Abstract
Pelagic–benthic coupling is relatively well studied in the marginal seas of the Arctic Ocean. Responses of meiofauna with regard to seasonal pulses of particulate organic matter are, however, rarely investigated. We examined the dynamics of metazoan meiofauna and assessed the strength of pelagic–benthic coupling in the Southeastern Beaufort Sea, during autumn 2003 and spring–summer 2004. Meiofauna abundance varied largely (range: 2.3 × 105 to 5 × 106 ind m−2), both spatially and temporally, and decreased with increasing depth (range: 24–549 m). Total meiofauna biomass exhibited similar temporal as well as spatial patterns as abundance and varied from 25 to 914 mg C m−2. Significant relationships between sediment photopigments and various representatives of meiofauna in summer and autumn likely indicate the use of sediment phytodetritus as food source for meiofauna. A carbon-based grazing model provided estimates of potential daily ingestion rates ranging from 32 to 723 mg C m−2. Estimated potential ingestion rates showed that meiofauna consumed from 11 to 477% of the sediment phytodetritus and that meiofauna were likely not food-restricted during spring and autumn. These results show that factors governing the distribution and abundance of metazoan meiofauna need to be better elucidated if we are to estimate the benthic carbon fluxes in marginal seas of the Arctic Ocean.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Meiofauna are an important component of benthic heterotrophic assemblages. They participate in the transfer of material and energy through the ecosystem and are an important link between primary producers and higher trophic levels in benthic systems (Giere 1993). Owing to their life cycles and high turnover rates, meiofauna are also particularly susceptible to respond rapidly to changes in food availability (Danovaro 1996).
Although a large number of studies have examined the ecology and the role of meiofauna in the energy transfer in various marine ecosystems (see Giere 1993 and references therein), little is known about polar-regions in this regard (Arntz et al. 1994). This is partly due to the fact that polar research involves highly sophisticated logistics and financial support that, until recently, were not always accessible (see Piepenburg 2005 and refs therein). Also, traditional research on polar benthos has been more concerned with macro- than meiofauna (see Piepenburg 2005 and refs therein). There have been, however, some descriptive and/or process-oriented studies on Antarctic shallow and deep-water meiofauna (Herman and Dahms 1992; Fabiano and Danovaro 1999; Vanhove et al. 2000; de Skowronski and Corbisier 2002; Gutzmann et al. 2004). Regarding Arctic waters, comparable published studies have been undertaken in the central Arctic Ocean (Soltwedel and Schewe 1998; Schewe and Soltwedel 1999; Schewe 2001), the Laptev Sea (Sheremetevsky 1977; Vanaverbeke et al. 1997), the northern Barents Sea and the Nansen Basin (Pfannkuche and Thiel 1987), the Barents Sea (Soltwedel et al. 2005), the Svalbard archipelago (Węsławski et al. 1997; Kotwicki et al. 2004; Urban-Malinga et al. 2005), and the Fram Strait (Schewe and Soltwedel 2003; Soltwedel et al. 2003).
Studies on the temporal dynamics of meiofauna in polar-regions are scarce. In an 18-month study on Antarctic shallow-water meiofauna, Vanhove et al. (2000) reported a relationship between the structure of meiofauna and the availability of food. In a synoptic study on various benthic components of the Northeast Water polynya during the summers of 1992 and 1993, Piepenburg et al. (1997) showed that nematode densities were correlated with water column and sediment pigment concentrations, thereby highlighting pelagic–benthic coupling in high latitude seas. The strength of pelagic–benthic coupling is thought to be more pronounced in the marginal seas of the Arctic Ocean since it is regulated by water-column factors which are constrained by a pronounced seasonality in light irradiance and ice coverage. Although pelagic–benthic coupling is relatively well studied in these regions, responses of the meiofaunal component of benthic systems with regard to seasonal pulses of particulate organic matter (POM) are still rarely investigated.
The present study was part of the Canadian Arctic Shelf Exchange Study (CASES) aiming at investigating the relationship between sea-ice variability and carbon fluxes between the Mackenzie Shelf and Cape Bathurst Polynya and the Canada Basin. The specific objectives of this study were to describe the seasonal dynamics of shallow and deep-water metazoan meiofauna (excluding foraminiferans) in the Southeastern Beaufort Sea, and to evaluate their responses to changes in food availability. A carbon-based grazing model was finally used to estimate the potential grazing impact of metazoan meiofauna on phytodetritus.
Materials and methods
Study area and sampling
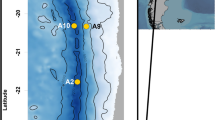
This study was conducted into the Southeastern Beaufort Sea, including the Mackenzie Shelf, Amundsen Gulf and Franklin Bay, from 1 October to 14 November 2003 and from 4 June to 10 August 2004 on board the icebreaker CGSS Amundsen. Phytoplankton, sediment photopigments and metazoan meiofauna samplings were carried out at 23 stations, located between 69° N and 71° N and between 122° W and 132° W (Fig. 1; Table 1). Stations 709 and 718 were repeatedly sampled during the season. Temperature data in near-bottom waters were obtained from a Seabird SBE911 + CTD. For the assessment of total phytoplankton chlorophyll a (chl a) biomass, water samples were collected with a rosette sampler (24 bottles of 12 l each) at selected depths of the water column (Brugel et al. 2005). For the determination of sediment photopigments (chlorophyll a and phaeopigment), meiofauna structure and composition, one to three USNEL-type box-cores hauls were performed at each station (Table 1). Sediments were composed of silt and clay. Sediment sub-cores were collected (generally 1 from each box-core and in areas with intact surface layers) for each parameter analysis using a 60-cm3 disposable syringe (i.d. 2.8 cm) with a cut off anterior end. On 16 occasions, however, only one box-core haul was performed per sampling station. Sediment sub-cores were therefore collected in this box-core for each parameter analysis (Table 1).
Laboratory analyses
Water samples for the determination of phytoplankton biomass were filtered (250 ml or more) onto Whatman GF/F filters. Chl a and pheopigment concentrations were calculated using equations of Holm-Hansen et al. (1965) after measuring fluorescence with a 10-AU Turner Designs fluorometer, following 24 h extraction in 90% acetone at 5°C in the dark without grinding (Parsons et al. 1984).
Sediment photopigments (chl a and pheopigments) were determined according to the slightly modified protocol of Riaux-Gobin and Klein (1993). The top first centimetre of each sediment sub-core was cut off and placed in a 50 ml polyethylene bottle with 30 ml of 90% acetone for a 48-h extraction of pigments at 5°C in the dark (Nozais et al. 2001). Sediment chl a and pheopigments concentrations were then measured using a 10-AU Turner Designs fluorometer and calculated using the adapted equations of Lorenzen (1966) (see Riaux-Gobin and Klein 1993 for further details). The sum of sediment chl a and pheopigments was referred as chloroplastic pigment equivalents (CPE) (Thiel 1978).
For the meiofaunal analyses, the top 1-cm layer of each sediment sub-core was preserved in a 1:500 (v/v) buffered freshwater formalin (5%) solution. Each sample was then passed through a 500 and a 63 μm sieve. Meiofauna were sorted from the 63 μm fraction, after staining with Rose Bengal, counted under a Wild M8 stereomicroscope, and identified to the lowest possible taxonomic level.
Meiofaunal biomass was estimated from size measurements of different animals. The length and width of a maximum of 30 organisms from each sediment sub-core were measured using a stereomicroscope MZ 16 Leica equipped with a camera Q imaging mono 10 bits and a graphic tablet. These measurements were used for further conversion into biomass, using the specific conversion factors for each taxonomic group.
Nematodes
The wet weight (WW in μg) was estimated according to Wieser (1960) and Warwick and Price (1979), using the empirical equation:
where 530 is a dimensionless conversion factor specific to nematodes, L is the total individual length (mm), W is the maximum width (mm) of an individual, and 1.13 is its specific gravity (μg nl−1) (Wieser 1960). Organic carbon content (Corg) was calculated assuming that Corg is equal to 12.4% of wet weight (Jensen 1984).
Copepods
The volume (V in nl) was calculated according to Warwick and Gee (1984), using the equation:
where L is the total length (mm), W is the width (mm), and C is a specific dimensionless conversion factor that depends on body shape, semi-cylindrical, (C = 560) semi-cylindrical compressed (C = 630), or pyriform (C = 400). The wet weight (WW in μg) of copepods was estimated according to Riemann et al. (1990):
where 0.9 is a dimensionless conversion factor, V is the volume of an individual copepod (nl), and 1.13 is its specific gravity (μg nl−1). Copepod dry weight was calculated as 22.5% of wet weight, following Gradinger et al. (1999). Organic carbon content (Corg) was estimated as 40% of dry weight (Feller and Warwick 1988).
Crustacean nauplii
The wet weight (WW in μg) was estimated according to Gradinger et al. (1999), using the equation:
where 360 is a conversion factor (μg mm−3), L is the length (mm) and W is the width (mm). Dry weight and organic carbon content were calculated as described above for copepods.
Turbellarians, kinorhynchs, and polychaetes: The wet weight (WW in μg) of all these groups was calculated according to Warwick and Price (1979) and Wieser (1960), using the equation:
where C is a dimensionless conversion factor (polychaetes: C = 530; kinorhynchs: C = 530; turbellarians: C = 550), L is the length (mm), W the width (mm), and 1.13 is the specific gravity (μg nl−1). Dry weight and organic carbon content were calculated as described above for copepods.
For the calculation of the maximum potential carbon ingestion rates of the meiofauna taxa, we used the allometric equation of Moloney and Field (1989) developed for planktonic organisms, and used also for sea-ice and benthic organisms (Vézina et al. 1997; Gradinger et al. 1999; Nozais et al. 2005):
where I max is the maximum potential ingestion rate (mg C m−2 d−1), 63 is a rate coefficient (in pg C0.25 d−1), M is the mass of a given individual (in pg C), B is the carbon biomass of the compartment (in mg C m−2), and T is the temperature at bottom waters measured at the time of sampling (in °C). All rates were calculated assuming a Q 10 value of 2 (Price and Warwick 1980). CPE concentration (in mg m−2) was expressed in chl a equivalents and was converted into autotrophic carbon (in mg C m−2) assuming a C:Chl a ratio of 47.6:1 (de Jonge 1980), and the percentage of CPE grazed daily by meiofauna was calculated as:
Data analysis
Abundance and biomass patterns of meiofauna, and vertically integrated phytoplankton concentrations in the Southeastern Beaufort Sea were presented using the Surfer software. The number of distance classes to be generated was calculated according to Sturge’s rule (Sherrer 1984). Spearman rank correlation analyses were used to infer relationships between variables. All statistical analyses were carried out using the SPSS 11 package (SPSS Inc., 2001), and considered as significant at a probability ≤0.05.
Results
Environmental characteristics
Temperature in near-bottom waters varied between −1.77 (Station 709, Otober 2003) and 0.45°C (Station 600, June 2004) (data not shown).
Vertically integrated chl a-concentrations varied greatly between 5.6 (Station 300, November 2003) and 369.7 mg m−2 (Station 303, June 2004) (Table 2). Highest values were recorded during late spring and early summer, in the northwestern part of Franklin Bay (Station 303) and in the eastern and western nearshore parts of the Mackenzie shelf (Stations 906, 912, 609 and 398) (Fig. 1; Table 2).
Sediment chl a-concentrations ranged from 0.06 (Station 303, June 2004) to 2.9 mg m−2 (Station 718, October 2003), averaging 0.8 ± 0.9 mg m−2 (Table 2). CPE concentrations varied between 1.2 (Station 500, October 2003) and 11 mg m−2 (Station 300, November 2003) (on average, 4.7 ± 2.6 mg m−2) (Table 2). On average, CPE concentrations reached a maximum in autumn (5.1 ± 3.2 mg m−2), declined the following spring (3.9 ± 2.2 mg m−2) and reached a second, smaller peak in summer (4.7 ± 2.4 mg m−2). Highest CPE concentrations occurred mostly in the Mackenzie shelf and in the northwestern part of Franklin Bay (Fig. 1; Table 2). Degraded pigments represented a large part of the CPE signal.
Meiofaunal abundance, carbon biomass and taxa
Total meiofaunal density varied between 2.3 × 105 (station 100, October 2004) and 5 × 106 ind m−2 (Station 609, June 2004), averaging 1.3 × 106 ± 0.2 × 106 ind m−2 (Fig. 2a; Table 3). Highest meiofaunal densities were recorded mostly during summer, in Franklin Bay and on the Mackenzie shelf (Fig. 2a). An analysis of the coefficient of variation, CV, was done within replicates from the same box-core (small-scale variability) and among box-cores (meso-scale variability). On average, CV values were lower among sub-cores from one box-core than among different box-cores (17.7 ± 7.9 and 29.1 ± 18.9%, respectively).
Metazoan meiofauna were represented by the following six groups: nematodes, copepods, crustacean nauplii, turbellarians, kinorhynchs and polychaetes. Nematodes were the most numerous group within the sediment, at all sampling periods and stations (Fig. 3a). They comprised, indeed, from 45.7% (Station 206, June 2004) to 87.8% (Station 709, June 2004) of the total meiofaunal abundance. Crustacean nauplii were in most instances the second most abundant group, averaging 10.4 ± 7.1%, followed by copepods (5.9 ± 2.9%), kinorhynchs (4.1 ± 4.0%), turbellarians (2.3 ± 1.9%) and polychaetes (0.2 ± 0.1%).
Total meiofauna carbon biomass showed similar temporal and spatial patterns as abundance (Fig. 2b; Table 4). It displayed values ranging from 25 (Station 117) to 914 mg C m−2 (Station 200) (on average, 167 ± 65.6 mg C m−2). Highest meiofaunal biomass values were recorded during summer in Franklin Bay and on the Mackenzie shelf (Fig. 2b).
Among the meiofaunal groups that occur on most occasions, nematodes, copepods and polychaetes were major contributors of total carbon biomass throughout the study period (Fig. 3b). On average, nematodes contributed 42.8 ± 18.9% to the total carbon biomass, followed by copepods (23.2 ± 16.2%) and polychaetes (17.2 ± 20.6%). Although present at most stations, crustacean nauplii, kinorhynchs and turbellarians weakly contributed to the total carbon biomass.
Meiofauna grazing on CPE
A carbon-based grazing model was applied to the total metazoan meiofauna to provide estimates of maximum potential daily ingestion rates. They ranged from 31.5 (Station 117) to 723.1 mg C m−2 (Station 200) (Table 2). Highest maximum potential daily ingestion rates were observed during summer in Franklin Bay, on the Mackenzie shelf and at the northern part of the Cape Bathurst polynya (Fig. 1; Table 2). Nematodes had the highest maximum potential daily ingestion rate followed by copepods, kinorhynchs, polychaetes, crustacean nauplii and turbellarians (Table 2). Nematodes and copepods contributed 14.6–80.9% (on average, 56.7 ± 16.8%), and 3.4–51.3% (on average, 19.5 ± 13%) of the maximum potential daily ingestion rate, respectively (Fig. 3c).
Assuming that meiofauna were feeding only on sediment photopigments, the potential daily grazing impact of the whole meiofaunal assemblage on the CPE standing stock was 11.2–476.8% (Table 4). Overall, it tended to be higher in summer (128.1 ± 136.7%) than in autumn (42.2 ± 28.9%) and spring (34.4 ± 11.3%).
Correlation analyses
Spearman rank-correlation analyses were done for data from seasons taken separately. Vertically integrated chl a concentrations were highly correlated with depth in autumn and with sediment chl a, pehopigment and CPE concentrations in summer and autumn (Table 5). Sediment chl a, indicating fresh phytodetritus, and CPE concentrations, decreased significantly with increasing water depth at all seasons but spring. Sediment pheopigments decreased also significantly with increasing water depth at all seasons except in spring. During spring, sediment pheopigments increased significantly with increasing CPE. A significant positive correlation between sediment chl a and sediment pheopigments during summer and autumn, likely indicated the relative freshness of phytodetritus.
At all seasons, there was a general tendency of a decrease of a given metazoan meiofauna taxa abundance with increasing water depth (Table 6). Nematodes, turbellarians and kinorhynchs decreased significantly with increasing depth in autumn and summer. In general, meiofaunal taxa abundance tended to increase with increasing sediment pigments. Total meiofauna abundance was significantly correlated with sediment chl a in autumn and with sediment pheopigments and CPE in spring and summer. No significant relationship was found between vertically integrated chl a concentrations and total meiofaunal abundances.
Discussion
The aim of this study was to investigate the dynamics of metazoan meiofauna in the Southeastern Beaufort Sea and to elucidate the strength of pelagic–benthic coupling in this region. Meiofauna densities were highly variable at the spatial and temporal scales with values varying between 0.2 × 106 and 5.2 × 106 ind m−2. Although the choice of a lower sieve size of 63 μm (instead of 32 μm) could have resulted in an underestimation of the metazoan meiofauna densities in the Southeastern Beaufort Sea (Soltwedel 2000), these values are in the range of those observed in different regions of the Arctic at comparable depths (Table 7). Highest meiofaunal densities were recorded in summer on the Mackenzie shelf and in Franklin Bay and could be related to food availability. Accordingly, a high input of organic matter was detected in the sediment at these stations that may contribute to the occurrence of abundant benthic meiofaunal communities.
Metazoan meiofauna were composed of nematodes, copepods, crustacean nauplii, turbellarians, kinorhynchs and polychaetes. Patterns in meiofaunal composition did not change over the three sampling seasons. Nematodes were, for instance, the major component of meiobenthic assemblages at all stations in the Southeastern Beaufort Sea, averaging 75.9 ± 9%, followed, in variable order at different stations, by crustacean nauplii, copepods, kinorhynchs, turbellarians and polychaetes. The dominance of nematodes at all stations in the Southeastern Beaufort Sea corroborated reports by Heip et al. (1985) as being a general feature in various areas and ecosystems.
As expected, a similar pattern to that observed in the abundance was found in the biomass distribution of meiofauna. Overall, the biomass of meiofauna in the Northeastern Beaufort Sea ranged between 25 and 914 mg C m−2, with a median of 167 mg C m−2 (Table 4). It is high, compared to those recorded in the Svalbard archipelago by Kotwicki et al. (2004) (Table 7).
In oceanic regions, the main food and energy source for heterotrophic benthic organisms depends on the input of particulate matter originating from the water column to the seafloor. In the marginal seas of the Arctic Ocean, pulses of phytodetritus to the seafloor are strongly seasonal for ice coverage and light conditions constrained primary production. Although a number of studies have reported tight linkages between pelagic and benthic processes (Ambrose and Renaud 1997; Piepenburg et al. 1997; Renaud et al. 2006), little has been reported, so far, with regard to the importance of this coupling with meiofaunal assemblages on a seasonal scale. As part of this project, we estimated the input of phytodetritus to the seafloor by determining the concentration of vertically integrated phytoplankton, and sediment chlorophyll a and pheopigments into the top first cm of the sedimentary column, and we assessed relationships between these factors and metazoan meiofauna abundances.
Highest vertically integrated chl a concentrations values were recorded during late spring and early summer in Franklin Bay and in the eastern nearshore part of the Mackenzie shelf. There were significant positive correlations between vertically integrated chl a concentrations, and sediment chl a and CPE concentrations during summer, suggesting a likely strong vertical transportation of particulate organic matter. Sediment chl a concentrations, which represent recent sedimentary input of organic matter in the form of phytodetritus, were low compared to pheopigment concentrations. On average, CPE concentrations reached a maximum in autumn, declined during spring and reached a second, smaller peak in summer. Decreasing sediment photopigment concentrations with increasing depth reflect gradual stages of degradation after the vertical passing of primary producers through the pelagic food webs.
Although not always significant, there was a general tendency of decreasing meiofaunal densities with increasing depth (Table 6). This trend has been already reported for other polar regions and is generally explained by the decreased availability and/or quality of organic matter with depth (see Vanaverbeke et al. 1997 and refs therein). In the Southeastern Beaufort Sea, we observed significant correlations between meiofauna standing stocks and measures for food availability at the sediment–water interface. For instance, turbellarians and kinorhynchs were correlated with fresh phytodetritus (i.e. sediment chl a concentration) in summer while a significant correlation was recorded in autumn between nematodes as well as copepods and turbellarians, and sediment chl a concentrations. This is indicative of a good ability of these organisms to use fresh phytodetritus. In summer, nematodes were positively correlated with sediment pheopigment standing stocks suggesting that nematodes were well adapted to more degraded phytodetritus. Positive correlations between CPE and total meiofauna in spring and summer may suggest that the latter are also well adapted overall to use phytodetritus of any quality. It is also possible, however, that degraded phytodetritus harbour-abundant bacterial populations that can contribute to the overall carbon flow by supporting bacterial-feeding meiofaunal populations.
So far, grazing rates of meiofaunal organisms have been estimated, both in the laboratory and in the field, using different techniques (see review by Montagna 1995). Duncan et al. (1974) employed pre-labeled bacteria with 14C-glucose to feed nematodes, while Admiraal et al. (1983) used 14C-algae (Navicula pygmea) for the same purpose. Using a similar technique Herman and Vranken (1988) estimated the assimilation efficiency of the nematode Monohystera disjuncta by coupling information on ingestion and defecation rates. More recently, Moens et al. (2002) used a combination of natural isotope signatures and experimental results to elucidate the grazing behavior of nematodes. Grazing rates of harpactidoid copepods were studied by using 3H-glucose and 3H-leucine pre-labeled bacteria as food source (Rieper 1978). Daro (1978) and Montagna (1984) employed 14C-labeling field techniques to determine total meiofauna grazing rates. Carman and Thistle (1985) also studied copepod-grazing rates in the field by injecting the sediment with 14C-bicarbonate and 14C-acetate; this allowed the identification of different feeding strategies for three different copepod species.
We estimated meiofaunal grazing rates by means of empirical allometric equations from Moloney and Field (1989). There is a large component of uncertainty in this type of calculations. For instance, this carbon-based grazing model provides a maximal grazing rate for meiofauna, and is likely to result in overestimates of in situ grazing activity. Our original motivation is, however, to provide estimates of meiofaunal potential grazing rate and impact on CPE concentrations. The total meiofauna had, on average, a daily potential ingestion rate of 70, 69.3 and 242.1 mg C m−2 during autumn, spring and summer, respectively. Highest daily potential ingestion rates were recorded in summer on the Mackenzie shelf and in Franklin Bay. Seasonal changes in grazing impact observed in the Southeastern Beaufort Sea follow the seasonal changes in meiofaunal biomass. Maximum daily grazing impact was observed during the summer period with values ranging from 23 to 477% and, overall, the potential daily grazing impact of the meiofauna community on the CPE biomass was highest on the Mackenzie shelf and in Franklin Bay. The major grazing impact of meiofauna on the bulk of phytodetritus in summer is confirmed by a strong positive correlation between total meiofauna abundance and CPE biomass during this period. During months when meiofauna abundance and biomass were lower, however, the grazing impact was lower. Overall, results of the present study show that meiofauna were likely not food-restricted during spring and autumn and that grazing on CPE was low during these seasons, averaging 36.8 and 47.8%, respectively.
In the present study, it was assumed that metazoan meiofauna fed only on phytodetritus. In fact they can feed on various food items. Turbellarians have been described as omnivores (McIntyre 1969), while nematodes and harpacticoid copepods are known as feeding on microfauna, algae, phytodetritus, bacteria, dissolved organic matter (DOM), and small metazoans (Boaden 1964; Montagna 1984; Rudnick 1989; Decho and Moriarty 1990; Tita et al. 2000; Moens et al. 1999; 2002).
In conclusion, consistent with previous studies, the abundance of meiofauna decreased with increasing depth. Additional data are however required to assess whether variability in the spatial distribution of meiofauna is partly governed by primary productivity levels in the upper layers of the euphotic zone. The results of this study also underline the importance of sediment phytodetritus as a potential food source for meiofauna in the Southeastern Beaufort Sea. This finding is consistent with previous studies on the strength of pelagic–benthic coupling on benthic processes in marginal seas of the Arctic Ocean. Our results showed that, on average, from a third to half of the bulk of sediment phytodetritus could be channeled through the meiofaunal component of heterotrophic benthic organisms in the Southeastern Beaufort Sea. These first estimates, which are based on allometric equations, should be now validated/revised by measurements on ingestion rates of sediment phytodetritus by meiofauna. This will have important consequences on the quantification of carbon fluxes and cycle at the sediment-interface in marginal seas of the Arctic Ocean.
References
Admiraal W, Bouwman LA, Hoekstra L, Romeyn K (1983) Qualitative and quantitative interactions between microphytobenthos and herbivorous meiofauna on a brackish intertidal mudflat. Int Rev Ges Hydrobiol 68:175–191
Ambrose WG Jr, Renaud PE (1997) Does a pulsed food supply to the benthos affect polychaete recruitment patterns in the Northeast Water Polynya? J Mar Syst 10:483–495
Arntz W, Brey T, Gallardo VA (1994) Antarctic zoobenthos. Oceanogr Mar Biol Ann Rev 32:241–304
Boaden PJS (1964) Grazing in the interstitial habitat: a review. In: Crisp DJ (ed) Grazing in terrestrial and marine environments. Blackwell, Oxford, pp 299–303
Brugel S, Ferreyra G, Nozais C, Demers S (2005) Phytoplankton production dynamics in the Beaufort Sea (Canadian Arctic): a comparison between the Mackenzie shelf and the Cape Bathurst polynya. American Society of Limnology and Oceanography, Saint-Jacques de Compostelle, Spain
Carman KR, Thistle D (1985). Microbial food partitioning by three species of benthic copepods. Mar Biol 88:143–148
Danovaro R (1996) Detritus–bacteria–meiofauna interactions in a seagrass bed (Posidonia oceanica) of the NW Mediterranean. Mar Biol 127:1–13
Daro MH (1978) A simplified 14C method for grazing measurements on natural planktonic populations. Helgol Wiss Meeresunters 31:241–248
Decho AW, Moriarty DJW (1990) Bacterial exopolymer utilization by a harpacticoid copepod: a methodology and results. Limnol Oceanogr 35:1039–1049
Duncan A, Schiemer F, Klekowski (1974) A preliminary study of feeding rates on bacterial food by adult females of a benthic nematode, Plectus palustris De Man, 1980. Pol Arch Hydrobiol l21:249–258
de Jonge VN (1980) Fluctuations in the organic carbon to chlorophyll-a ratios for estuarine benthic diatom populations. Mar Ecol Prog Ser 2:345–353
Fabiano M, Danovaro R (1999) Meiofauna distribution and mesoscale variability in two sites of the Ross Sea (Antarctica) with contrasting food supply. Polar Biol 22:115–123
Feller RJ, Warwick RM (1988) 13. Energetics. In: Higgins RP, Thiel H (eds) Introduction to the study of meiofauna. Smithsonian Institution Press, Washington, pp 181–196
Giere O (1993) Meiobenthology—the microscopic fauna in aquatic sediments. Springer, Berlin
Gradinger R, Friedrich C, Spindler M (1999) Abundance, biomass and composition of the sea ice biota of the Greenland Sea pack ice. Deep-sea Res Part II Top Stud Oceanogr 46:1457–1472
Gutzmann E, Martínez Arbizu, Rose A, Veit-Köhler G (2004) Meiofauna communities along an abyssal depth gradient in the Drake Passage. Deep Sea Res II 51:1617–1628
Heip C, Vincx M, Vranken G (1985) The ecology of marine nematodes. Oceanogr Mar Biol Annu Rev 23:399–489
Herman MJ, Vranken G (1988). Studies of the life-history and energetics of marine and brackish-water nematodes. II. Production, respiration and food uptake by Monohystera disjuncta. Oecologia 77:457–463
Hermann RL, Dahms HU (1992) Meiofauna communities along a depth transect of Halley Bay (Weddell Sea, Antarctica). Polar Biol 12:313–320
Holm-Hansen O, Lorenzen CJ, Holmes RW, Strickland JDH (1965) Fluorometric determination of chlorophyll. J Cons Perm Int Explor Mer 30:1–15
Jensen P (1984) Measuring carbon content in nematodes. Helgol Meeresunters 38:83–86
Kotwicki L, Szymelfenig M, De Troch, Zajączkowski M (2004) Distribution of meiofauna in Kongsfjorden, Spitsbergen. Polar Biol 27:661–669
Lorenzen CJ (1966) A method for the continuous measurement of in vivo chlorophyll concentration. Deep Sea Res 13:223–227
McIntyre AD (1969) Ecology of marine meiobenthos. Biol Rev 44:245–290
Moens T, Verbeeck L, Vincx M (1999) Feeding biology of a predatory and a facultatively predatory nematode (Enoploides longispiculosus and Adoncholaimus fuscus). Mar Biol 134:585–593
Moens T, Luyten C, Middelburg JJ, Herman PMJ, Vincx M (2002) Tracing organic matter sources of estuarine tidal flat nematodes with stable carbon isotopes. Mar Ecol Prog Ser 234:127–137
Moloney CL, Field JG (1989) General allometric equations for rates of nutrient uptake, ingestion and respiration in plankton organisms. Limnol Oceanogr 34:1290–1299
Montagna PA (1984) In situ measurements of meiobenthic grazing rates on sediment bacteria and edaphic diatoms. Mar Ecol Prog Ser 18:119–130
Montagna PA (1995) Rates of metazoan meiofaunal microbivory: a review. Vie Milieu 45:1–9
Nozais C, Perissinotto R, Mundree S (2001) Annual cycle of microalgal biomass in a South African temporarily-open estuary: nutrient versus light limitation. Mar Ecol Prog Ser 223:39–48
Nozais C, Perissinotto R, Tita G (2005) Seasonal dynamics of meiofauna in a South African temporarily open/closed estuary (Mdloti Estuary, Indian Ocean). Estuar Coast Shelf Sci 62:325–338
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, Toronto
Pfannkuche O, Thiel H (1987) Meiobenthic standing stocks and benthic activity on the NE Svalbard Shelf and in the Nansen Basin. Polar Biol 7:253–266
Piepenburg D, Ambrose WG Jr, Brandt A, Renaud PE, Ahrens MJ, Jensen P (1997) Benthic community patterns reflect water column processes in the Northeast Water polynya (Greenland). J Mar Syst 10:467–482
Piepenburg D (2005) Recent research on Arctic benthos: common notions need to be revised. Polar Biol 28:733–755
Price R, Warwick RM (1980) The effect of temperature on the respiration rate of meiofauna. Oecologia 44:145–148
Renaud P, Riedel A, Michel C, Morata N, Gosselin M, Juul-Pedersen T, Chiuchiolo A (2006) Seasonal variation in benthic community oxygen demand: a response to an ice algal bloom in the Beaufort Sea, Canadian Arctic? J Mar Syst doi:10.1016/j.jmarsys.2006.07.006
Riaux-Gobin C, Klein B (1993) Microphytobenthic biomass measurement using HPLC and conventional pigment analysis. In: Kemp PF, Sherr BF, Sherr EB, Cole JJ (eds) Handbook of methods in aquatic microbial ecology. Lewis Publishers, Boca Raton, pp 369–376
Riemann F, Ernst W, Ernst R (1990) Acetate uptake from ambient water by the free-living marine nematode Adoncholaimus thalassophygas. Mar Biol 104:453–457
Rieper M (1978) Bacteria as food for marine harpacticoid copepods. Mar Biol 45:337–345
Rudnick DT (1989) Time lags between the deposition and meiobenthic assimilation of phytodetritus. Mar Ecol Prof Ser 50:231–240
Schewe I (2001) Small-sized benthic organisms of the Alpha Ridge, Central Arctic Ocean. Int Rev Hydrobiol 86:317–335
Schewe I, Soltwedel T (1999) Deep-sea meiobenthos of the central Arctic Ocean: distribution patterns and size–structure under extreme oligotrophic conditions. Vie Milieu 49:79–92
Schewe I, Soltwedel T (2003) Benthic response to ice-edge-induced particle flux in the Arctic Ocean. Polar Biol 26:610–620
Sheremetevsky AM (1977) Meiobenthos in the intertidal zone of the Laptev Sea and the New Siberian Islands. Gidrobiol Zh 13:63–70
Sherrer (1984) Biostatistique. Gaëtan Morin, Montréal Paris Casablanca
Skowronski RSP de, Corbisier TN (2002) Meiofauna distribution in Martel Inlet, King George Island (Antarctica): sediment features versus food availability. Polar Biol 25:126–134
Soltwedel T (2000) Metazoan meiobenthos along continental margins: a review. Prog Oceanogr 46:59–84
Soltwedel T, Schewe I (1998) Activity and biomass of the small benthic biota under permanent ice-coverage in the central Arctic Ocean. Polar Biol 19:52–62
Soltwedel T, Miljutina M, Mokievsky V, Thistle D, Vopel K (2003) The meiobenthos of the Molloy Deep (5,600 m) Fram strait, Arctic Ocean. Vie Milieu 53:1–13
Soltwedel T, Portnova D, Kolar I, Mokievsky, Schewe I (2005) The small-sized benthic biota of the Hakon Mosby Mud Volcano (SW Barents Sea slope). J Mar Syst 55:271–290
Thiel H (1978) Benthos in upwelling regions. In: Boje R, Tomczak M (eds) Upwelling ecosystems. Springer, Heidelberg, pp 124–138
Tita G, Desrosiers G, Vincx M, Nozais C (2000) Predation and sediment disturbance effects of the intertidal polychaete Nereis virens (Sars) on associated meiofaunal assemblages. J Exp Mar Biol Ecol 243:261–282
Urban-Malinga B, Wiktor J, Jabłońska A, Moens T (2005) Intertidal meiofauna of a high-latitude glacial Arctic fjord (Kongsfjorden, Svalbard) with emphasis on the structure of free-living nematode communities. Polar Biol 28:940–950
Vanaverbeke J, Martinez Arbizu P, Dahms H-U, Schminke HK (1997) The metazoan meiobenthos along a depth gradient in the Arctic Laptev Sea with special attention to nematode communities. Polar Biol 18:391–401
Vanhove S, Beghyn M, Van Gansbeke D, Bullough LW, Vincx M (2000) A seasonally varying biotope at Signy Island, Antarctic: implications for meiofaunal structure. Mar Ecol Prog Ser 202:13–25
Vézina AF, Demers S, Laurion I, Sime-Ngando T, Juniper SK, Devine L (1997) Carbon flows through the microbial food web of first-year ice in Resolute Passage (Canadian High Arctic). J Mar Syst 11:173–189
Warwick RM, Price R (1979) Ecological and metabolic studies on free-living nematodes from an estuarine mud-flat. Estuar Coast Shelf Sci 9:257–271
Warwick RM, Gee JM (1984) Community of estuarine meiobenthos. Mar Ecol Prog Ser 18:97–111
Węsławski JM, Zajączkowski M, Wiktor J, Szymelfenig M (1997) Intertidal zone of Svalbard. 3. Littoral of a subarctic, oceanic island: Bjornoya Polar Biol 18:45–52
Wieser W (1960) Benthic studies in Buzzards Bay. II. The meiofauna. Limnol Oceanogr 5:121–137
Acknowledgments
This research, as part of the Canadian Arctic Shelf Exchange Study (CASES), was supported by grants from the Natural Sciences and Engineering Research Council (NSERC) of Canada to S.D. and C.N. We are grateful to the Canadian Coast Guard officers and crews of the scienfitic icebreaker ‘Amundsen’ for their skilful support during the expedition. We thank Richard Cloutier and Julien Lambrey de Souza for video analyses, and three anonymous referees for their constructive comments on the manuscript. This is a contribution to the Institut des sciences de la mer de Rimouski, BioNord and the Canadian Arctic Shelf Exchange Study research programs.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is dedicated to the memory of our dear friend and colleague Gaston Desrosiers who contributed so much to benthic ecology. We will continue in his spirit.
Rights and permissions
About this article
Cite this article
Bessière, A., Nozais, C., Brugel, S. et al. Metazoan meiofauna dynamics and pelagic–benthic coupling in the Southeastern Beaufort Sea, Arctic Ocean. Polar Biol 30, 1123–1135 (2007). https://doi.org/10.1007/s00300-007-0270-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-007-0270-6