Abstract
As part of a monitoring study of Adélie and Gentoo penguin colonies, birds occupying nests with eggs and chicks in crèches were counted annually from the 1995/1996 to the 2006/2007 seasons at Stranger Point, Isla 25 de Mayo (King George Island), Antarctica. During the study period the Adélie penguin population showed a decrease of 62%. The number of chicks in crèches followed a similar trend, the smallest number occurring in 2002, when it was 63% lower than in 1995/1996. In contrast, the Gentoo breeding population size increased by 68%, while chicks produced increased by 63%. Despite the opposing trends in population size between species, there was a positive relation in their interannual variation, although the extent, and for some years the direction, of the change observed always favoured Gentoo penguins. Breeding success (chicks in crèches/nests with eggs) fluctuated between 0.65 and 1.26 for Adélies and between 0.76 and 1.27 for Gentoo penguins, and did not differ significantly between species. The similar breeding success of these species suggests that the contrasting population trends observed were driven by factors operating over winter. We suggest that current changes in environmental conditions may affect adult birds of both species during the previous winter with different intensity but in a roughly similar way, but that juvenile survival of both species and thus the recruitment of new breeders might be affected differentially, with a much lower survival rate of juvenile Adélie penguins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Changes in population size are indicative of the quality of the environment on which a population relies for food and are also important for understanding and predicting the effects of environmental change (Croxall et al. 2002). There is growing evidence that climate development in the Antarctic Peninsula region (APR) has moved from a relatively cold regime to an increasingly warm regime during the past 100 years (Smith et al. 1999; Hughes 2000; Ingolfsson et al. 2003). Air temperature records for the area show an increase of 2–3°C over the past 50 years (King 1994; Smith et al. 1996) and the retreat of a number of ice shelves and glaciers with increasing speed since the 1980s (Skvarca et al. 1999). There is general consensus that the recent changes in regional climate are already affecting species distribution and phenology (Hughes 2000). In the APR, a decreased frequency of cold years, with the associated reduction of extensive sea ice (Fraser et al. 1992) and its effects on krill abundance, have been observed (Siegel and Loeb 1995; Loeb et al. 1997; Fraser and Hofmann 2003; Ducklow et al. 2007), and concurrent responses of penguin populations have been recorded (Fraser et al. 1992; Fraser and Trivelpiece 1996; Loeb et al. 1997; Fraser and Hofmann 2003; Sander et al. 2007a, b; Hinke et al. 2007). However, the ecological mechanisms underlying the observed changes and the links to climate warming are not totally clear (Croxall et al. 2002).
At Stranger Point, Isla 25 de Mayo (King George Island), two pygoscelid penguins, Pygoscelis adeliae and P. papua, breed sympatrically. Adélie penguins are the most widely distributed of the Antarctic penguins, showing a circumpolar distribution and the southernmost breeding range (Williams 1995). Gentoo penguins breed on sub-Antarctic islands and along the Antarctic Peninsula to approximately 65°S latitude (Williams 1995). Both species show marked differences in their affinity for sea ice during winter. Adélie penguins are obligate inhabitants of the pack ice, whereas the Gentoo penguin is a non-migratory species that winters close to their breeding sites (Trivelpiece et al. 1987).
In the present study, we compare the breeding population size and the breeding success of Adélie and Gentoo penguins breeding at Stranger Point, Isla 25 de Mayo, describe the variation in the population parameters over a 12-year period and discuss the potential causes of the observed results.
Materials and methods
Study area
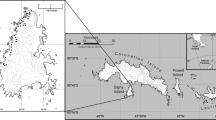
The present study was carried out at Stranger Point, Isla 25 de Mayo (King George) (62°16′S, 58°37′W) within the Antarctic Specially Protected Area No. 132 during 12 austral summers from 1995/96 to 2006/07. The area holds breeding groups (here defined as a group of penguins breeding as a geographically continuous unit located within a larger area) of Adélie and Gentoo penguins (Fig. 1).
Pygoscelis adeliae colony
In the 1995/1996 season the population of the colony consisted of 50 breeding groups ranging from 4 to more than 3,000 nests. Of these groups, 46 (the nests of which represented about half the total number of nests in the colony) were monitored in every season from 1995/1996 to 2006/2007. In 1995/1996, these 46 groups had fewer than 100 nests (n = 26), 100–200 nests (n = 15), 200–300 nests (n = 3) and 300–400 nests (n = 2) nests. The remaining four breeding groups are codified as “B” (Fig. 1) and were monitored in 1996/1997 and 2006/2007. In 1996/1997, they were composed of fewer than 100 nests (n = 1), 100–200 nests (n = 2) and more than 3,000 nests (n = 1).
Adélie breeding population size and chicks crèched
Penguins occupying nests with eggs and chicks crèched were counted in the 46 breeding groups on two occasions during the season as follows: 1 week after the peak of egg-laying (14–20 November) and when at least two-thirds of the chicks were in crèches (2–12 January). Nests with eggs were counted in the remaining four breeding groups in 1996/1997 and 2006/2007. Three separate counts were made for each breeding group according to standard CCAMLR Ecosystem Monitoring Program Methods (CCAMLR 2003) and the average values were recorded. We assume that the number of chicks in crèches represents the number of chicks that fledge, since once the crèche stage is reached chicks are able to cope with less frequent feeds and show low mortality rates (Davis and McCaffrey 1986; Clarke et al. 2002).
Pygoscelis papua colony
Breeding groups of P. papua are less conspicuous at Stranger Point than those of P. adeliae. Owing to that, counts were carried out for the colony as a whole.
Gentoo breeding population size and chicks crèched
For the P. papua colony, penguins occupying nests with eggs and chicks crèched were counted on two occasions during the season as follows: 1 week after peak egg-laying (11–23 November) and when at least two-thirds of the chicks were in crèches (12–17 January). Counts were made from the 1995/1996 to the 2006/2007 seasons (except for 1998/1999 and 1999/00). Chicks crèched were not counted during 1995/1996.
Breeding success
Breeding success for both species was estimated by measuring an index (IBS) (chicks crèched/nests with eggs) for each year when data for both parameters were available.
Interannual variation in population size
To compare the interannual variation in the breeding population size of both species we used the percentage annual change in the total number of breeding pairs (nests with eggs). The magnitude and direction (increasing or decreasing) is calculated relative to the preceding year’s census for each species.
Statistical analyses
Values are given as mean ± standard deviation (SD) and results were considered to be significant at P < 0.05 level. A comparative analysis of the decline observed was done between Adélie breeding groups (>200 versus <200 nests) using chi-square analysis after Yates’ correction. The strength of the association between pairs of variables was measured using Pearson product–moment correlations. We compared the IBS index using Student’s t tests. Finally, we used simple linear regressions to test for trends in the abundance of penguins.
Results
Breeding population size of Adélie penguins
The trend in breeding population size for the 46 breeding groups counted every year is shown in Fig. 2. The breeding population showed a decrease of about 15% between 1995/1996 and 1996/1997, increasing during 1997/1998 and 1998/1999 when it reached the 1995/1996 level. From 1998/1999 to 2005/2006, the breeding population showed a continuous decrease with a slight increase between 2005/2006 and 2006/2007. The decline in breeding Adélies was 73% throughout the study period (−369 pairs per year, F (1,10) = 133.1, P < 0.0001, R 2 = 0.93). From the original 46 breeding groups counted in 1995/1996, 24 had no breeding birds by 2006/2007 (Fig. 1).
Trends in breeding population size (nests with eggs) of Adélie (filled squares) and Gentoo penguins (cross) at Stranger Point, Isla 25 de Mayo from the 1995/1996 to the 2006/2007 seasons. Data for Adélie penguins come from the 46 breeding groups monitored every year while those for Gentoo penguins come from all the breeding rookery
The four breeding groups counted for the first time during 1996/1997 also showed a decrease in their breeding population size. As a whole, breeding groups denoted by “B” (Fig. 1) showed a decrease of 42% from 1996/1997 to 2006/2007 (3,570 versus 2,089 nests with eggs). For these groups, the number of breeding pairs can be estimated for the 1995/1996 season, assuming that the decline between the 1995/1996 and 1996/1997 seasons was similar to that observed in the 46 breeding groups actually counted in both seasons. If so, total numbers of nests with eggs for the “B” breeding groups during 1995/1996 would be 4,191 and the decrease for the study period (1995/1996 to 2006/2007) would be 49.9%. Based on the above assumption, the overall decline in the population size for the 50 breeding groups would be 62.4% (9,087 versus 3,412 nests with eggs) between 1995/1996 and 2006/2007.
Considering the size of the 50 breeding groups in 1995/1996, the overall decline (1995/1996–2006/2007) in breeding groups of up to 200 breeding pairs (69%) was significantly higher than that observed in the breeding groups of more than 200 breeding pairs (58%) (χ 2 = 118.0, P < 0.0001).
Breeding population size of Gentoo penguins
The breeding population showed a slight decrease between 1995/1996 and 1996/1997. Then it increased until 2002/2003 and after some fluctuations (2003/2004–2005/2006) reached the highest number in 2006/2007 (Fig. 2). The number of Gentoo penguins increased during the study period (140 pairs per year, F (1,8) = 62.0, P < 0.0001, R 2 = 0.88). Overall, the number of breeding pairs increased from 2,236 to 3,764 (68%).
Interannual variation in population size
There was a positive correlation between species in their interannual variation in population size (r = 0.75, n = 8, t = 2.7, P < 0.05) (Fig. 3).
Percentage annual change in the total number of breeding pairs (nests with eggs) of Adélie and Gentoo penguins on Stranger Point, Isla 25 de Mayo. The magnitude and direction (increasing or decreasing) is calculated relative to the preceding year’s census for each species. Annual change (%) = ((PY(i + 1)/PY(i)) − 1) × 100, PY: population size
Chicks crèched of Adélie penguins
The number of chicks crèched was lower in all the seasons in relation to the number recorded in 1995/96 (Table 1). During 2006/2007 the number of chicks crèched was only about 24% of those fledged in 1995/1996.
Chicks crèched of Gentoo penguins
The number of chicks crèched was higher in all the seasons in relation to the number recorded in 1996/97 (Table 1). The highest number of chicks was counted in 2005/2006 (4,402 chicks, representing an increase of 60.3%). Overall, the number of chicks crèched increased by 20% from 1996/97 to 2006/2007.
Breeding success
Breeding success for both species (chicks in crèches/nests with eggs) is shown in Fig. 4. The breeding success index for Adélie penguins fluctuated between 0.65 and 1.26 while that for Gentoo ranged between 0.76 and 1.27. Overall, the index for Adélies was not significantly different from that for Gentoos (0.98 ± 0.20, n = 12 versus 1.03 ± 0.19, n = 9, respectively, t test, P > 0.6). If the comparison is made only for the seasons in which the data for both species are available, the result is similar (0.95 ± 0.20, n = 9 versus 1.03 ± 0.19, n = 9; t test, P > 0.4). Breeding success between species was not significantly correlated throughout the study period (P > 0.1).
Discussion
Population changes can be driven by a number of factors acting in the same or opposing directions throughout the birds’ annual cycle and/or with different intensity during their life. Below we discuss possible causes of the contrasting population trends in Adélie and Gentoo penguin populations observed at Stranger Point.
The breeding success of a population can affect breeding population size since penguins tend to reproduce in their natal colony and, in an important proportion, at their natal site (Ainley et al. 1983). Reduced breeding success will result in a lower number of chicks coming back to breed, which could in turn have an adverse effect on the breeding population trend. At Stranger Point, the average breeding success of Adélies throughout the study was not significantly different from that of Gentoos. However, there was no correlation in the IBS between Adélie and Gentoo penguins. This seems to indicate that the species would occupy well-differentiated trophic niches that would account for their differential breeding success in a given season. Interestingly, although fish constituted a relatively important portion of the diet of Gentoo penguins in other localities such as Esperanza/Hope Bay (Antarctic Peninsula), krill was the bulk of the summer diet for both species at Stranger Point (N.R. Coria, unpublished data). Even though these species have different foraging behaviours, Gentoo penguins being deep divers and therefore able to exploit a niche unavailable to its congener (Trivelpiece et al. 1987), the comparatively large amount of food that Gentoo penguins need to rear chicks and their small foraging range suggest that, overall, availability of resources during summer was not a major cause of the contrasting population changes observed in these sympatric penguins. Moreover, the IBS of Adélie penguins falls well within the ranges observed in other Adélie penguin populations. For instance, overall breeding success at Esperanza Bay, Antarctic Peninsula (Carlini et al. 2007; A.R. Carlini and N.R. Coria, unpublished data for the 2005/2006 and 2006/2007 seasons) during almost the same period (1995–2005) was not significantly different from that observed at Stranger Point in those seasons for which concurrent data were available (1.18 ± 0.22, n = 9 versus 1.02 ± 0.18, n = 9, respectively, t test P > 0.1), while breeding population size showed a much lower decline (35%) than at Stranger Point (62%). At Laurie Island, South Orkney Islands the IBS was also similar to that reported at Stranger Point for the 1995–2006 period in those seasons for which concurrent data were available (0.95 ± 0.25, n = 11 versus 0.95 ± 0.19, n = 11; t test P > 0.9) (A.R. Carlini and N.R. Coria, unpublished data), while the breeding population size exhibited an increase of 2.8%, although there were no trends in the abundance of Adélie penguins (F (1,9) = 2.6, P > 0.1). As the breeding success of the Stranger Point Adélie penguin breeding colony did not significantly differ from that observed at Esperanza Bay and Laurie Island or from that of Gentoo penguins, we suggest that the breeding success of Adélie penguins during summer would be sufficient for their population to remain stable. Therefore, other factors, such as redistribution of breeding pairs or survival of chicks and/or adults must be taken into account to explain the differences in the population trends observed.
The redistribution of breeding pairs can be linked to changes in the microclimate of the breeding places and/or human disturbance. It has been shown that the disappearance of a breeding group can take only 2–3 years of breeding failure (Yeates 1975). This implies that changes in the distribution of breeding pairs could be very rapid in response to changes in local conditions (Trivelpiece and Fraser 1996). During the study, the Adélie IBS fluctuated between 0.6 and 1.2 chicks per breeding pair and did not show consecutive years of very low breeding success (Fig. 4). Additionally, nesting locations at Stranger Point are situated on well-drained slopes with porous substrate and good water runoff, suggesting that the overall Adélie population trend at Stranger Point was not driven by the loss of breeding habitat. Interspecific competition for breeding places, as was reported for Adélie and Chinstrap penguins (Trivelpiece and Volkman 1979; Carlini et al. 2005), has not been recorded between Adélie and Gentoo penguins at Stranger Point up to now. Both species nest in well-differentiated, although in many cases close, places. Moreover, and due to the decline in the number of Adélie penguins, of the original 50 breeding groups present in 1995/1996, 25 had no breeding birds by 2006/2007 and none of these sites were occupied by Gentoo penguins (Fig. 1). This indicates that competition for breeding places between species has not occurred up to date at Stranger Point. Human disturbance, which may affect mainly the recruitment of pre-breeders (Woehler et al. 1991, 1994) and/or breeding success (Giese 1996), is an unlikely explanation for the Adélie population decline observed at Stranger Point, since this colony is situated within the Specially Protected Area No. 132 and can be visited only under permit. The dynamics of a population could also be affected by density-dependent processes (Leirs et al. 1997). Barbraud and Weimerskirch (2003) found that, when climatic conditions become unfavourable, survival decreases at low population densities (although it remains high enough to avoid a major population decline), but survival declines dramatically at high population densities. They suggest that competition acting through low food availability at sea may be the mechanism leading to the association between survival rates and density. Since Adélie breeding populations are declining all over the APR region (see below), density-dependent effects do not appear to be involved in the population decline observed at Stranger Point.
Since the potential causes examined above do not seem to explain the contrasting population trends observed at Stranger Point, it is possible to infer that the differences between the two species could be mainly driven by the differential survival rates of adults and/or chicks operating during winter. Breeding population size is a measure of overwinter survival and also an indicator of how successful the year’s potential breeders were in the previous winter (Fraser et al. 1992). Life history strategies between these penguin species differ markedly during winter and thus current environmental changes could affect them differentially. Since the hypothesis presented by Fraser et al. (1992), which suggested that a decrease in winter sea ice in the western Antarctic Peninsula was a major factor driving long-term changes in the abundance of some regional krill-dependent populations, mounting evidence has been gathered (Fraser and Trivelpiece 1996; Trathan et al. 1996; Smith et al. 1999; Fraser and Hofmann 2003; Forcada et al. 2006; Hinke et al. 2007; Sander et al. 2007a, b). Hinke et al. (2007) working at Admiralty Bay, 16 km from Stranger Point, also found divergent population trends in Adélie and Gentoo penguins. Those authors found that cohort and total recruitment of Adélie penguins declined over the 1980–2005 period, whereas recruitment of Gentoo penguins did not trend. These facts, together with the similar summer breeding success of these species, allow them to conclude that differential over-winter survival could be the most plausible explanation for the divergent population trends observed. Our results support this hypothesis. Surprisingly, however, even though both populations showed an opposite trend in their breeding population size (Fig. 2), there was a positive relation in their interannual variation, although the extent, and for some years the direction, of the change observed always favoured Gentoo penguins (Fig. 3). It is possible that variability in sea ice conditions and their effect on krill abundance may affect adult birds of both species during the previous winter with different intensity but in a roughly similar way (thus explaining the positive relation in the interannual variation), but that juvenile survival of both species and thus the recruitment of new breeders might be affected differentially, with a much lower survival rate of juvenile Adélie penguins (thus explaining the divergent population trend observed).
Finally, while there seems to be a common factor driving the Adélie population changes in the APR, there may be additional local factors influencing the variation observed. The decline in the Adélie penguin population reported here was faster than that observed at Hope Bay on the tip of the Antarctic Peninsula (Carlini et al. 2007), while no trend was observed at Laurie Island (see above). At Stranger Point, an important level of predation by brown skuas (Catharacta antarctica lonnbergi) on Adélie penguins (Santos et al. 2008) and the relatively small size of most of the Adélie breeding groups could also play a role in the dramatic decline observed.
References
Ainley DG, LeResche RE, Sladen WJL (1983) Breeding ecology of the Adélie penguin. University of California Press, Berkeley
Barbraud C, Weimerskirch H (2003) Climate and density shape population dynamics of a marine top predator. Proc R Soc Lond B Biol Sci 270:2111–2116. doi:10.1098/rspb.2003.2488
Carlini AR, Coria RN, Santos MM, Buján SM (2005) The effect of Chinstrap penguins on the breeding performance of Adélie penguins. Folia Zool (Brno) 54:147–158
Carlini AR, Coria RN, Santos MM, Libertelli MM, Donini G (2007) Breeding success and population trends in Adélie penguins in areas with low and high levels of human disturbance. Polar Biol 30:917–924. doi:10.1007/s00300-006-0251-1
CCAMLR (2003) Standard methods for monitoring parameters of predator species. CCAMLR Ecosystem Monitoring Program. CCAMLR, Hobart
Clarke J, Kerry K, Irvine L, Phillips B (2002) Chick provisioning and breeding success of Adélie penguins at Bechervaise Island over eight successive seasons. Polar Biol 25:21–30. doi:10.1007/s003000100307
Croxall JP, Trathan PN, Murphy EJ (2002) Environmental change and Antarctic seabird populations. Science 297:1510–1514. doi:10.1126/science.1071987
Davis LS, McCaffrey FT (1986) Survival analysis of eggs and chicks of Adélie penguins (Pygoscelis adeliae). Auk 103:379–388
Ducklow HW, Baker K, Martinson DG, Quetin LB, Ross RM, Smith RC, Stammerjohn SE, Vernet M, Fraser W (2007) Marine pelagic ecosystems: the West Antarctic Peninsula. Philos Trans R Soc B 362:67–94. doi:10.1098/rstb.2006.1955
Forcada J, Trathan PN, Reid K, Murphy EJ, Croxall JP (2006) Contrasting population changes in sympatric penguin species in association with climate warming. Glob Chang Biol 12:411–423. doi:10.1111/j.1365-2486.2006.01108.x
Fraser W, Hofmann E (2003) A predator’s perspective on causal links between climate change, physical forcing and ecosystem response. Mar Ecol Prog Ser 265:1–15. doi:10.3354/meps265001
Fraser WR, Trivelpiece WZ (1996) Factors controlling the distribution of seabirds: winter–summer heterogeneity in the distribution of Adélie penguin populations. Foundations for ecological research west of the Antarctic Peninsula. Antarct Res Ser 70:257–272
Fraser WR, Trivelpiece WZ, Ainley D, Trivelpiece SG (1992) Increases in Antarctic penguin populations: reduced competition with whales or a loss of ice due to environmental warming? Polar Biol 11:525–531. doi:10.1007/BF00237945
Giese M (1996) Effects of human activity on Adélie penguin Pygoscelis adeliae breeding success. Biol Conserv 75:157–164. doi:10.1016/0006-3207(95)00060-7
Hinke JT, Salwicka K, Trivelpiece SG, Watters GM, Trivelpiece WZ (2007) Divergent responses in Pygoscelis penguins reveal a common environmental driver. Oecologia 153:845–855. doi:10.1007/s00442-007-0781-4
Hughes L (2000) Biological consequences of global warming, is the signal already apparent? Trends Ecol Evol 15:369–381. doi:10.1016/S0169-5347(00)01940-6
Ingolfsson O, Hjort C, Humlum O (2003) Glacial and climate history of the Antarctic Peninsula since the last glacial maximum. Arct Antarct Alp Res 35:175–186. doi:10.1657/1523-0430(2003)035[0175:GACHOT]2.0.CO;2
King JC (1994) Recent climate variability in the vicinity of the Antarctic Peninsula. Int J Climatol 14:357–369. doi:10.1002/joc.3370140402
Leirs H, Stenseth NC, Nichols JD, Hines JE, Verhagen R, Verheyen W (1997) Stochastic seasonality and nonlinear density-dependent factors regulate population size in an African rodent. Nature 389:176–180. doi:10.1038/38271
Loeb V, Siegel V, Holm-Hansen O, Hewitt R, Fraser W, Trivelpiece W, Trivelpiece S (1997) Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature 387:897–900. doi:10.1038/43174
Sander M, Balbão TC, Polito MJ, Costa ES, Carneiro APB (2007a) Recent decrease in chinstrap penguin (Pygoscelis antarctica) populations at two of Admiralty Bay’s islets on King George Island, South Shetland Islands, Antarctica. Polar Biol 30:659–661. doi:10.1007/s00300-007-0259-1
Sander M, Balbão TC, Costa ES, dos Santos CR, Petra MV (2007b) Decline of the breeding population of Pygoscelis antarctica and Pygoscelis adeliae on Penguin Island, South Shetlands, Antarctica. Polar Biol 30:651–664. doi:10.1007/s00300-006-0218-2
Santos MM, Juares MA, Moreira ME, Coria NR, Carlini AR (2008) Éxito reproductivo en el pingüino adelia: es la predación del skua pardo un factor importante? In: Proceedings of the XII Reunión Argentina de Ornitología. Neuquén, Argentina, p 169
Siegel V, Loeb V (1995) Recruitment of Antarctic Krill Euphausia superba and possible causes for its variability. Mar Ecol Prog Ser 123:45–56. doi:10.3354/meps123045
Skvarca P, Rack W, Rott H, Donangelo TL (1999) Climatic trends and the retreat and disintegration of ice shelves on the Antarctic Peninsula: an overview. Polar Res 18:151–157. doi:10.1111/j.1751-8369.1999.tb00287.x
Smith RC, Stammerjohn SE, Baker KS (1996) Surface air temperature variations in the western Antarctic Peninsula Region. Foundations for ecological research west of Antarctic Peninsula. Antarct Res Ser 70:105–121
Smith RC, Ainley D, Baker K, Domack E, Emslie S, Fraser S, Kennet A, Mosley-Thomson E, Stammerjohn S, Vernet M (1999) Marine ecosystem sensitivity to climate change. Bioscience 49:393–404. doi:10.2307/1313632
Trathan PN, Croxall JP, Murphy EJ (1996) Dynamics of Antarctic penguin populations in relation to inter-annual variability in sea-ice distribution. Polar Biol 16:321–330. doi:10.1007/BF02342178
Trivelpiece WZ, Fraser W (1996) The breeding biology and distribution of Adélie penguins: adaptations to environmental variability. Foundation for ecological research west of the Antarctic Peninsula. Antarct Res Ser 70:273–285
Trivelpiece WZ, Volkman N (1979) Nest-site competition between Adélie and Chinstrap penguins: an ecological interpretation. Auk 96:675–681
Trivelpiece WZ, Trivelpiece SG, Volkman NJ (1987) Ecological segregation of Adélie, Gentoo and Chinstrap penguins at King George Island, Antarctica. Ecology 68:351–361. doi:10.2307/1939266
Williams TD (1995) The penguins. Oxford University Press, Oxford
Woehler EJ, Slip DJ, Robertson LM, Fullagar PJ, Burton HR (1991) The distribution, abundance and status of Adélie penguins Pygoscelis adeliae at the Windmill Island, Wilkes Land, Antarctica. Mar Ornithol 19:1–19
Woehler EJ, Penney RL, Creet SM, Burton HR (1994) Impact of human visitors on breeding success and long-term population trends in Adélie penguins at Casey, Antarctica. Polar Biol 14:269–274. doi:10.1007/BF00239175
Yeates GW (1975) Microclimate, climate, and breeding success in Antarctic penguins. In: Stonehouse B (ed) The biology of penguins. University Park Press, Baltimore, pp 397–409
Acknowledgments
We want to thank S. Poljak, R Montiel, E. Soibelzon, M. Ciancio, M. Gasco, J. Mennucci and L. Balboni for field assistance. The permit for this work was granted by the Dirección Nacional del Antártico (Environmental Office). This work was supported by the Dirección Nacional del Antártico, Instituto Antártico Argentino. We thank Dr. W. Fraser and two anonymous reviewers for their helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carlini, A.R., Coria, N.R., Santos, M.M. et al. Responses of Pygoscelis adeliae and P. papua populations to environmental changes at Isla 25 de Mayo (King George Island). Polar Biol 32, 1427–1433 (2009). https://doi.org/10.1007/s00300-009-0637-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-009-0637-y