Abstract
Habitat heterogeneity is one of the major factors shaping community structure and diversity of many fauna. The present study aimed to reveal the influence of habitat heterogeneity on the nematode community structures and diversity in a seasonally hypoxic bay (Omura Bay of Nagasaki in Western Kyushu, Japan). The severity of hypoxia varies typically along north–south axis of the bay, which is intensified southwardly. Nematode abundance and diversity were highest in the northern site than the other sites, and nematode communities were clustered into three groups by sampling site. There were significant differences in composition (Two-way ANOSIM, Rho = 0.726, p < 0.05) and in feeding types (Two-way ANOSIM, Rho = 0.589, p < 0.05) among the groups. Organic matter content alone was the best predictor for the shift in nematode compositions (BIOENV procedure, Correlation = 0.666, p < 0.05), whereas the combination of salinity and DO correlated well with the shift in nematode feeding types (Correlation = 0.568, p < 0.05). These findings strongly suggest that the diversity and the structures of nematode assemblages were strongly affected by the habitat heterogeneity in terms of seasonal DO availability, salinity change and persistent food availability (organic carbon accumulation) over the surface sediment of the bay.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Dissolved oxygen (DO) concentration of bottom water is one of the critical environmental factors directly affecting benthic community structures (Levin 2003; Gooday et al. 2010; Jessen et al. 2017). For most macro benthic fauna, low DO concentration can adversely affect their survival, and lead to a shift in the benthic communities between hypoxic and normoxic conditions (Diaz and Rosenberg 2008; Neira et al. 2013). Contrary to the sensitivities of macrofauna to low oxygen stress, some meiofaunal taxa, particularly nematodes, are more tolerant to deoxygenation (Levin et al. 1991; Neira et al. 2013). Nematodes are one of the most abundant marine benthos and plays important roles in marine ecosystem functioning such as organic matter recycling, and/or trophic links between the micro- and macro-organisms (Higgins and Thiel 1988). Therefore, the effects of deoxygenation on nematodes need to be fully understood to make better predictions of the ecosystem response to a global trend in deoxygenation (Breitburg and Levin 2018). Because of greater abundance, biodiversity and resilience over other meiobenthos, free-living nematodes have been used as sensitive means to examine the impacts of hypoxia on the spatial distribution of meiofauna (Neira et al. 2013).
In addition to DO conditions, habitat heterogeneity in other environmental factors, such as organic matter load or salinity, also plays important roles in shaping macro- and meiofauna community diversity and structures at a regional (basin-wide) scale (Levin et al. 2010; Kovalenko et al. 2012; Stein et al. 2014). Therefore, dynamics of nematode community composition in deoxygenated coastal areas should be addressed in conjunction with the heterogeneity of bottom environments to make more accurate understanding and realistic predictions for the ecosystem impacts of deoxygenation (Giere 2009; Traunspurger and Majdi 2017).
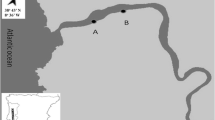
Omura Bay is located at the center of Nagasaki Prefecture in western Kyushu. The bay is strictly enclosed and covers an area of 320 km2, with an average depth of 14.7 m (Iizuka and Min 1989) (Fig. 1). As the bay is connected to the open sea (East China Sea) by only two extremely narrow channels at its northwestern corner, the water exchange between Omura Bay and the outer sea is strictly limited. Severe bottom hypoxia occurs every summer usually at the central part of the bay and it expands to any directions. However, the northern well-mixed area is less affected by hypoxia due to its proximity to the bay mouth compared with the center and south-east areas. As the south-east site is farthest from the bay mouth and close to the closed-off section of the bay, hypoxic condition can remain for longer period than other areas (Yokoyama 1995; Fukumoto and Kobayashi 2005; Takahashi et al. 2009). Therefore, Omura Bay provides a suitable opportunity to investigate how benthic organisms are affected along with gradients in oxygen availability and other environmental factors across the basin.
Yokoyama (1995) found that the DO availability and sediment characteristics of the bay controlled the macrofaunal communities. Nguyen et al. (2018) reported that nematode community structures temporally shifted between normoxic and hypoxic conditions in Omura Bay. However, analyses on horizontal distribution of nematode communities, and how the nematode assemblages respond to the development of hypoxia across the basin of the bay have yet to be done. In this study, to address the questions raised by the previous study, and to gain more comprehensive insights into how deoxygenation and habitat heterogeneity control the diversity and structures of nematode community in Omura Bay, we conducted environmental surveys of the water and sediment and quantitative samplings of nematode community occurring in the surface sediment at the stations set along the longitudinal direction of the bay. We report the results of these surveys and samplings and discuss effects of the habitat heterogeneity in DO availability and other physico-chemical parameters in water column and sediment on the nematode community in the bay.
2 Materials and methods
2.1 Sample collection, and environmental parameters monitoring
Sediment core samples of Omura Bay were obtained with a multiple core sampler, Ashura (RIGOSHA Co., Ltd.) on a training ship, Kakuyo Maru, Nagasaki University at four sampling sites: (1) The north (St. N), (2) the center (St. C), (3) the south west (St. SW), and (4) the south east of the bay (St. SE) between June and October 2017 (Fig. 1). Detailed locations and dates of sampling are summarized in Table 1. At each site, triplicate sediment cores (82-mm inner diameter) were collected. Besides those cores, extra triplicate core sample were taken in June, August, and October for the analysis of sediment parameters (gain size, organic matter, and sediment chlorophyll a). Sediment cores were extruded and horizontally sliced into layers with a pair of clean stainless-steel straight blades on board a ship. Each sediment layer was fixed immediately and preserved in 5% buffered seawater formalin containing borax (final conc. = 30–40 g L−1) and rose bengal (final conc. = 1 g L−1). All formalin-fixed samples were maintained at approximately 25 °C, and carefully transported to the laboratory within 1–2 days after sampling to avoid direct exposure to sunlight and other physical disturbances (Nguyen et al. 2018). Water column parameters (DO concentration, temperature, salinity, and water chlorophyll a) in overlying water 1 m above the sediment surface were monitored with a multi-parameter monitoring device (AAQ, JFE-Alec) at each sampling site.
2.2 Sediment gain size, organic matter (OM), and chlorophyll a (Chl a) analysis
As suggested by Nguyen et al. (2018), hypoxic conditions in the bay fully developed in August, while normoxic conditions occurred in June and October. Therefore, a portion of the sediment core samples from 3 months (June, August and October) were examined for grain size, organic matter and chlorophyll a content. Small amounts of sediment (approximately 3 ml) were taken from each sample to determine median grain sizes, which were measured in triplicate using a laser diffraction particle size analyzer (SALD-3100, Shimadzu Corp., Kyoto, Japan). The samples for organic matter analysis were placed into plastic bags and kept frozen at − 20 °C. Sediment samples in glass cupboard were dried at 105 °C for 1 h, cooled down to room temperature and determined their dry weights. Dried sediments were gradually heated until they reached temperatures of 500 °C, and kept for 4 h. The samples were then put into a desiccator and weighed at room temperatures. The amount of organic matter (Loss on ignition; LOI) is the weight difference between the dry matter and the 500 °C ash (Vereș and Daniel 2002). For extracting sediment Chl a., a small amount (approximately 1 ml) of each sediment sample was put into 5 ml N,N-dimethylformamide (DMF). Concentrations of Chl a were then measured fluorometrically in triplicate using a 10-AU Field Fluorometer (Turner Designs, USA).
2.3 Sample processing and nematode identification
As nematodes in the top layer (0–10 mm depth) seemed to be most strongly influenced by low DO conditions compared to the subsurface (Nguyen et al. 2018), the top layer was examined across all the sediment cores. Samples were divided into quarters using a Folsom plankton splitter prior to the laboratory sieving processes, and either a 1/8 or 1/4 of the sediment was treated until nematode numbers exceeded 50 (Setoguchi et al. 2014). Sieving and sorting of specimens were done as described in a previous paper (Nguyen et al. 2018).
Up to 50 randomly-picked nematodes were identified to the genus level by referring to Warwick et al. (1998a, b), Schmidt-Rhaesa (2013), and the keys on the NeMys online identification system (http://nemys.ugent.be/). Finally, based on the morphology of the buccal cavity, the identified nematodes were categorized into four feeding types: selective deposit feeders (type 1A), non-selective deposit feeders (type 1B), epistrate (diatom) feeders (type 2A), and predators/omnivores (type 2B) (Lambshead 1986).
2.4 Statistical analysis
To test whether there were any differences in means of the environmental parameters (sediment and water column parameters), the pooled data of nematode abundance or diversity between sampling sites or months, Kruskal–Wallis test was performed (Kruskal and Wallis 1952). Multiple pairwise comparisons were made to further compare all pairs of medians for each factor using the Steel–Dwass–Critchlow–Fligner test (Spurrier 2006). Those tests were done using XLSTAT software (XLSTAT 2018: Data Analysis and Statistical Solution for Microsoft Excel. Addinsoft, Paris, France).
Multivariate analyses, calculations of diversity, and BIOENV procedure were performed using the PRIMER6 software (PRIMER-E Ltd., Plymouth, UK). Abundance data were square root transformed to weigh down the effects of the abundant genera. To visualize similarities in the nematode community compositions and feeding types in the four stations (St. N, C, SW and SE) or 5 months (June–October), non-metric multidimensional scaling (nMDS) was used. A two-way analysis of similarities (ANOSIM) without replication were made to make comparisons between the means of groups of data, where sampling sites and months were considered. Furthermore, one-way ANOSIM to indicate pairwise differences between the means of each group like sampling sites or months. Similarity percentage analyses (SIMPER) were conducted to identify the group of nematode taxa or morphotypes that was contributing most to any similarity or dissimilarity. DIVERSE analyses were performed to calculate a set of univariate biodiversity measures (Shannon–Weiner index, H’ and Simpson diversity index, 1-lambda) among sampling sites. To find the best variable combinations between nematode community structure and environmental parameters among sampling sites, the BIOENV procedure was conducted using weighted Spearman rank correlation (pw) (Clarke and Warwick 2001).
3 Results
3.1 Environmental parameters
3.1.1 Bottom water parameters
Figure 2 illustrates changes in four bottom water parameters at four sampling sites of Omura Bay between Jun and Oct 2017. Temperature of the bottom water began to increase from June to August (from around 19 °C to 26 °C), and then declined from September to October (from around 26 °C to 23 °C) across the sampling sites. There was no significant difference in the water temperature among the sampling sites (Kruskal–Wallis test, p > 0.05). Salinity of the bottom water at St. N, C and SW remained rather constant ranging from 30 to 32, while at St. SE it fluctuated notably with a peak (32) in July followed by a decline to 24 in October. However, statistical tests failed to show significant difference among the four sites (Kruskal–Wallis test, p > 0.05) in salinity. In contrast, there was a significant difference among sampling months (Kruskal–Wallis test, p < 0.05), although the pairwise test did not detect any significant differences in salinity between months (Steel–Dwass–Critchlow–Fligner test, p > 0.05). In the present study, hypoxia was defined as DO concentrations of less than 3 mg L−1 (Wada et al. 2012). DO concentration at St. N mostly remained normoxic, while the St. SE suffered from persistent hypoxia. The difference in the overall mean of DO between St. N and St. SE was significant (Steel–Dwass–Critchlow–Fligner test, p < 0.05). DO concentration at St. C and St. SW ranged widely; DO started to fall below 3 mg L−1 after July and hypoxic conditions developed from August until September, while it recovered over 3 mg L−1 by October. The Chl a concentration of the bottom water ranged from 0.73 through 5.26 μg L−1 at St. N, C and SW. The St. SE showed the large fluctuation of Chl a concentration with the highest in July (11.44 μg L−1) and the lowest in October (0.18 μg L−1). However, there were no significant differences in the mean Chl a concentration of the bottom water in the five sampling occasions between June and October among the sampling sites (Kruskal–Wallis test, p > 0.05).
Four parameters (DO concentration, water temperature, salinity, and water chlorophyll a) of the bottom water at the four sampling sites in Omura Bay between June and October 2017. The dashed lines show the transition point of DO concentration (3 mg L−1) between hypoxia and normoxia. Hypoxic months were underlined to separate from normoxic ones
3.1.2 Sediment parameters
Sediment grain size (the mean values of the median diameter of the sediment particles) tended to increase from 8.77 to 18.64 μm across the sampling sites between June and October, but difference among the sites was not significant (Kruskal–Wallis test, p > 0.05) (Fig. 3). The mean values of LOI at St. N (from 13.4 to 14.8%) were lower and significantly different from other stations across the sampling occasions (Steel–Dwass–Critchlow–Fligner test, p < 0.05). There was no significant difference in LOI among the sampling sites (Kruskal–Wallis test, p > 0.05). The Chl a content of the sediment ranged from 640 to 2000 mg kg−1 at the three sampling sites (St. C, SW, and SE) between June and October. The highest value of the Chl a content of the sediment (6212 mg kg −1) was found at St. N in June. There was no significant difference in sediment Chl a among the sites (Kruskal–Wallis test, p > 0.05).
3.2 Abundance, genus composition, and genus diversity of nematode community
3.2.1 Nematode abundance
The variations of the mean abundance among the different sampling sites at 5 months (June–October) were statistically significant (Kruskal–Wallis test, p < 0.05). The Steel–Dwass–Critchlow–Fligner test showed that the overall mean of nematode abundance throughout the study period at St. N (626 ind. 10 cm−2) was significantly larger than those observed at St. C (215 ind. 10 cm−2, p < 0.05) and SW (226 ind. 10 cm−2, p < 0.05) and SE (310 ind. 10 cm−2, p < 0.05). There was a decreasing trend for the nematode mean abundance from 480 to 266 ind. 10 cm−2 across the sampling sites between July and September (Fig. 4). The variations of the mean abundance among the different sampling months at four stations were statistically significant (Kruskal–Wallis test, p < 0.05); however, the pairwise test did not detect any significant differences in abundance between months (Steel–Dwass–Critchlow–Fligner test, p > 0.05).
Nematode abundance at the four sampling sites in Omura Bay between June and October in 2017. The error bars represent the standard deviation of nematode abundance. The round-dot lines indicate changes in the nematode abundance over the four sampling sites. Hypoxic months were underlined to separate from normoxic ones
3.2.2 Genus composition of the nematode community
As shown in Table 2 and Table S1, Neotonchus (176 out of 733 individuals, 24.0%) and Axonolaimus (338 out of 749 individuals, 45.1%) were found to be the most abundant genera at St. N and St. SE, respectively. Chromadorina dominated at St. C (195 out of 743 individuals, 26.2%) and SW (215 out of 737 individuals, 29.2%). A clear distinction between St. N ad SE was largely attributed to the predominance of Neotonchus, one of the epistrate feeding nematodes (2A), at St. N and that of Axonolaimus, one of the non-selective deposit feeding nematodes (1B), at St. SE. Three nematode genera, Chromadonina, Axonolaimus and Pseudolella remained and accounted for more than 50% of the nematode population during hypoxic conditions.
There was a significant difference in the genus-level composition among the sampling sites (two-way ANOSIM, Rho = 0.726, p < 0.05). The pairwise test showed that genus-level composition at St. N was significantly different from other three sites (p < 0.05), and that of St. SE was significantly different from St. C (p < 0.05). Further analyses showed that the nematode communities noted for the five sampling occasions at the four sampling sites were clustered into three different groups with more than 60% similarity except one at St. N in October (Fig. 5). Group I consisted of most plots at St. N (except October), in which Neotonchus was the most abundant. Group II included all of the plots at St. SE (dominated by Axonolaimus) and August at St. SW (dominated by Chromadorina). Group III consists of the rest of plots at St. C and SW (dominated by Chromadorina) at the similarity level of 60%. However, there were no significant differences in the composition among sampling months (Rho = 0.176, p > 0.05).
Non-metric MDS plot of nematode community structure at the four sampling sites in Omura Bay between June and October in 2017 with superimposed clusters based on the Bray–Curtis similarity (standardize sample by total; transformation: square root). The round-dot lines refer to similarity levels of 60%. Hypoxic months were underlined to separate from normoxic ones
The SIMPER analysis indicated that the within group similarity was the largest at St. SE (67.9%), followed by St. C (65.1%), St. SW (62.5%) and St. N (62.3%) (Table S2). The genus that showed the greatest contribution to the group similarity at St. SE (contrib % = 24.2%) was Axonolaimus. Chromadorina contributed most to the average similarities at St. C and St. SW (contrib % = 18.3% and contrib % = 22.9%, respectively), while Neotonchus contributed most to the average similarities at St. N (contrib % = 17.8%).
3.2.3 Genus diversity of the nematode
At St. N, the genus-level species richness (Hʹ) and the evenness (1 − Lambda) were on average 2.51 and 0.88, respectively, and highest among the four sampling sites. On the other hand, lowest values for the diversity indices were found at St. SE (on average 1.87 for Hʹ and 0.71 for 1 − Lambda) (Fig. 6). It was also noted that variations of the diversity were smallest at St. N, whereas they were largest at St. SE. The diversity indices at St. C and SW were intermediate between N and SE. The Steel–Dwass–Critchlow–Fligner test indicated that H’ at St. N was significantly different from that at SW (p < 0.05). These results demonstrated that St. N had higher species richness (genus-level) and evenness, while the nematode community at St. SE was less rich and the genus was not equally abundant. These findings were also consistent with the rank abundance of nematode genera (Table 2). Seven genera are needed to achieve 70% cumulative percentage of the community abundance in St. N, whereas only four genera occupied more than 70% in St. SE. Neither of the two diversity indices were statistically different among sampling months (Kruskal–Wallis test, p > 0.05).
Shannon and Simpson indexes of nematode diversity at the four sampling sites of Omura Bay between June and October in 2017. The crosses indicate the means. The central horizontal bars denote the medians. The lower and upper limits of the box plot are the first and third quartiles, respectively. Points above or below the whiskers’ upper and lower bounds are considered as outliers
3.2.4 Nematode feeding types
There was a significant difference in the feeding type compositions among the four sampling sites (two-way ANOSIM, Rho = 0.589, p < 0.05). The pairwise test detected that the feeding type compositions in St. SE were significantly different from other three sites (one-way ANOSIM, p < 0.05). Further analyses revealed that most of the nematode communities based on the feeding type were clustered into three different groups, except October at St. N, which was not included in any of the groups (Fig. 7). Group I consisted of all the communities at St. SE and one from August at St. SW, which were dominated by type 1B nematodes. Group II included most of the communities at St. N, the one in July at St. SW, and the two other communities at St. C (July and October). They were dominated by type 2A nematodes. Group III consists of the rest of the communities at St. C and SW. The level of dominance for type 2A and 1B was comparable in this group. The SIMPER analysis indicated that the within group similarity was the largest at St. N (90.7%), followed by St. SW (87.7%), St. C (86.8%) and St. SE (86.6%). The feeding type that showed the greatest contribution to the group similarity at St. N (39.3%) was 2A. 2A group also contributed most to the average similarities at St. SW (30%) and C (33.1%). The 1B type nematodes contributed most to the average similarities at St. SE (46.1%) (Table S3).
Non-metric MDS plot of nematode feeding types at the four sampling sites in Omura Bay between June and October in 2017 with superimposed clusters based on the Bray–Curtis similarity (standardize sample by total; transformation: square root). Continuous lines refer to similarity levels of 60%. Hypoxic months were underlined to separate from normoxic ones
As shown in Fig. 8, the relative percentage of the nematodes with teeth (types 2A and 2B, 63.9%) was higher than that of toothless nematodes (types 1A and 1B, 36.1%) at St. N. On the other hand, the relative percentage of toothless nematodes (78.3%) was higher than that of the nematodes with teeth (21.7%) at St. SE. Type 2A (50.3%) was more dominant than 2B (13.6%) among the nematodes with teeth at St. N, while type 1B (61.3%) dominated over 1A (17.0%) within the toothless nematodes at St. SE throughout the study period. Relative abundance of toothless nematodes reached a maximum in August at St. C (59.7%) and SW (72.3%). Among the toothless nematodes, 1B type predominated at all sites but the relative abundance of 1A type tended to increase in August through September, except at St. C.
Relative abundance of nematode feeding types (1A, 1B, 2A, and 2B) at the four sampling sites of Omura Bay between June and October 2017. The round-dot lines indicate changes in the relative percentage of toothless nematodes (types 1A and 1B) and nematodes with teeth (types 2A and 2B) over the four sampling sites. Selective deposit feeders (1A), non-selective deposit feeders (1B), epistrate (diatom) feeders (2A), and predators/omnivores (2B). Hypoxic months were underlined to separate from normoxic ones
3.2.5 Correlation between nematode community structure and environmental variables
To correlate the patterns of the nematode community structure (nematode composition and feeding types) and the environmental variables, BioEnv analysis was conducted. As shown in Table 3, loss on ignition (LOI) showed the best match (pw = 0.666) with the MDS ordination of the nematode community composition. A combination of LOI and sediment chlorophyll a content yielded the second highest correlation (pw = 0.41). On the other hand, a combination of all the variables but water temperature gave rise to the highest correlation (pw = 0.598) with the ordination plot of the nematode feeding type. The best 2-variable combination involved salinity and DO (pw = 0.568).
4 Discussion
Basin-wide heterogeneity of the environmental conditions in Omura Bay was clearly demonstrated in relation to the bottom-water DO and sediment organic matter (LOI) between bay mouth (St. N) and closed section of the bay (St. SE). In contrast to St. N, hypoxia and enrichment of organic matter was apparent at St. SE. This is consistent with a notion that sediment organic matter is well-preserved under low DO conditions in the bottom water (Jessen et al. 2017; Mori et al. 2018). On the other hand, a greater fluctuation of DO concentration at St. C and SW during the study period demonstrates the seasonal transition between normoxia and hypoxia in the bay. Such seasonality of DO condition was less pronounced at St. N and SE. Highly fluctuating, lower values of salinity at St. SE also indicated a kind of heterogeneity of the bottom environment in Omura Bay. This is attributable to the influence of freshwater discharge from the nearby coast. The peak of Chl a concentration of the bottom water coinciding with that of salinity at St. SE in July may, therefore, reflect an algal growth promoted by extra input of nutrients such as nitrogen and phosphorous from terrestrial origin to the inner bay in early summer (during June to July). The Chl a content of the sediment can be an indicator for the input of easily degradable organic matter from upper layer of the water column (Boon and Duineveld 1998). A greater amount of sediment Chl a found in June at St. N than other sites may, therefore, indicate downward flux of potential food resource to benthic fauna was transient, but larger at St. N. A report on the aerosol particles (> 0.3 microns) transported from Eurasia to Nagasaki atmosphere (Takatsuji et al. 2017) suggests that amount of aerosols increased during summer in 2017. This may help explain the apparent increase in the sediment grain size found from June through October in Omura Bay.
A significant difference in the overall mean of nematode abundance between St. N and other sampling sites (Fig. 4) suggests that the extent to which hypoxic condition restricts population growth of nematodes varies considerably along the north and south axis of Omura Bay. Significant differences in the nematode community structure based on the genus-level composition among the sampling sites (Fig. 5, Table 2) further suggest that the variations of DO and food availability along the axis play important roles in shaping the community compositions and the trophic diversity. In the previous report (Nguyen et al. 2018), neither Chromadorina, nor Pseudolella was shown as a major group of nematodes at St. C regardless of the sampling months during the 3 consecutive years (2013–2015), except that Axonolaimus remained in the higher rank making up of more than 22% of the nematode population (Nguyen et al. 2018). The differences in the dominant nematode composition between 2017 and 2013–2015 at St. C, therefore, suggest that the faunal replacement at genus level had stochastically occurred after 2015. The results also supported the previous notion that Axonolaimus are well adapted to low oxygen stress in Omura Bay.
In contrast to the significant differences between the north and southeast communities, there were no significant differences between the center (St. C) and southwest (St. SW) communities. At these sites, toothless nematodes (types 1A and 1B) predominated in hypoxic conditions, while the nematodes with teeth (types 2A and 2B) prevailed in normoxic conditions (Fig. 8). The very similar patterns on trophic diversity between St. C and SW suggests a gradient of environmental parameters were negligible between the two sites. Even though a two-way ANOSIM failed to detect significant differences in nematode feeding types between sampling months (Fig. 7), a horizontal gradient of nematode trophic diversity was obvious across the north and south axis of the bay (Fig. 8).
According to the results of BIOENV analysis (Table 3), it was clearly demonstrated that the gradient of organic matter content across the basin has profound impacts on the horizontal pattern of genus-level community composition. As the LOI varied more significantly among sampling sites than sampling months (Fig. 3), it should represent the bulk of organic matter that is built up by long-term processes of sedimentation and degradation throughout the year. In contrast, a single abiotic variable did not provide a very successful match with the ordination pattern of the feeding type. However, the combination of salinity and DO yielded much better correlation than any other 2-variable subset. In the previous study, it was noted that seasonal decline in DO availability would select for toothless nematodes (1A, 1B types) during the stratified period of Omura Bay (Nguyen et al. 2018). However, the present results strongly suggest that horizontal gradient of DO conditions also play important roles in determining the extent of relative contribution of the feeding types in nematode community. Salinity may impact the nematode community structure through tolerance or preference to fluctuating salinity (Platt 1977). Limitation to dispersion of nematode (Broman et al. 2018) may also be taken into consideration in our study sites.
Our results support the previous finding that gradients of organic matter influenced nematode community structure (Adão et al. 2009; Warwick 1971). Furthermore, our study provided the first insight into how strongly the habitat heterogeneity in terms of seasonal DO availability, changes in salinity and persistent food availability, which varies along with the unique geomorphological characteristics of enclosed bay, affects the diversity and the structures of nematode assemblages. These findings will help us to understand how meiofaunal community response to present-day environmental conditions in coastal areas and make better predictions about future responses to environmental disturbances caused by recent climate change.
References
Adão H, Alves AS, Patrício J, Neto JM, Costa MJ, Marques JC (2009) Spatial distribution of subtidal Nematoda communities along the salinity gradient in southern European estuaries. Acta Oecologica 35(2):287–300
Boon AR, Duineveld GCA (1998) Chlorophyll a as a marker for bioturbation and carbon flux in southern and central North Sea sediments. Mar Ecol Prog Ser 162:33–43
Breitburg D, Levin LA et al. (2018) Declining oxygen in the global ocean and coastal waters. Science 359(6371)
Broman E, Raymond C, Sommer C, Gunnarsson JS, Creer S, Nascimento FJA (2018) Salinity drives meiofaunal community structure dynamics across the Baltic ecosystem. Mol Ecol 28(16):3813–3829
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. Primer-E, Plymouth
Diaz RJ, Rosenberg R (2008) Spreading dead zones and consequences for marine ecosystems. Science 321(5891):926–929
Fukumoto T, Kobayashi N (2005) Bottom stratification and water exchange in enclosed bay with narrow entrance. J Coast Res 21:135–145
Giere O (2009) Meiobenthology: the microscopic motile fauna of aquatic sediments. Springer, Berlin
Gooday AJ, Bett BJ et al (2010) Habitat heterogeneity and its influence on benthic biodiversity in oxygen minimum zones. Mar Ecol 31(1):125–147
Higgins RP, Thiel H (1988) Introduction to the study of meiofauna. Smithsonian Institution Press, Washington DC, pp 80–81
Iizuka S, Min SH (1989) Formation of anoxic bottom waters in Omura Bay. J Oceanogr Soc Jpn 26:75–86 (in Japanese)
Jessen GL, Lichtschlag A et al (2017) Hypoxia causes preservation of labile organic matter and changes seafloor microbial community composition (Black Sea). Sci Adv 3(e1601897):1–14
Kovalenko KE, Thomaz SM, Warfe DM (2012) Habitat complexity: approaches and future directions. Hydrobiologia 685(1):1–17
Kruskal WH, Wallis WA (1952) Use of ranks in one-criterion variance analysis. J Am Stat Assoc 47(260):583–621
Lambshead PJD (1986) Sub-catastrophic sewage and industrial waste contamination as revealed by marine nematode faunal analysis. Mar Ecol Prog Ser 29(29):247–260
Levin LA (2003) Oxygen minimum zone benthos: adaptation and community response to hypoxia. Oceanogr Mar Biol Annu Rev 41:1–45
Levin LA, Huggett CL, Wishner KF (1991) Control of deep-sea benthic community structure by oxygen and organic-matter gradients in the eastern Pacific Ocean. J Mar Res 49(4):763–800
Levin LA, Sibuet M, Gooday AJ, Smith CR, Vanreusel A (2010) The roles of habitat heterogeneity in generating and maintaining biodiversity on continental margins: an introduction. Mar Ecol 31(1):1–5
Mori F, Umezawa Y, Kondo R, Wada M (2018) Effects of bottom-water hypoxia on sediment bacterial community composition in a seasonally hypoxic enclosed bay (Omura Bay, West Kyushu, Japan). FEMS Microbiol Ecol 94(5):1–14
Neira C, King I, Mendoza G, Sellanes J, De Ley P, and Levin LA (2013) Nematode community structure along a central Chile margin transect influenced by the oxygen minimum zone
Nguyen QTD, Ueda R, Mori F, Kang T, Kim D, Shimanaga M, Wada M (2018) Response of nematode community structure to hypoxia in an enclosed coastal sea, Omura Bay, for three consecutive years. Plankton Benthos Res 13(2):59–65
Platt HM (1977) Vertical and horizontal distribution of free-living marine nematodes from Strangford Lough, Northern Ireland. Cahiers de Biologie Mar 18(1959):261–273
Schmidt-Rhaesa A (2013) Gastrotricha, Cycloneuralia and Gnathifera, Nematoda, vol 2. De Gruyter, Berlin, pp 128–140
Setoguchi Y, Nomaki H, Kitahashi T, Watanabe H, Inoue K, Ogawa NO, Shimanaga M (2014) Nematode community composition in hydrothermal vent and adjacent non-vent fields around Myojin Knoll, a seamount on the Izu-Ogasawara Arc in the western North Pacific Ocean. Mar Biol 161(8):1775–1785
Spurrier JD (2006) Additional tables for Steel-Dwass-Critchlow-Fligner distribution-free multiple comparisons of three treatments. Commun Stat Simul Comput 35(2):441–446
Stein A, Gerstner K, Kreft H (2014) Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol Lett 17(7):866–880
Takahashi T, Nakata H, Hirano K, Matsuoka K, Iwataki M, Yamaguchi H, Kasuya T (2009) Upwelling of oxygen-depleted water (Sumishio) in Omura Bay, Japan. J Oceanogr 65:113–120
Takatsuji T, Nakashima T, Furui K, and Sera K (2017) Relations among Stable and Radioactive Elements Contained in Aerosol and Trajectories of the Nagasaki Atmosphere from 2013 to 2017. NMCC Annual Report 24
Traunspurger W, Majdi N (2017) Meiofauna. Methods in Stream. Ecology 1:273–295
Vereș DS (2002) A comparative study between loss on ignition and total carbon analysis on mineralogenic sediments. Studia UBB Geologia 47(1):171–182
Wada M, Suzuki S, Nara T, Umezawa Y, Shimanaga M, Matsuoka K, Nakata H (2012) Microbial community respiration and structure of dead zone sediments of Omura Bay, Japan. J Oceanogr 68(6):857–867
Warwick RM (1971) Nematode associations in the exe estuary. J Mar Biol Assoc UK 51(2):439–454
Warwick RM, Platt HM, and Somerfield PJ (1998a) Free-living Marine Nematodes (Part 2: British Chromadorids) - FSC Field Studies Council, Shrewsbury
Warwick RM, Platt HM, and Somerfield PJ (1998b) Free-living Ma- rine Nematodes (Part 3: Monhysterids) - FSC Field Studies Council, Shrewsbury
Yokoyama H (1995) Macrobenthic assemblages in Omura Bay I: community parameters versus bottom environmental factors (in Japanese). Bull Natl Res Inst Aquacult 24:43–53 (in Japanese with English abstract)
Acknowledgements
This study was partially supported by JSPS KAKENHI (Grant numbers, JP17H03854 and JP18K19236), MEXT, Japan. We thank the help provided by Dr. S. Takeuchi and Dr. F. Mori with the grain size analyses. We would like to thank Dr. S. Takeda for valuable advice on Chlorophyll a concentration measurement. Finally, the authors appreciate the editor and anonymous reviewers whose valuable comments improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10872_2020_558_MOESM1_ESM.pptx
Supplementary material 1 (PPTX 84 kb) Fig. S1 Shannon and Simpson indexes of nematode diversity at the four sampling sites of Omura Bay between June and October in 2017. Hypoxic months were underlined to separate from normoxic ones
Rights and permissions
About this article
Cite this article
Nguyen, Q.T.D., Kim, D., Shimanaga, M. et al. Horizontal distribution of nematode communities in a seasonally-hypoxic enclosed sea (Omura Bay, Japan). J Oceanogr 76, 479–489 (2020). https://doi.org/10.1007/s10872-020-00558-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10872-020-00558-2