arctic Ocean. Deep-Sea Res 54:109–129. https://doi.org/10.1016/j.dsr.2006.09.007
Iken K, Brey T, Wand U, Voigt J, Junghans P (2001) Food web structure of the benthic community at the Porcupine Abyssal Plain (NE Atlantic): a stable isotope analysis. Prog Oceanogr 50:383–405
Iken K, Bluhm B, Dunton K (2010) Benthic food-web structure under differing water mass properties in the southern Chukchi Sea. Deep-Sea Res II 57:71–85
Ingels J, Van den Driessche P, De Mesel I, Vanhove S, Moens T, Vanreusel A (2010) Preferred use of bacteria over phytoplankton by deep-sea nematodes in polar regions. Mar Ecol Prog Ser 406:121–133
Jensen P (1984) Ecology of benthic and epiphytic nematodes in brackish waters. Hydrobiologia 108:201–217. https://doi.org/10.1007/BF00006329
Leduc D, Probert PK, Berkenbusch K, Nodder SD, Pilditch CA (2010) Abundance of small individuals influences the effectiveness of processing techniques for deep-sea nematodes. Deep-Sea Res 57:1363–1371. https://doi.org/10.1016/j.dsr.2010.07.002
Lin R, Huang D, Guo Y, Chang Y, Cao Y, Wang J (2014) Abundance and distribution of meiofauna in the Chukchi Sea. Acta Oceanol Sin 33:90–94. https://doi.org/10.1007/s13131-014-0493-7
Logerwell E, Rand K, Weingartner TJ (2011) Oceanographic characteristics of the habitat of benthic fish and invertebrates in the Beaufort Sea. Polar Biol 34:1783–1796
Lovvorn JR, North CA, Kolts JM, Grebmeier JM, Cooper LW, Cui X (2016) Projecting the effects of climate-driven changes in organic matter supply on benthic food webs in the northern Bering Sea. Mar Ecol Prog Ser 548:11–30
Majewski AR, Atchison S, MacPhee S, Eert J, Niemi A, Michel C, Reist JD (2017) Marine fish community structure and habitat associations on the Canadian Beaufort shelf and slope. Deep-Sea Res 121:169–182. https://doi.org/10.1016/j.dsr.2017.01.009
McLachlan A, Brown A (2006) The ecology of sandy shores. Elsevier, USA, p 373
McLaughlin FA, Carmack EC, Macdonald RW, Bishop JKB (1996) Physical and geochemical properties across the Atlantic/Pacific water mass front in the southern Canadian Basin. J Geophys Res-Oceans 101:1183–1197. https://doi.org/10.1029/95jc02634
Miljutin DM, Miljutina MA, Tchesunov AV, Mokievsky VO (2014) Nematode assemblages from the Kandalaksha Depression (White Sea, 251–288 m water depth). Helgoland Mar Res 68:99–111. https://doi.org/10.1007/s10152-013-0371-2
Mincks SL, Smith CR, DeMaster DJ (2005) Persistence of labile organic matter and microbial biomass in Antarctic shelf sediments: evidence of a sediment “food bank.” Mar Ecol Prog Ser 300:3–19
Moens T, Vincx M (1997) Observations on the feeding ecology of estuarine nematodes. J Mar Biol Ass UK 77:211–227. https://doi.org/10.1017/S0025315400033889
Moens T, Braeckman U, Derycke S, Fonseca G, Gallucci F, Gingold R, Guilini K, Ingels J, Leduc D, Vanaverbeke J, Colen CV, Vanreusel A, Vincx M (2013) Ecology of free-living marine nematodes. In: Schmidt-Rhaesa A (ed) Handbook of zoology, vol 2. vol 2. De Gruyter, Berlin, pp 109–152
Neira C, King I, Mendoza G, Sellanes J, De Ley P, Levin LA (2013) Nematode community structure along a central Chile margin transect influenced by the oxygen minimum zone. Deep-Sea Res 78:1–15. https://doi.org/10.1016/j.dsr.2013.04.002
Nelson RJ, Ashjian CJ, Bluhm BA, Conlan KE, Gradinger RR, Grebmeier JM, Hill VJ, Hopcroft RR, Hunt BP, Joo HM, Kirchman DL, Kosobokova KN, Lee SH, Li WK, Lovejoy C, Poulin M, Sherr E, Young KV (2014) Biodiversity and biogeography of the lower trophic taxa of the Pacific Arctic Region: sensitivities to climate change. In: Grebmeier JM, Maslowski W (eds) The Pacific Arctic Region. Springer, pp 269–336. https://doi.org/10.1007/978-94-017-8863-2_10
Nephin J, Juniper SK, Archambault P (2014) Diversity, abundance and community structure of benthic macro- and megafauna on the Beaufort Shelf and Slope. PLoS ONE 9:e101556. https://doi.org/10.1371/journal.pone.0101556
Niemi A, Michel C, Dempsey M, Eert J, Reist J, Williams W (2015) Physical, chemical and biological oceanographic data from the Beaufort Regional Environmental Assessment: Marine Fishes Project, August-September (2013) Canadian Data Report of Hydrogrography and Ocean Sciences 198. Fisheries and Oceans Canada, Ottawa
Pape E, Bezerra T, Jones D, Vanreusel A (2013) Unravelling the environmental drivers of deep-sea nematode biodiversity and its relation with carbon mineralisation along a longitudinal primary productivity gradient. Biogeosciences 10:3127–3143. https://doi.org/10.5194/bg-10-3127-2013
Pearson TH, Rosenberg R (1978) Macrobenthic succession in relation to organic enrichment and pollution in the marine environment. Oceanogr Mar Biol Annu Rev 16:229–311
Pereira TJ, De Santiago A, Schuelke T, Hardy SM, Bik HM (2020) The impact of intragenomic rRNA variation on metabarcoding-derived diversity estimates: a case study from marine nematodes. Environmental DNA 2:519–534. https://doi.org/10.1002/edn3.77
Pickart RS, Weingartner TJ, Pratt LJ, Zimmermann S, Torres DJ (2005) Flow of winter-transformed Pacific water into the Western Arctic. Deep-Sea Res II 52:3175–3198
Piepenburg D, Blackburn TH, von Dorrien CF, Gutt J, Hall POJ, Hulth S, Kendall MA, Opalinski KW, Rachor E, Schmid MK (1995) Partitioning of benthic community respiration in the Arctic (northwest Barents Sea). Mar Ecol Prog Ser 118:119–213
Piepenburg D, Ambrose WG Jr, Brandt A, Renaud PE, Ahrens MJ, Jensen P (1997) Benthic community patterns reflect water column processes in the Northeast Water polynya (Greenland). J Mar Syst 10:467–482
Platt HM, Warwick RM (1983) Freeliving marine nematodes. Part 1: British enoplids. Pictorial key to world genera and notes for the identification of British species. Cambridge University Press, for the Linnean Society of London and the estuarine and brackish-water sciences association, Cambridge,
Platt HM, Shaw KM, Lambshead PJD (1984) Nematode species abundance patterns and their use in the detection of environmental perturbations. Hydrobiologia 118:59–66. https://doi.org/10.1007/BF00031788
Portnova DA, Garlitska LA, Udalov AA, Kondar DV (2017) Meiobenthos and nematode community in Yenisei Bay and adjacent parts of the Kara Sea shelf. Oceanology 57:130–143. https://doi.org/10.1134/S0001437017010155
Pusceddu A, Gambi C, Corinaldesi C, Scopa M, Danovaro R (2014) Relationships between meiofaunal biodiversity and prokaryotic heterotrophic production in different tropical habitats and oceanic regions. PLoS ONE 9:e91056. https://doi.org/10.1371/journal.pone.0091056
Rand KM, Logerwell EA (2011) The first demersal trawl survey of benthic fish and invertebrates in the Beaufort Sea since the late 1970s. Polar Biol 34:475–488
Ravelo AM, Konar B, Bluhm BA (2015) Spatial variability of epibenthic communities on the Alaska Beaufort Shelf. Polar Biol 38:1783–1804. https://doi.org/10.1007/s00300-015-1741-9
Ravelo AM, Bluhm BA, Foster N, Iken K (2020) Biogeography of epibenthic assemblages in the central Beaufort Sea. Mar Biodivers 50:8. https://doi.org/10.1007/s12526-019-01036-9
Renaud PE, Ambrose WG, Vanreusel A, Clough LM (2006) Nematode and macrofaunal diversity in central Arctic Ocean benthos. J Exp Mar Biol Ecol 330:297–306. https://doi.org/10.1016/j.jembe.2005.12.035
Rex MA, Etter RJ, Morris JS, Crouse J, McClain CR, Johnson NA, Stuart CT, Deming JW, Thies R, Avery R (2006) Global bathymetric patterns of standing stock and body size in the deep-sea benthos. Mar Ecol Prog Ser 317:1–8
Riemann F (1974) On hemisessile nematodes with flagelliform tails living in marine soft bottoms and on micro-tubes found in deep sea sediments. In: Mikrofauna des Meeresbodens, vol 40. Steiner, pp 1–15
Riemann F, Thiermann F, Bock L (2003) Leptonemella species (Desmodoridae, Stilbonematinae), benthic marine nematodes with ectosymbiotic bacteria, from littoral sand of the North Sea island of Sylt: taxonomy and ecological aspects. Helgoland Mar Res 57:118–131. https://doi.org/10.1007/s10152-003-0149-z
Roy V, Iken K, Archambault P (2014) Environmental drivers of the Canadian Arctic megabenthic communities. PLoS ONE 9:e100900. https://doi.org/10.1371%2Fjournal.pone.0100900
Roy V, Iken K, Gosselin M, Tremblay J-É, Bélanger S, Archambault P (2015) Benthic faunal assimilation pathways and depth-related changes in food-web structure across the Canadian Arctic. Deep-Sea Res 102:55–71. https://doi.org/10.1016/j.dsr.2015.04.009
Schmidt-Rhaesa A (ed) (2014) Handbook of zoology: gastrotricha, cycloneuralia and gnathifera, vol. 2: Nematoda. Walter de Gruyter GmBH, Berlin/Boston, p 759
Schratzberger M, Ingels J (2018) Meiofauna matters: the roles of meiofauna in benthic ecosystems. J Exp Mar Biol Ecol 502:12–25. https://doi.org/10.1016/j.jembe.2017.01.007
Schratzberger M, Gee JM, Rees HL, Boyd SE, Wall CM (2000) The structure and taxonomic composition of sublittoral meiofauna assemblages as an indicator of the status of marine environments. J Mar Biol Ass U K 80:969–980
Schratzberger M, Warr K, Rogers SI (2007) Functional diversity of nematode communities in the southwestern North Sea. Mar Environ Res 63:368–389. https://doi.org/10.1016/j.marenvres.2006.10.006
Schratzberger M, Lampadariou N, Somerfield PJ, Vandepitte L, Vanden Berghe E (2009) The impact of seabed disturbance on nematode communities: linking field and laboratory observations. Mar Biol 156:709–724. https://doi.org/10.1007/s00227-008-1122-9
Seinhorst J (1959) A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica 4:67–69
Semprucci F, Cesaroni L, Guidi L, Balsamo M (2018) Do the morphological and functional traits of free-living marine nematodes mirror taxonomical diversity? Mar Environ Res 135:114–122. https://doi.org/10.1016/j.marenvres.2018.02.001
Sharma J, Baguley JG, Montagna PA, Rowe GT (2012) Assessment of longitudinal gradients in nematode communities in the deep northern Gulf of Mexico and concordance with benthic taxa. Int J Oceanogr. https://doi.org/10.1155/2012/903018
Smoot CA, Hopcroft RR (2017a) Cross-shelf gradients of epipelagic zooplankton communities of the Beaufort Sea and the influence of localized hydrographic features. J Plankton Res 39:65–78. https://doi.org/10.1093/plankt/fbw080
Smoot CA, Hopcroft RR (2017b) Depth-stratified community structure of Beaufort Sea slope zooplankton and its relations to water masses. J Plankton Res 39:79–91. https://doi.org/10.1093/plankt/fbw087
Soetaert K, Heip C (1990) Sample-size dependence of diversity indices and the determination of sufficient sample size in a high-diversity deep-sea environment. Mar Ecol Prog Ser 59:305–307
Soetaert K, Heip C (1995) Nematode assemblages of deep-sea and shelf break sites in the North Atlantic and Mediterranean Sea. Mar Ecol Prog Ser 125:171–183
Soetaert K, Vincx M, Heip C (1995) Nematode community structure along a Mediterranean shelf-slope gradient. Mar Ecol 16:189–206. https://doi.org/10.1111/j.1439-0485.1995.tb00405.x
Soetaert K, Muthumbi A, Heip C (2002) Size and shape of ocean margin nematodes: morphological diversity and depth-related patterns. Mar Ecol Prog Ser 242:179–193
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research. Freeman and Company, New York, W.H, p 887
Somerfield PJ, Clarke KR (1995) Taxonomic levels, in marine community studies, revisited. Mar Ecol Prog Ser 127:113–119
Somerfield PJ, Clarke KR (1997) A comparison of some methods commonly used for the collection of sublittoral sediments and their associated fauna. Mar Environ Res 43:145–156. https://doi.org/10.1016/0141-1136(96)00083-9
Somerfield PJ, Warwick RM (2013) Meiofauna techniques. In: Eleftheriou A (ed) Methods for the study of marine benthos. Wiley, pp 253–284
Somerfield PJ, Cochrane SJ, Dahle S, Pearson TH (2006) Free-living nematodes and macrobenthos in a high-latitude glacial fjord. J Exp Mar Biol Ecol 330:284–296. https://doi.org/10.1016/j.jembe.2005.12.034
Stasko AD, Bluhm BA, Michel C, Archambault P, Majewski A, Reist JD, Swanson H, Power M (2018a) Benthic-pelagic trophic coupling in an Arctic marine food web along vertical water mass and organic matter gradients. Mar Ecol Prog Ser 594:1–19
Stasko AD, Bluhm BA, Reist JD, Swanson H, Power M (2018b) Relationships between depth and δ15N of Arctic benthos vary among regions and trophic functional groups. Deep-Sea Res 135:56–64. https://doi.org/10.1016/j.dsr.2018.03.010
Thistle D, Lambshead PJD, Sherman KM (1995) Nematode tail-shape groups respond to environmental differences in the deep sea. Vie et milieu 45:107–115
Tita G, Vincx M, Desrosiers G (1999) Size spectra, body width and morphotypes of intertidal nematodes: an ecological interpretation. J Mar Biol Ass U K 79:1007–1015. https://doi.org/10.1017/S0025315499001241
Urban-Malinga B, Hedtkamp SIC, van Beusekom JEE, Wiktor J, Węsławski JM (2006) Comparison of nematode communities in Baltic and North Sea sublittoral, permeable sands—diversity and environmental control. Est Coast Shelf Sci 70:224–238. https://doi.org/10.1016/j.ecss.2006.06.020
Vanaverbeke J, Arbizu PM, Dahms H-U, Schminke HK (1997) The metazoan meiobenthos along a depth gradient in the Arctic Laptev Sea with special attention to nematode communities. Polar Biol 18:391–401. https://doi.org/10.1007/s003000050205
Vanaverbeke J, Steyaert M, Soetaert K, Rousseau V, Van Gansbeke D, Parent J-Y, Vincx M (2004) Changes in structural and functional diversity of nematode communities during a spring phytoplankton bloom in the southern North Sea. J Sea Res 52:281–292. https://doi.org/10.1016/j.seares.2004.02.004
Vanhove S, Arntz W, Vincx M (1999) Comparative study of the nematode communities on the southeastern Weddell Sea shelf and slope (Antarctica). Mar Ecol Prog Ser 181:237–256
Vanreusel A, Vincx M, Van Gansbeke D, Gijselinck W (1992) Structural analysis of the meiobenthos communities of the shelf break area in two stations of the Gulf of Biscay (NE Atlantic). Belg J Zool 122:185–202
Vanreusel A, Clough L, Jacobsen K, Ambrose W, Jutamas J, Ryheul V, Herman R, Vincx M (2000) Meiobenthos of the central Arctic Ocean with special emphasis on the nematode community structure. Deep-Sea Res 47:1855–1879. https://doi.org/10.1016/S0967-0637(00)00007-8
Vanreusel A, Fonseca G, Danovaro R, Silva MCd, Esteves AM, Ferrero T, Gad G, Galtsova V, Gambi C, Genevois VdF, Ingels J, Ingole B, Lampadariou N, Merckx B, Miljutin D, Miljutina M, Muthumbi A, Netto S, Portnova D, Radziejewska T, Raes M, Tchesunov A, Vanaverbeke J, Gaever SV, Venekey V, Bezerra TN, Flint H, Copley J, Pape E, Zeppilli D, Martinez PA, Galeron J (2010) The contribution of deep-sea macrohabitat heterogeneity to global nematode diversity. Mar Ecol 31:6–20
Walker AM, Leigh MB, Mincks SL (in press) Structure and composition of bacteria and archaea provide novel insights into biogeochemical processes in Arctic marine surface sediments from the Beaufort Sea shelf and slope. Front Microbiol
Wassmann P, Reigstad M (2011) Future Arctic Ocean seasonal ice zones and implications for pelagic-benthic coupling. Oceanography 24:220–231
Weingartner T, Aagaard K, Woodgate R, Danielson S, Sasaki Y, Cavalieri D (2005) Circulation on the north central Chukchi Sea shelf. Deep-Sea Res II 52:3150–3174
Weingartner T, Dobbins E, Danielson S, Winsor P, Potter R, Statscewich H (2013) Hydrographic variability over the northeastern Chukchi Sea shelf in summer-fall 2008–2010. Cont Shelf Res 67:5–22. https://doi.org/10.1016/j.csr.2013.03.012
Wei C-L, Rowe GT, Escobar-Briones E, Boetius A, Soltwedel T, Caley MJ, Soliman Y, Huettmann F, Qu F, Yu Z, Pitcher CR, Haedrich RL, Wicksten MK, Rex MA, Baguley JG, Sharma J, Danovaro R, MacDonald IR, Nunnally CC, Deming JW, Montagna P, Levesque M, Weslawski JM, Wlodarska-Kowalczuk M, Ingole BS, Bett BJ, Billett DSM, Yool A, Bluhm BA, Iken K, Narayanaswamy BE (2010) Global patterns and predictions of seafloor biomass using random forests. PLoS ONE 5:e15323
Wentworth CK (1922) A scale of grade and class terms for clastic sediments. J Geol 30:377–392
Whitehouse GA, Aydin K, Essington T, Hunt G Jr (2014) A trophic mass balance model of the eastern Chukchi Sea with comparisons to other high-latitude systems. Polar Biol 37:911–939. https://doi.org/10.1007/s00300-014-1490-1
Wieser W (1953) Die Beziehungen zwischen Mundhohlengestalt, Ernahrungsweise und Vorkommen bei freilebenden marinen Nematoden. Ark Zool 4:439–484
Winsor P, Chapman DC (2004) Pathways of Pacific water across the Chukchi Sea: a numerical model study. J Geophys Res C 109:C03002. https://doi.org/10.1029/2003JC001962
Zeppilli D, Sarrazin J, Leduc D, Arbizu P, Fontaneto D, Fontanier C, Gooday A, Kristensen R, Ivanenko V, Sørensen M, Vanreusel A, Thébault J, Mea M, Allio N, Andro T, Arvigo A, Castrec J, Danielo M, Foulon V, Fumeron R, Hermabessiere L, Hulot V, James T, Langonne-Augen R, Le Bot T, Long M, Mahabror D, Morel Q, Pantalos M, Pouplard E, Raimondeau L, Rio-Cabello A, Seite S, Traisnel G, Urvoy K, Van Der Stegen T, Weyand M, Fernandes D (2015) Is the meiofauna a good indicator for climate change and anthropogenic impacts? Mar Biodivers 45:505–535. https://doi.org/10.1007/s12526-015-0359-z
Acknowledgements
We gratefully acknowledge Alexis Walker and Marissa Hadjuk for sorting sediment samples to remove the nematodes examined here. We also thank scientists and crew who participated in the CSESP, USTB, and BREA field programs for collecting samples used here. We appreciate the valuable comments of three anonymous reviewers which greatly improved the manuscript. Funding for this work was provided by North Pacific Research Board (Project #1303).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mincks, S.L., Pereira, T.J., Sharma, J. et al. Composition of marine nematode communities across broad longitudinal and bathymetric gradients in the Northeast Chukchi and Beaufort Seas. Polar Biol 44, 85–103 (2021). https://doi.org/10.1007/s00300-020-02777-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-020-02777-1