Abstract
The plasticity in the trophic ecology, the breeding phenology, and the low nesting site fidelity would be the reasons why most of the gentoo penguin (Pygoscelis papua) populations breeding in the western Antarctic Peninsula and islands of Scotia Arc remained stable or increasing, despite the changing environmental conditions. Regardless of the overall trend, recent studies revealed a decline in the abundance of some gentoo penguin populations at those regions. Therefore, it is highly valuable to update the population size of each colony in order to confirm the current trends of individual colonies and generate a comprehensive overview of the population status. We report the abundance and breeding performance of gentoo penguins at the Stranger Point/Cabo Funes colony from 2000/2001 to 2018/2019. During the last season, 5383 breeding pairs and 5545 chicks in crèche were counted. Despite there were considerable inter-annual fluctuations over the study period, the total number of gentoo penguin breeding pairs increased by 74.6% (+ 3.1% per annum), while the number of chicks crèched increased by 60.0% (+ 2.6% per annum). However, the index of breeding success remained relatively constant over time, varying between 0.74 and 1.23 chicks in crèche/breeding pairs. Gentoo penguins have life-history strategies that are advantageous to face the environmental variability, allowing the species to maintain their breeding performance stable over time and enhance their resilience, which can favour the population growth at Stranger Point.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The western Antarctic Peninsula (WAP) and Scotia Arc regions have been experiencing a marked environmental variability, driven by a rapid climate warming and consequent sea-ice loss (Kerr et al. 2018 and references therein; but see Turner et al. 2016). This variability in marine and terrestrial conditions impacts, direct or indirectly, on population dynamics of predators such as pygoscelid penguins (Fraser et al. 1992, 2013; Forcada et al. 2006; Hinke et al. 2007, 2012; Trathan et al. 2007; Trivelpiece et al. 2011; Lynch et al. 2012a; Juáres et al. 2015). However, the biological responses of these predators will be species-specific according to their life-history strategies, and to regional and local conditions (e.g. Forcada et al. 2006; Hinke et al. 2007; Trathan et al. 2007; Carlini et al. 2009; Lynch et al. 2010, 2012a; Trivelpiece et al. 2011; Fraser et al. 2013; Casanovas et al. 2015; Juáres et al. 2015; Dunn et al. 2016, 2019).
Changes in the abundance and breeding performance of penguins can be used as bioindicators of shifts in their ecosystem (CCAMLR 2014). Gentoo penguin (Pygoscelis papua) populations have remained stable or increased in abundance throughout the WAP and islands of Scotia Arc (e.g. Carlini et al. 2009; Lynch et al. 2012a; González-Zevallos et al. 2013; Korczak-Abshire et al. 2013; Casanovas et al. 2015; Dunn et al. 2016; Braun et al. 2017; Hinke et al. 2017). This less ice-adapted penguin species exhibits an ecological plasticity that can buffer the potential impact of ecosystem changes. The greater flexibility in their feeding and breeding behaviour as well as a low nesting site fidelity contributes to maintain relatively constant their breeding success despite the adverse effects of environmental variability on their main prey—the Antarctic krill (Euphausia superba)—and on local conditions in the breeding site, such as the increase in the frequency of years with heavy snowfall (Miller et al. 2009; Hinke et al. 2012; Lynch et al. 2012b; Juáres et al. 2013). Nevertheless, more recent studies have shown a decline in the abundance of some gentoo penguin populations in this region both in colonies exposed and not exposed to human disturbance (Petry et al. 2018; Dunn et al. 2019). Therefore, it is valuable to update the population size of each monitored colony in order to confirm the current trends. Additionally, the update of the population size of gentoo penguins contributes to current estimates of the total predator populations in the region, which is crucial for the management and conservation of Antarctic marine living resources (Santos et al. 2018).
For the Stranger Point/Cabo Funes colony, the available information on the population size and breeding performance of gentoo penguins is outdated by a decade (see Carlini et al. 2009). In order to update information on the abundance and the breeding performance of gentoo penguins nesting at Stranger Point, and to assess whether the population trajectory previously reported have persisted over time, we examined the direction and magnitude of changes in the number of breeding pairs and chicks in crèche from ground counts carried out between 2000/2001 and 2018/2019. Additionally, we evaluated the long-term changes over a 54-year period from historical records of the number of breeding pairs reported by this colony (Aguirre 1995).
Materials and methods
Study area
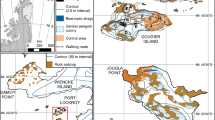
Fieldwork was carried out at Stranger Point/Cabo Funes, (62°16′ S, 58°37′ W. 25 de Mayo/King George Island, South Shetland Islands, Antarctica; Fig. 1) during 19 consecutive breeding seasons (i.e. from 2000/2001 to 2018/2019).
This research is part of a long-term monitoring of gentoo penguins conducted by the Argentine Ecosystem Monitoring Program (Argentine Antarctic Institute). Data were collected, whenever possible, according to the standard protocols defined by the Ecosystem Monitoring Program (CEMP) of the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR 2014).
Breeding population size and chicks crèched
In this study, a breeding group was defined as an assemblage of birds nesting as a geographically continuous unit within a colony (Carlini et al. 2009). Annually, all breeding groups of gentoo penguins were surveyed from direct ground counts. The total number of occupied nests (i.e. the sum of the nests with eggs and pairs occupying an empty nest) was counted approximately 1 week after the peak of egg-laying (typically from October 28 to December 14), while the total number of chicks crèched was counted when at least two-thirds of the chicks had entered crèche (typically from December 30 to January 18). Due to the continual arrival of gentoo penguin pairs at the breeding site that compensates the loss of nests, the penguin count will remain flat for a wider period, i.e. the time interval in which the censuses can be carried out is broader and may be less critical than in their congeners (Lynch et al. 2009).
In both censuses, three counts were made on each breeding group and the average values were calculated. From the sum of the average values recorded in each breeding groups, we estimated the breeding population size (except in 2003/2004 and 2007/2008) from all the occupied nests (i.e. all breeding pairs) and the productivity using the total number of chicks that survived until crèche stage. Additionally, considering information on previous censuses (taken from Aguirre 1995, Table 1), the overall fluctuations in the number of breeding pairs were analysed over a broader period (54 years; Table 1). Carlini et al. (2009) reported the population size of gentoo penguins from 1995/1996 to 2006/2007; however, those authors only considered the number of nest with eggs. In order to avoid an overestimation of the population increase, these data were not included.
Following Dunn et al. (2019), we analysed the direction and magnitude of population changes. The percentage annual change in the total number of breeding pairs and in the number of chicks reared to crèche was calculated as
where PS is the population size or chicks crèched in season s.
We calculated the annual intrinsic rate of population changes:
where lnPS is the natural logarithm of the population size or chicks crèched in season s and T is the time interval between counts.
Furthermore, the coefficient of variation (CV) of the abundance was calculated to evaluate its temporal fluctuation.
Index of breeding success
The index of breeding success (IBS) was calculated as total number of chicks in crèche/total number of occupied nests (CCAMLR 2014). This index was estimated for each season when both parameters were available.
Simple linear regressions of counts against time were used to visualize the general trend in the number of breeding pairs, the number of chicks in crèche, and the index of breeding success.
Results
The total number of gentoo penguin breeding pairs present on Stranger Point increased by 74.6% between 2000/2001 (3083 pairs) and 2018/2019 (5383 pairs) at an annual rate of + 3.1% (R2 = 0.69; Fig. 2). However, inter-annual fluctuations in the number of breeding pairs were evident (Fig. 2; Online Resource 1). Overall, the breeding population increased by 40.2% (+ 4.2% per annum) between 2000/2001 and 2008/2009, decreased 26.1% in the 2009/2010 season, and increased by 68.6% (+ 5.8% per annum) between 2009/2010 and 2018/2019. The number of breeding pairs counted in 2000/2001 and 2009/2010 seasons represented the lowest values recorded throughout our study period (i.e. 3083 and 3192 nests, respectively).
The number of chicks in crèche increased by 60.0% between 2000/2001 (3466 chicks) and 2018/2019 (5545 chicks) at an annual rate of + 2.6% (R2 = 0.48; Fig. 2). In comparison with the number of breeding pairs, the total number of chicks showed a higher inter-annual variability (Fig. 2; CV = 16.8 vs. 23.6 for breeding pairs and chicks crèched, respectively). The number of chicks in crèche observed during the 2002/2003 and 2009/2010 breeding seasons represented the lowest values recorded throughout our study period (i.e. 2780 and 2652 chicks, respectively).
From 2000/2001 to 2018/2019, the mean IBS was 0.93 ± 0.14 (range = 0.74–1.23), which represents an average breeding performance of 46.6% (range = 36.8–61.4%). The IBS estimated for the 2002/2003 and 2014/2015 were the lowest of the study period (0.74 and 0.77, respectively). Nevertheless, the reproductive performance remained relatively stable through the study period (R2 = 0.007; Fig. 3).
Breeding population size over a broader period
Between 1965/1966 and 2018/2019, the total number of breeding pairs of gentoo penguins increased by 84.3% (+ 1.2% per annum). However, the population experienced periods of decline and increase over these 54 years. The number of breeding pairs decreased by 20.4% (− 1.0% per annum) between 1965/1966 and 1988/1989 (Table 1), increased by 32.6% (+ 2.4% per annum) from 1988/1989 to 2000/2001 and since then, the population increased at a faster rate of change per year (i.e. + 3.1%).
In 1988/1989, the gentoo penguin colony consisted of 2325 pairs settled in 57 breeding groups (Aguirre 1995). After 30 years, we recorded 5383 pairs breeding in 69 groups varying in size from 7 to 365 nests. During our study period, there was colonization of new breeding areas and re-occupation of sites previously abandoned (see Juáres et al. 2013).
Discussion
Given that some gentoo penguin populations are undergoing local declines both in colonies exposed and not exposed to human disturbance (Chwedorzewska and Korczak 2010; Petry et al. 2018; Dunn et al. 2019), there is a growing interest in updating the breeding population size and describe the population trajectory of individual colonies in order to understand the mechanisms underlying such changes and the large-scale processes (e.g. Dunn et al. 2016, 2019; Petry et al. 2018). Here, we report the present status of the abundance and breeding performance of gentoo penguins nesting at Stranger Point, 25 de Mayo/King George Island. During the 2018/2019 season, 5383 breeding pairs and 5545 chicks in crèche of gentoo penguins were counted. Despite the inter-annual variability observed, our results reveal an increasing trend of more than 60% in the abundance of gentoo penguins between 2000/2001 and 2018/2019, while the breeding performance remained relatively constant over time (Fig. 3). The IBS (chicks crèched/breeding pairs) observed at Stranger Point fluctuated between 0.74 (in 2002/2003) and 1.23 (in 2005/2006). Not surprisingly our values fell within the range of IBS observed in other colonies which exhibited an increase (e.g. Signy Island: 0.05–1.27 chicks crèched/pairs; Dunn et al. 2016) or even a decline (e.g. Goudier Island, WAP: 0.2–1.4 chicks crèched/pairs; Dunn et al. 2019) in their population sizes. Nevertheless, years of extremely low breeding performance have not been observed at Stranger Point.
Although this population has undergone a phase of decline since the mid-1960s, a similar pattern of the long-term population trajectories was recorded by Korczak-Abshire et al. (2013) and Sierakowski et al. (2017) in gentoo colonies also located in 25 de Mayo/King George Island. Nevertheless, different trajectories in the long-term trends were observed in other colonies, such as Signy Island (South Orkney Islands; Dunn et al. 2016) and Danco Coast (WAP; Quintana et al. 2000) whose populations showed an increase of gentoo breeding pairs since the 1960s.
In a broad scale, there are differences among colonies and years in the direction and/or magnitude of long-term population changes (e.g. Humphries et al. 2017) which difficult the identification of the factors driving such trends. Evidences suggest that fluctuations in pygoscelid penguin populations are related to changes in suitable habitats, prey availability, and to the potential impact of human activities such as tourism and krill fishery (e.g. Forcada et al. 2006; Ainley et al. 2007; Trathan et al. 2007, 2008; Trivelpiece et al. 2011; Lynch et al. 2012a; Juáres et al. 2013; Korczak-Abshire et al. 2013; Casanovas et al. 2015; Dunn et al. 2016, 2019). However, the regional complexity also highlights the importance of considering how local conditions impact on the population dynamics (e.g. Trathan et al. 2008; Casanovas et al. 2015; Dunn et al. 2019).
According to the “krill surplus” hypothesis, krill-dependent populations grew during the 1970s in response to an increase in the food availability due to the over-exploitation of whales (Ainley et al. 2007). However, at Stranger Point a declining population trend was observed between 1965/1966 and 1988/1989. Despite the limitations and potential biases of the early counts, this negative trend is in line with those previously reported in near breeding colonies (Korczak-Abshire et al. 2013; Sierakowski et al. 2017). Since the late 1980s, gentoo penguin populations increased in many places of this region (Chwedorzewska and Korczak 2010; González-Zevallos et al. 2013; Korczak-Abshire et al. 2013; Casanovas et al. 2015; Dunn et al. 2016; Braun et al. 2017). In a scenario of shifts in krill availability—related to ice loss and changes in primary productivity driven by climate warming (e.g. see Kerr et al. 2018)—along with the recovery of whale populations (that increase competition for krill), a decline in gentoo populations would be expected as in their congeners (Trivelpiece et al. 2011; Lynch et al. 2012a). Thus, the positive trajectories observed in gentoo penguins appear to be determined by their life-history strategies in face of the influence of environmental variability. Gentoo penguins are ice-avoiding species; therefore, the availability of their breeding and foraging habitats can increase due to the reduction of sea ice (Forcada et al. 2006; Trathan et al. 2007; Lynch et al. 2012a). Furthermore, the plasticity in their feeding strategies allows them to withstand shifts in the abundance and availability of Antarctic krill (Miller et al. 2009) and by being inshore-foraging predators they could be less affected by the recovery of whales (Lynch et al. 2012a).
On the other hand, breeding gentoo penguins are constrained by nest attendance and the foraging distance (e.g. Hinke et al. 2017). Thus, both krill fishery and tourism—which have increased in recent years—could negatively impact on penguin populations through competition for the same prey resource or the high disturbance generated at the breeding site and/or at the feeding areas (Hinke et al. 2017; Dunn et al. 2019). In the Stranger Point colony, the potential interaction between gentoo penguins and krill fishery has not yet been evaluated (although, up to now, the breeding performance does not seem to be affected) and the human disturbance can be considered minimal given that this colony is located within a protected area (Fig. 1) and does not receive tourists.
Furthermore, unusual weather conditions can be reflected in the inter-annual fluctuations of abundance and breeding performance of gentoo penguins, as recorded in other annually monitored colonies that also experienced an overall increase (Lynch et al. 2010; Dunn et al. 2016). Snowfall can impact on the breeding population size and/or breeding performance (Trathan et al. 2008; Lynch et al. 2010; Hinke et al. 2012). At Stranger Point, a high accumulation and persistence of snow was observed during the austral summer of 2009/2010 and 2012/2013 (Juáres et al. 2013, 2015). Although a low number of breeding pairs and chicks in crèche were recorded in 2009/2010, the breeding performance of gentoo penguins remained relatively constant and the number of breeding pairs did recover in the following season. During seasons of heavy snowfall, several breeding pairs moved to previously unoccupied higher places (Juáres et al. 2013; unpublished data). Thus, the plasticity in the breeding phenology (specifically in the timing of egg-laying) and the relocation of breeding groups allow gentoo penguins to cope with this local variability maintaining a relatively constant breeding success (Juáres et al. 2013) and consequently, reflecting the high resilience of this species.
In the last decades, the WAP and Scotia Sea areas have experienced an increase both in environmental variability and in human activities (i.e. tourism and krill fishing). These factors, alone or combined, may have profound implications on population dynamics of pygoscelid penguins. Thus, all updated information will help to improve our understanding on ecological processes underlying the changes in penguin populations observed in this region.
References
Aguirre CA (1995) Distribution and abundance of birds at Potter Peninsula, 25 de Mayo (King George) Island, South Shetland Islands, Antarctica. Mar Ornithol 23:23–31
Ainley D, Ballard G, Ackley S, Blight LK, Eastman JT, Emslie ED, Lescroël A, Olmastroni S, Townsend SE, Tynan CT, Wilson P, Woehler E (2007) Paradigm lost, or is top-down forcing no longer significant in the Antarctic marine ecosystem? Antarct Sci 19:283–290
Braun C, Esefeld J, Peter HU (2017) Monitoring the consequences of local climate change on the natural resources of the ice-free regions of Maxwell Bay (King George Island, Antarctic). Umweltbundesamt. Texte 26/2017. Dessau-Roßlau, Germany
Carlini AR, Coria NR, Santos MM, Negrete J, Juáres MA, Daneri GA (2009) Responses of Pygoscelis adeliae and P. papua populations to environmental changes at Isla 25 de Mayo (King George Island). Polar Biol 32:1427–1433
Casanovas P, Naveen R, Forrest S, Poncet J, Lynch HJ (2015) A comprehensive coastal seabird survey maps out the front lines of ecological change on the western Antarctic Peninsula. Polar Biol 38:927–940
CCAMLR (2014) CCAMLR ecosystem monitoring program standard methods. Commission for the Conservation of Antarctic Marine Living Resources, Hobart
Chwedorzewska KJ, Korczak M (2010) Human impact upon the environment in the vicinity of Arctowski Station, King George Island, Antarctica. Pol Polar Res 31(1):45–60
Croxall JP, Kirkwood ED (1979) The distribution of penguins on the Antarctic Peninsula and Islands of the Scotia Sea. Life Science Division, British Antarctic Survey, Cambridge, p 186
Dunn MJ, Jackson JA, Adlard S, Lynnes AS, Briggs DR, Fox D, Waluda CM (2016) Population size and decadal trends of three penguin species nesting at Signy Island, South Orkney Islands. PLoS ONE 11(10):e0164025. https://doi.org/10.1371/journal.pone.0164025
Dunn MJ, Forcada J, Jackson JA, Waluda CM, Nichol C, Trathan PN (2019) A long-term study of gentoo penguins (Pygoscelis papua) population trends at a major Antarctic tourist site, Goudier Island, Port Lockroy. Biodivers Conserv 28:37–53
Forcada J, Trathan PN, Reid K, Murphy EJ, Croxall JP (2006) Contrasting population changes in sympatric penguin species in association with climate warming. Glob Change Biol 12:411–423
Fraser WR, Trivelpiece WZ, Ainley D, Trivelpiece SG (1992) Increases in Antarctic penguin populations: reduced competition with whales or a loss of ice due to environmental warning? Polar Biol 11:525–531
Fraser WR, Patterson-Fraser DL, Ribic CA, Schofield O, Ducklow H (2013) A nonmarine source of variability in Adélie penguin demography. Oceanography 26:207–209
González-Zevallos D, Santos MM, Rombolá EF, Juáres MA, Coria NR (2013) Abundance and breeding distribution of seabirds in the northern part of the Danco Coast, Antarctic Peninsula. Polar Res 32:11133. https://doi.org/10.3402/polar.v32i0.11133
Hinke JT, Salwicka K, Trivelpiece SG, Watters GM, Trivelpiece WZ (2007) Divergent responses in Pygoscelis penguins reveal a common environmental driver. Oecologia 153:845–855
Hinke JT, Polito MJ, Reiss CS, Trivelpiece SG, Trivelpiece WZ (2012) Flexible reproductive timing can buffer reproductive success of Pygoscelis spp. Penguins in the Antarctic Peninsula region. Mar Ecol Prog Ser 454:91–104
Hinke JT, Cossio AM, Goebel ME, Reiss C, Trivelpiece WZ, Watters GM (2017) Identifying risk: concurrent overlap of the Antarctic krill fishery with krill-dependent predators in the Scotia Sea. PLoS ONE 12(1):e0170132. https://doi.org/10.1371/journal.pone.0170132
Humphries GRW, Che-Castaldo C, Naveen R, Schwaller M, McDowall P, Schrimpf M, Lynch HJ (2017) Mapping application for penguin populations and projected dynamics. www.penguinmap.com. Accessed 23 Apr 2019
Jabłoński B (1984) Distribution and numbers of penguins in the region of Admiralty Bay (King George Island, South Shetland Islands) in the breeding season 1980/1981. Pol Polar Res 5:17–30
Juáres MA, Santos MM, Negrete J, Santos MR, Mennucci JA, Rombolá E, Longarzo L, Coria NR, Carlini AR (2013) Better late than never? Interannual and seasonal variability in breeding chronology of gentoo penguins at Stranger Point, Antarctica. Polar Res 32:18448. https://doi.org/10.3402/polar.v32i0.18448
Juáres MA, Santos M, Negrete J, Mennucci JA, Perchivale PJ, Casaux R, Coria NR (2015) Adélie penguin population changes at stranger point: 19 years of monitoring. Antarct Sci 27(5):455–461
Kerr R, Mata MM, Mendes CRB, Secchi ER (2018) Northern Antarctic Peninsula: a marine climate hotspot of rapid changes on ecosystems and ocean dynamics. Deep-Sea Res II 149:4–9
Korczak-Abshire M, Wegrzyn M, Angiel PJ, Lisowska M (2013) Pygoscelid penguins breeding distribution and population trends at Lions Rump rookery, King George Island. Pol Polar Res 34:87–99
Lynch HJ, Fagan WF, Naveen R (2010) Population trends and reproductive success at a frequently visited penguin colony on the western Antarctic Peninsula. Polar Biol 33:493–503
Lynch HJ, Fagan WF, Naveen R, Trivelpiece SG, Trivelpiece WZ (2009) Timing of clutch initiation in Pygoscelis penguins on the Antarctic Peninsula: towards an improved understanding of off-peak census correction factors. CCAMLR Sci 16:149–165
Lynch HJ, Naveen R, Trathan PN, Fagan WF (2012a) Spatially integrated assessment reveals widespread changes in penguin populations on the Antarctic Peninsula. Ecology 93:1367–1377
Lynch HJ, Fagan WF, Naveen R, Trivelpiece SG, Trivelpiece WZ (2012b) Differential advancement of breeding phenology in response to climate may alter staggered breeding among sympatric Pygoscelid penguins. Mar Ecol Prog Ser 454:135–145
Miller AK, Karnovsky NJ, Trivelpiece WZ (2009) Flexible foraging strategies of gentoo penguins Pygoscelis papua over 5 years in the South Shetland Islands, Antarctica. Mar Biol 156:2527–2537
Petry MV, Valls FCL, Petersen ES, Finger JVG, Krüger L (2018) Population trends of seabirds at Stinker Point, Elephant Island, Maritime Antarctica. Antarct Sci 30:220–226
Quintana RD, Cirelli V, Orgeira JL (2000) Abundance and spatial distribution of bird populations at Cierva Point, Antarctic Peninsula. Mar Biol 28:21–27
Santos MM, Hinke JT, Coria NR, Fusaro B, Silvestro A, Juáres MA (2018) Abundance estimation of Adélie penguins at the Esperanza/Hope Bay mega colony. Polar Biol 41:2337–2342
Sierakowski K, Korczak-Abshire M, Jadwiszczak P (2017) Changes in bird communities of Admiralty Bay, King George Island (West Antarctic): insights from monitoring data (1977–1996). Pol Polar Res 38(2):231–262
Trathan PN, Forcada J, Murphy EJ (2007) Environmental forcing and Southern Ocean marine predator populations: effects of climate change and variability. Phil Trans R Soc B 362:2351–2365
Trathan PN, Forcada J, Atkinson R, Downie RH, Shears JR (2008) Population assessments of gentoo penguins (Pygoscelis papua) breeding at an important Antarctic tourist site, Goudier Island, Port Lockroy, Palmer Archipelago, Antarctica. Biol Conserv 141:3019–3028
Trivelpiece WZ, Hinke JT, Miller AK, Reiss CS, Trivelpiece SG, Watters GM (2011) Variability in krill biomass links harvesting and climate warming to penguin population changes in Antarctica. Proc Natl Acad Sci 108:7625–7628
Turner J, Lu H, White I, King JC, Phillips T, Hosking JS, Bracegirdle TJ, Marshall GJ, Mulvaney R, Pranab D (2016) Absence of 21st century warming on Antarctic Peninsula consistent with natural variability. Nature 535:411–415
Acknowledgements
We thank all field workers involved in the penguin monitoring. The permit for this work was granted by the Dirección Nacional del Antártico (Environmental Office). The FONCYT—Agencia Nacional de Promoción Científica y Tecnológica (Grant: PICTO 2010-0111) and the Instituto Antártico Argentino—Dirección Nacional del Antártico (PINST-05) provided financial and logistical support. We also thank Dr Maria Virginia Petry, two anonymous reviewers and editors for their helpful comments and suggestions on improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
All research involving gentoo penguins was conducted under appropriate international, national and/or institutional guidelines for the care and use of animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Juáres, M.A., Casaux, R., Negrete, J. et al. Update of the population size and breeding performance of gentoo penguins (Pygoscelis papua) at Stranger Point/Cabo Funes, South Shetland Islands. Polar Biol 43, 123–129 (2020). https://doi.org/10.1007/s00300-019-02614-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-019-02614-0