Abstract
Climate change and direct impacts of human activity are often considered among the main drivers of ecosystem dynamics; however, their relative importance for high-Arctic marine systems is not clearly understood. The Baydaratskaya Bay (southwestern part of the Kara Sea) was not subject to any human activity until 2011, when the construction of the underwater portion of a gas pipeline began; thus, it is a good place to investigate this issue. We used data on the macrobenthos from 1945 to 2013 to assess temporal variability and reveal either decadal-scale trends or construction impacts on the ecosystem. Until 2013, no evidence of long-term changes was detected in biomass, diversity, species composition, functional structure, and biogeographic attributes of the fauna. The ecological status was permanently evaluated as undisturbed. In 2013, however, an extensive shift occurred in the deep-sea area close to the pipeline trench. Benthic biomass and diversity decreased abruptly; while total abundance increased. The functional structure changed. Large bivalves almost completely disappeared, abundance of gastropods and amphipods dropped sharply, while the abundance of small opportunistic polychaetes increased abruptly. Benthic quality assessment indices indicated the deterioration of the community. The most likely reason for such catastrophic changes was the direct impact of dredging and dumping of sediments during pipeline burial from 2012 to 2013. We conclude that the benthic macrofauna in Baydaratskaya Bay has remained relatively stable over the last several decades without any signs of climate-driven borealization. Recent human activity, however, caused local but rapid and strong response, indicating the high sensitivity and vulnerability of this ecosystem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Background and objectives of the study
Climate-driven changes in communities and ecosystems, both observed and predicted, have drawn growing attention for the past decades. Such studies are currently of particular interest in the Arctic because the region is expected to be strongly influenced both by climate shifts and rapidly increasing human activities (Wassmann et al. 2011; Renaud et al. 2015). Although there is growing evidence of Arctic climate changes, the relative importance of global warming versus natural variability and the direct impacts of human activity are not clearly understood (Piepenburg 2005; Bowler et al. 2017). Drivers related to climate change, such as warming, ice decline, and acidification, affect the benthic communities on a pan-Arctic scale, while trawling, water pollution, river/glacier discharge, and invasive species have significant impacts on regional or local scales (Kortsch et al. 2012; Josefson et al. 2013; Jørgensen et al. 2017). These direct human impacts may intensify the effect of climate shifts (Huntington et al. 2007).
Despite considerable interest in understanding high-latitude marine ecosystem responses to these impacts, there are few long-term studies addressing these issues (Wassmann et al. 2011). Current monitoring efforts focus on some well-studied regions, such as the Barents Sea or Chukchi Sea, which are also highly impacted by humans, while in many other Arctic areas, systematic long-term ecological research is rare. Well-documented examples of long-term changes in the Arctic are still scarce (see reviews by Wassmann et al. 2011; Renaud et al. 2015). Moreover, some studies did not show any long-term trends. For instance, high temporal stability of benthic communities was observed during the twentieth and the early twenty-first centuries in Van Mijenfjord, Svalbard (Renaud et al. 2007), several areas of the White Sea (Solyanko et al. 2011), and the southwestern Kara Sea (Kozlovskiy et al. 2011). However, it is not clear whether the relative isolation and low anthropogenic pressure on these ecosystems are responsible for their steady states or whether current climate changes are not yet evident.
Baydaratskaya Bay (in the southwestern part of the Kara Sea) is an example of an area with a low level of human activity until the beginning of the twenty-first century, when underwater trenching began in 2011 to build the buried subsea transition piece of a pipeline. We used the data on benthic macrofauna collected from 1945 to 2013 to assess the temporal variability and long-term dynamics of diversity, faunal composition, biogeographic, taxonomic and functional structure, spatial distribution, and ecological quality of the macrozoobenthos in this area. Since the beginning of the construction is exactly known, these data provide a good opportunity to evaluate the relative role of long-term (climatic-driven) and direct local-scale impacts on the benthic ecosystem. The research questions were as follows:
-
(i)
could any decadal-scale trends in macrofaunal structure be revealed before construction work started?
-
(ii)
did this work have an influence on the benthic communities?
We examined whether temporal patterns of macrofaunal communities were correlated with local and regional climatic indices in order to elucidate the possible relationships between environmental forcing and biological response. We also tested the applicability of several estimators to assess benthic ecological quality. The size structure of communities was estimated using the abundance/biomass comparison (ABC) method, which is sensitive to changes in the community associated with pollution or disturbance resulted in predominance of small-sized opportunistic organisms (Warwick 1986). The AZTI Marine Biotic Index (AMBI) and its multivariate extension, M-AMBI, are based upon the well-known ecological models of ecological succession in stressed environments (Borja et al. 2000). The AMBI was originally designed to detect the response of soft-bottom communities to changes in water and sediment quality, mainly organic pollution, along European coasts and has further been successfully verified in relation to various natural and human-induced impact sources. The index has been applied in various regions throughout the Atlantic Ocean, Baltic, Mediterranean, and North Seas, and in Hong Kong, Uruguay, and Brazil; however, there have been only a few attempts to apply it in the Arctic (Borja et al. 2015).
These retrospective data on this high-Arctic ecosystem could be used in future studies as a baseline for assessing long-term changes and their underlying drivers.
Description of study area
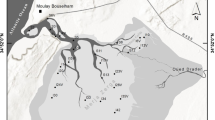
Baydaratskaya Bay, situated in the southwestern part of the Kara Sea (69°N, 67°E), is a large (180 km long, up to 80 km wide), shallow (with maximum depths of 46 m in the inner part), semiclosed bay that runs deeply inland (Fig. 1a). It has a low amount of freshwater runoff and consequently has high water salinity. For 8–10 months (usually October–June), the bay is covered by ice. The bottom is impacted by ice-hummock scouring, which is most intensive in the shallow part down to 10 m depth (Geptner 1997). High-density, cold (down to − 2 °C), and saline (34–35 psu) water is formed in winter. In summer, water is vertically stratified, with the thermocline situated at depths of 10–14 m. As a result, in coastal zone shallower the 15 m, the summer bottom-water temperature is + 3.6 ± 2.1 °C, sometimes reaching 9.4 °C at a depth of 15 m (Ermakova and Novikhin 2011). In the central part of the bay, which is deeper than 20 m, the average bottom-water temperature is − 0.3 ± 0.9 °C even in July–August (Geptner 1997; Jørgensen et al. 1999; Kozlovskiy et al. 2011). In the coastal part, the bottom sediments are formed by fine-grained sands (mean particle size 0.2–0.1 mm); the proportion of the silty fraction increases with depth from 30 up to 90%, and the sediments in the central part are mainly represented by silts and clayed mud (Geptner 1997; Motychko et al. 2013). Long-term (since 1914) dynamics of water temperature and salinity show quasi-periodical oscillations without considerable permanent trends (Geptner 1997).
Until the beginning of the twenty-first century, Baydaratskaya Bay has been an area without the environmental impact of human activities such as settlement, commercial fishing, and industrial infrastructure. However, in 2011, the construction of the Yamal–Center gas pipeline began. The underwater part of the pipeline, which is 67 km long, crosses the central bay area at depths of 22–23 m (Fig. 1b). In 2012, the first twin lines of large-diameter pipes had been laid; two more lines had been completed in 2014; and the third twin pipeline is under construction. Since 2012, intensive pipeline-burying operations, including pipeline trench dredging by dredge hopper vessels, dumping of sediments, and ditch backfilling, have continued in this area during every ice-free period.
The benthic macrofauna of the Baydaratskaya Bay were first studied from 1945 to 1946 (Filatova and Zenkevich 1957) and then 30 years later in 1975 (Antipova and Semenov 1989). In the 1980s and early 1990s, a few expeditions collected benthic samples in the Bay, providing new data on the zoobenthos in the inner part of the bay (Denisenko et al. 1993; Jørgensen et al. 1999; Stepanova 2000; Denisenko et al. 2003). Systematic benthic surveys were performed from 1992 to 1994 and in 2007 by expeditions organized by the P.P. Shirshov Institute of Oceanology as a part of the gas pipeline design and survey work (Geptner 1997; Vozzhinskaya et al. 1997; Kucheruk et al. 1998; Kozlovskiy et al. 2011). From 2012 to 2013, benthic surveys were conducted as a part of the impact assessment program during pipeline construction (Kokarev et al. 2015). The material collected during these surveys was the basis of this study.
Materials and methods
Data
The data for this study were compiled from several sources. In total, data from 76 stations (216 quantitative grab samples) were used (Fig. 1b, Table 1).
The data from the 1945–1946 surveys were used along with others to compile a schematic map of the Kara Sea benthic communities. The detailed results have never been published, so we used the data from four grab stations that were presented in the handwritten technical report (Grishina and Filatova 1948) archived at the Institute of Oceanology RAS.
The 1975 survey in the southwestern part of the Kara Sea was organized by the Polar Research Institute of Marine Fisheries and Oceanography (Antipova and Semenov 1989). Of the 40 benthic stations, five were located in Baydaratskaya Bay. However, detailed station-based data were not published, so the averaged data from these five stations were used in the analysis.
The data collected in the 1980s were also not presented in detail, and reliable information was obtained for only the averaged biomass and composition of the zoobenthos at the five stations sampled in 1987 (Stepanova 2000). The exact coordinates of these stations are unknown, so they are not shown on the map (Fig. 1b).
From 1992 to 1994, benthic surveys in the Bay were performed by expeditions organized by the P.P. Shirshov Institute of Oceanology (19 stations in total).
In 2006, macrobenthos samples in the planned pipeline area were collected by the trained staff of the pipeline engineering corporation Peter Gaz. The results were presented in a technical report (Ecological Survey 2006). As detailed data on species composition were lacking in the report, only total biomass values were used in the analysis.
In 2007, during a voyage of the R/V Professor Boiko, data were collected at 31 stations (93 samples) at depths from 5 to 25 m (Kozlovskiy et al. 2011). From 2012 to 13, data were collected at several stations along the pipeline and in the dumping area (Kokarev et al. 2015).
Data harmonization and quality control
Careful quality control was undertaken to harmonize the datasets. To avoid possible misidentifications, the material collected from 1993 to 1994 and 2007 to 2013 and currently stored at the Institute of Oceanology was re-examined, and all organisms were identified to the lowest possible taxonomic level using a stereo microscope. All species lists were carefully checked for validity, spelling errors, obsolete taxon names, synonyms, and/or later-ascertained misidentifications. Taxonomic assignment was unified according to the scientific names contained within the World Registry of Marine Species database (WoRMS, https://www.marinespecies.org). Earlier doubtful records, which were possibly inaccurate or problematic taxa identifications, were reduced to higher taxonomic levels (genus or family). In particular, most amphipods and some Polychaeta species (e.g., Cirratulidae, Dorvilleidae, Flabelligeridae, Sabellidae) were reduced to the family level. Oligochaetes and nemerteans were not identified to species and were considered in toto.
For zoogeographic classification, all species were subdivided into one of the following main zoogeographic groups (Jørgensen et al. 1999; Denisenko et al. 2003): Arctic, High boreal–Arctic, boreal–Arctic, and widespread (from the tropics or subtropics to the Arctic). Each species was assigned to a zoogeographic group based on many literature and Internet resources, which are listed in Online Resource 1 (Supplementary Table S1).
Because a single grab per station was taken during the early period (1945–1978, see Table 1), replicated grabs from each station in later datasets were averaged, and all measures of community structure were calculated for these pooled samples. Species abundance and biomass values were calculated per m2. At one station in 2013 that was close to the pipeline (marked as an inverted filled triangle in Fig. 1b), the macrofauna were absent except for a few nematodes, so the station was excluded from further analysis of community structure but was considered when appropriate, i.e., in average biomass calculations or the index of ecological quality estimations (see below).
For each station, sampling depth and the type of sediments were used as habitat characteristics. As only a qualitative description of sediments was available in most cases, we used three sediment categories: sand, mixed (silty sands and sandy silts) and silty sediments (silt, clay, and mud). Because the data design was strongly unbalanced (i.e., not all depths had been sampled in all the years), we pooled the yearly data into four longer periods: 1945–1975, 1992–1994, 2007–2012, and 2013. The latter year was considered a separate period to evaluate the potential impact of sediment dredging.
Environmental data
We used three climate indices potentially affecting the benthic ecosystem. Two regional (pan-Arctic) synoptic factors were the Arctic Oscillation (AO) and the Arctic Climate Regime Index (ACRI). The AO is the first principal component of the sea level pressure field at latitudes greater than 20°N, while the ACRI measures variations in Arctic Ocean and ice circulation based on the sea level height anomaly at the North Pole. The local synoptic factor was the annual sea surface temperature (SST) averaged for the three warmest months (July–September) at the Baydaratskaya Bay neighborhoods. Data for the AO and for SST were obtained from the National Oceanic and Atmospheric Administration Earth System Research laboratory (NOAA ESRL) website (AO: https://www.cpc.ncep.noaa.gov/products/precip/CWlink/daily_ao_index/ao.shtml; SST: https://www1.ncdc.noaa.gov/pub/data/cmb/ersst/v5/ascii/), and for the ACRI from (https://www.whoi.edu/page.do?pid=66578). Each index was used in two forms: the values for a present year (AO-1, ACRI-1, and SST-1), and 3-year running means (two previous and present year; AO-3, ACRI-3, and SST-3), to account for possible delay in benthic response.
Statistical analysis
To estimate the role of each species in a community, we used its relative respiration rate R (Azovsky et al. 2000)
where Bi and Ni are biomass (g wet wt m–2) and abundance (ind. m–2) of the ith species and Ai is the taxon-specific coefficient of respiration intensity (Kokarev et al. 2017). Because this measure combines the abundance and biomass of each species, it provides an ecologically relevant compromise between both measures and ensures a balanced contribution of small but abundant species and large species with low abundance but high biomass (Azovsky et al. 2000). Prior to the analysis, respiration values were standardized by station totals in order to operate with percentages.
The similarities between stations were estimated by the Bray–Curtis index (Clarke and Warwick 2001):
where Rij and Rik are the ith species relative respiratory rates in samples j and k, respectively.
Differences in the community structure across time and environmental factors were tested using a non-parametric permutation analysis of variance (PERMANOVA) on a Bray–Curtis similarity matrix (Anderson et al. 2008). The PERMANOVA model included the terms “depth” (fixed, 3 levels), “sediment” (fixed, 3 levels), “period” (fixed, 4 sampling periods), “year” (random, nested in period), and the interaction term, “period × depth.” The significance probabilities of the effects were estimated both by 999 permutations of the residuals under a reduced model (P(perm)) and by a Monte Carlo randomization procedure (P(MC)). In pairwise post hoc PERMANOVA tests, the Benjamini–Hochberg correction for multiple comparisons was used to reduce the possibility of false-positive results. The PERMDISP tests for possible dispersion heterogeneity were performed for each of the main effects revealed by PERMANOVA. To visualize the differences in community structure, an ordination of stations was performed using non-metric multidimensional scaling (nMDS) of the resulting similarity matrix.
We used the expected species number per 100 individuals, ES(100) as measure of species diversity. This index is suitable for comparison of samples with different sampling efforts (Clarke and Warwick 2001).
Non-parametric distance-based regression analysis (DistLM) was used to explore the relationships between variations in the benthic features (community structure, diversity, and biomass) and three climate indices. Since some of these indices are positively dependent (e.g., AO and ACRI, or 1- and 3-year indices), this leads to potential dependencies between multiple tests. Therefore, to estimate the significance of the relationships, we applied the Benjamini–Hochberg multiple testing correction procedure with false discovery rate of 0.1, modified under the tests’ dependency (Benjamini and Yekutieli 2001).
Comparing the species lists, special attention was paid to species that were present in one period but absent in another period. To estimate the probability of their absence due to statistical reasons (i.e., rarity), we used the following estimator (Azovsky 2018):
where NPRES is the number of stations with the presence of a species in period A; NA and NB are the total numbers of stations collected in periods A and B, respectively; and P(B=0) is the probability of accidentally overlooking this species in period B.
Functional structure (biological traits analysis)
The application of biological traits to study community structure is of particular interest in polar regions because it allows changes in ecosystem functioning to be traced, which are not always related to changes in taxonomic composition (Frid and Caswell 2015; Dergen et al. 2018). Moreover, phylogenetically related taxa can share most of the traits, so the effects of cryptic diversity or possible taxonomic misidentification on the data are less pronounced. We considered seven traits with 29 related modalities (Table 2). Species were assigned to trait modalities using a fuzzy coding approach based on information found in literature (see Kokarev et al. (2017) for the references); body size classes were specified using the mean individual weight according our data. We used the respiration values for each species multiplied by the respective trait codes to obtain the traits × station matrix, which was used as a base for fuzzy correspondence analysis (FCA) (Kokarev et al. 2017). The values from the matrix were standardized by trait for each station before analysis.
Assessment of ecological quality
To assess benthic ecological quality, we applied several estimators.
To quantify size structure of communities, we used a W-index (Clarke 1990)
where Bi and Ai are the cumulative sums of the first i species ranked by biomass and abundance, respectively, and S is the total number of species. This index takes values from – 1 to 1, with negative values indicating the predominance of small organisms and interpreted as a disturbed or stressed community, and positive values indicating the dominance of larger organisms (an undisturbed state).
We also used the AZTI Marine Biotic Index (AMBI) (Borja et al. 2000) and its multivariate extension, M-AMBI, recently introduced as a refinement of AMBI (Muxika et al. 2007). Its computation involves a factorial correspondence analysis based on AMBI, species richness, and the Shannon diversity index. Both indices were calculated using species respiration values instead of numerical abundances. The calculations were performed using AMBI software (version 5.0, downloadable from www.azti.es) and a species list that was last updated in June 2017, including 8,400 taxa. When applying the AMBI, species not yet specifically classified were given the same sensitivity value as most species in the same genus. Neither AMBI nor M-AMBI class boundaries have been calibrated in Arctic waters, so we used the default reference values from the AMBI software.
All multivariate analyses and W statistic calculations were performed using the software PRIMER ver. 6.1.15 with the PERMANOVA add-on (PRIMER-E Ltd., Anderson et al. 2008). Biological trait analysis (FCA) was carried out using R ver. 3.4.4 and the ade-4 package 1.7-10.
Results
Faunal composition, biomass and diversity
The total list included 219 unique taxonomic units (see Online Resource 1, Supplementary Table S1). One hundred seventy taxa were identified at the species level. Polychaetes were the richest group (82 taxa), followed by crustaceans (47 taxa) and mollusks (33 bivalves and 31 gastropods). Polychaetes generally dominated in abundance, while bivalve mollusks dominated in biomass. The roles of gastropods and crustaceans (mainly amphipods, cumaceans, and isopods) were also noticeable, while other groups were of minor importance. A geographic distribution could be assigned to 146 species, and most of them (71.2%) were boreal–Arctic or even more widely distributed (subtropical boreal–Arctic or cosmopolitan) forms; high boreal–Arctic (20.6%); and Arctic (8.2%) forms were less numerous, while there were no species with purely boreal distributions.
Thirty-seven taxa identified to the species level were found in the early period (1945–1994) only. Of these species, 68% have boreal–Arctic distributions, and 32% are high boreal–Arctic or Arctic forms. Sixteen species were first found after 2000; 65% of them are boreal–Arctic, and 35% are high boreal–Arctic or Arctic. Thus, neither a noticeable loss in total richness nor any sign of borealization has been observed. Moreover, most species that were not found during certain periods in the bay were reported in this period from other parts of the Kara Sea. This presence/absence pattern should, therefore, be treated as local species turnover rather than regional extinction/colonization events. Probabilistic estimation shows that for almost half of these species, their “appearance” or “disappearance” could be explained by their rarity (see Online Resource 1, Supplementary Table S1). This “null” hypothesis can be rejected with 95% probability for 21 species reported only in the twentieth century but for no more than four species first found in the twenty-first century (viz., gastropods Marsenina glabra, Neptunea despecta, and Retusa obtusa and polychaeta Manayunkia polaris).
We considered the dynamics of total biomass and diversity separately for three depth ranges (shallower than 10 m, 10–20 m, and deeper than 20 m) for the reasons presented in the next section. Biomass generally increased with depth, varying from 10 to 60 g/m2, from 65 to 180 g/m2, and from 70 to 200 g/m2 in shallow, intermediate, and deep zones, respectively, without any clear temporal trends (Fig. 2). Some increase in mid-depth biomass in the twenty-first century is statistically insignificant because of high between-station variations. The only noticeable change occurred in 2013 when the biomass declined drastically in the deep zone. The diversity remained relatively constant most of the time, being slightly higher in the deep zone. Again, the decline in diversity in 2013 in the deepest zone was the only apparent outlier (Fig. 3).
Taxonomic structure
First, a PERMANOVA was performed on the whole dataset to evaluate the potential sources of variability. The results (Table 3) indicated that there was significant variability among depths (explaining 24.1% of the total variance) and sampling periods, but the variation among years was not detected over and above the period-level variability (i.e., the term “year(period)” is not statistically significant, p = 0.239). The effect of sediment type was also non-significant (p = 0.596), and the estimate of its component of variation was negative. Furthermore, the interaction term “period × depth” was highly significant, while the “period” term per se showed only marginal significance, although both terms explained similar portions of the variation (13.5% and 14.4%, respectively). This result indicated that temporal variations in community structure were strongly affected by depth. Since our data design was evidently unbalanced, we performed the special PERMDISP tests for possible dispersion heterogeneity for each of the main effects revealed by PERMANOVA. These did not reveal any significant differences in dispersion, neither for depth (p = 0.559) nor for period (p = 0.192). Moreover, not one of the individual pairwise PERMDISP tests showed a significant difference (the results are not performed). The significant effects of depth and time period detected by the PERMANOVA can therefore now be interpretable to be purely (in essence) the location effects, i.e., caused by changes in community structure rather than by differences in within-group variability. These results confirm the consistency and reliability of our previous analysis.
The results of the MDS ordination of the whole dataset (Fig. 4) did not reveal temporal clustering but confirmed that stations from different bathymetric zones formed clearly distinct, although partially overlapping, clusters.
The five dominant species summed to half of the total metabolism for each community are listed in Table 4. These lists were similar for all three communities, and the only difference was in the dominance order. The shallow area was occupied by a Nephtys longosetosa–Astarte borealis–Liocyma fluctuosa community, and the intermediate depths were occupied by a Serripes groenlandicus–Ciliatocardium ciliatum–A. borealis community. An A. borealis–C. ciliatum–Micronephthys minuta community was situated at deeper depths. Interestingly, taxonomically related species with different geographical distributions had different bathymetrical distributions. For instance, the polychaete genus Nephtys was replaced by the closely related genus Micronephthys on the depth gradient. Arctic–boreal N. longosetosa and N. ciliate were abundant (over 50 ind. m−2 on average) on sandy sediments in the shallow, relatively warm zones but rarely occurred below the thermocline (2 ind. m−2 on average). In contrast, the Arctic species M. minuta was highly abundant in colder water deeper than 20 m (485 ind. m−2 on average) but almost disappeared in the shallower water (2 ind. m−2). Among the three isopods of the genus Saduria, the average abundance of the two Arctic species, S. sabini and S. sibirica, increased threefold with depth, while the boreal–Arctic S. entomon occurred only in the shallowest area. A similar pattern was found for ophiuroids: three species with a high boreal–Arctic distribution (Amphiura sundevalli, Ophiocten sericeum, and Ophiura robusta) were almost absent in the shallow zone but consistently increased in abundance with depth, while the wide-ranging boreal–Arctic Stegophiura nodosa was most abundant above 20 meters. As a result, the contribution of species with high-latitude distributions increased, but that of widespread species decreased toward the deepest and coldest waters, and the boreal–Arctic species permanently dominated at every depth (Fig. 5).
Considering the depth dependence of temporal variability revealed by the PERMANOVA, we further analyzed the community dynamics in each bathymetric zone separately. When the general PERMANOVA test showed a significant effect of the sampling period, pairwise tests for interperiodic differences were also performed (Table 5). To reduce the possibility of false-positive results of these multiple comparisons, the Benjamini–Hochberg correction of the p values was applied. This procedure kept all the results unchanged, with all significant differences remaining significant after correction. In the shallowest zone, data from periods II (1992–1994) and III (2007–2012) were only available, and the difference between these periods was non-significant, indicating no temporal shifts in community structure. At the intermediate depths, the only significant difference was observed between periods II and III–IV. This difference was conditioned by several stations from 1993 to 1994, with a low abundance of the bivalves Ciliatocardiumciliatum and Yoldia hyperborea, which were both mainly represented by juveniles; thus, Astarte borealis and polychaetes, particularly Nephtyidae and Terebellidae, reached there the top positions along with Serripes groenlandicus. Later, from 2007–2013, the assemblage returned to the bivalve-dominant state (Table 6). Finally, in the deepest zone, the latest period IV differed significantly from all other periods, indicating that community structure was stable throughout the research period but changed drastically in 2013. This distinct shift is quite apparent on the MDS ordination diagram (Fig. 6).
In order to link the changes in benthic communities to long-term environmental variability, we have performed a set of DistLM analyses of relationships between the community structure, diversity, and total biomass and three climate indices. The separate analyses were performed for each depth zone and for both current year and 3-year indices (see Materials and Methods). The results are presented in Table 7. Only two formally significant (observed p values < 0.01) correlations were discovered, both relating community properties in the deepest zone with the upward trend in 3-year sea surface temperature (SST-3). The first one correlated this trend with shifts in community structure and was attributable to changes in abundance of dominating bivalve species. Specifically, abundance of clams Astarte borealis and A. montagui decreased while abundance of hairy cockle Ciliatocardium ciliatum increased in time. The second significant correlation was found between increasing SST and decreasing total biomass. This correlation was mainly influenced by the abrupt biomass drop in 2013 but disappeared if this year was excluded from the analysis. After correction for multiplicity, however, not one null hypothesis could be rejected, i.e., not one correlation from Table 7 could actually be considered “significant,” despite the rather liberal chosen level for proportion of errors (10%).
The dramatic changes that occurred in the deepest zone in 2013 are worthy of special note. At these four stations, large bivalves (A. borealis, Y. hyperborea) were completely absent, and other previously common bivalves (S. groenlandicus, C. ciliatum, Mya truncata) were represented by juveniles only. Many common crustaceans, such as the cumacean Brachydiastylis resima, the amphipods Byblis spp. and Haploops spp., the isopods Synidotea bicuspida and S. nodulosa, as well as all gastropods, also disappeared. At one station, macrofauna were absent except for a few nematodes. At the same time, the abundance of small polychaetes increased abruptly (fivefold to one 100-fold). In particular, the abundance of Micronephthys minuta increased from 174 ind. m−2 (the average for the previous surveys) to 2450 ind. m−2, Cossura sp. gr. longocirrata increased from 21.6 up to 3250 ind. m−2, Cirratulidae spp.—from 87 up to 490 ind. m−2, and Levinsenia gracilis (Paraonidae)—from 10 up to 920 ind. m−2. The previously rare polychaete Capitella capitata reached levels of 140 ind. m−2. Notably, the former species is assigned to the “indifferent to disturbance” ecological group according to the AZTI classification, and the four other increased species are assigned to the “tolerant to disturbance” or “opportunistic” groups (WoRMS, https://www.marinespecies.org). Except for cirratulids, all these polychaetes are mobile subsurface deposit feeders with planktonic larvae. The other species that increased in abundance were the cumacean Brachydiastylis resima (from 24 to 150 ind. m−2) and the ophiuroid Ophiocten sericeum (from 1 to 5 ind. m−2); both species are classified as “indifferent to disturbance”. M. minuta was the most abundant species at all four stations.
Functional structure
The first two axes of the fuzzy correspondence analysis explained only 51.6% of the total variation (37.8% and 13.8%, respectively). The variation on the first axis was mainly associated with body design, feeding habit, mobility, and body size (Table 8). This axis separated the stations into two main groups (Fig. 7a, b). The first group on the positive end of the axis mainly included stations belonging to the intermediate and deep zones, with dominance of discretely mobile and large suspension-feeding bivalves. The second axis revealed that at the deeper stations in this group, a benthic reproduction strategy was more common. Most of the shallow stations were grouped on the negative end of the first axis along with some deeper stations with a less prominent dominance of bivalves. The deeper stations in 2013, which showed considerable taxonomic changes, appeared to be similar to the shallower stations with respect to the dominance of mobile-segmented worms with subsurface deposit-feeding and carnivore/omnivore habits. Moreover, the second axis revealed that small organisms were mainly associated with several stations in 2013. Overall, despite the low variance explained by the first two axes of the FCA, they clearly reflected the depth-related community structure, unlike any temporal trends.
Ordination of a stations and b traits in the first two FCA axes based on respiration-weighed biological traits composition. Traits are marked by upper-case letters: BD body design, P environmental position, FH feeding habit, LH living habit, S maximum adult size, M mobility, RS reproduction strategy; see Table 2 for notation of trait modalities
Indicators of disturbance
The ABC index of stress (W statistic) was substantially positive for all stations until 2013 (Fig. 8), representing the undisturbed conditions of the benthic communities with a prevalence of large-bodied forms. In 2013, however, the W statistic for the deepest stations dropped sharply to negative values, which indicated the impacted state characterized by the prevalence of few small-bodied 'opportunist' species.
Validation of our species list in accordance with the latest version of the AZTI Species List (AMBI 5.0) revealed the high compatibility of these lists. The percentage of taxa not assigned to any ecological group varied between years, from 0.4 to 6.9% of the total abundance. This is far less than the 20% limit of “not assigned” taxa recommended by Borja and Muxika (2005) for obtaining a reliable evaluation.
The AMBI index evaluated the community status as “undisturbed” until 2012, but it clearly indicated some loss of ecological quality in 2013 (down to “slightly disturbed,” Fig. 9a). The M-AMBI showed similar dynamics, assigning lower statuses (“good” but not “high”) to the years 1975 and 2012–13 (Fig. 9b). The low level in 1975 was due to insufficient sampling effort (five grab samples only), which consequently resulted in a low number of registered species.
Discussion
Faunal composition and the vertical distribution of communities
Our total list combined records over a long time period (1945–2013) and included 219 taxa; polychaete worms and mollusks accounted for 37% and 29% of the total richness, respectively. We should emphasize, however, that many crustaceans, particularly amphipods, were assigned to family level only to ensure the compatibility of the records throughout the sampling period. If the lists were categorized at the species level, the number of crustaceans would reasonably be expected to increase from 47 to 60–70 taxa. These figures confirm the previous estimations of macrofauna diversity and composition in this region. The prevalence of bivalves and polychaetes, along with the relatively minor importance of other groups, was previously noted in the southern part of the Kara Sea (Antipova and Semenov 1989; Denisenko et al. 1993). Jørgensen et al. (1999) reported nearly 500 macrobenthic taxa from the southern part of the Kara Sea consisting mainly of Crustacea, Polychaeta, and Mollusca. One hundred sixty-seven of these species were registered in Baydaratskaya Bay. Later, Denisenko et al. (2003) increased this figure to 223 identified species, including those inhabiting the offshore area. Polychaetes dominated in species richness, abundance, and biomass.
With respect to biogeographical structure, there was a strong dominance of species with a boreal–Arctic or wider distribution, while high boreal–Arctic and true Arctic forms constituted less than 30%. The proportions of Arctic and boreal–Arctic forms were similar among species found in only the 20th or twenty-first centuries. In the adjacent, more easterly Kara Sea shelf, Jørgensen et al. (1999) and Denisenko et al. (2003) reported approximately 20% true Arctic species, approximately 70% widely distributed boreal species, and the rest as dominated by boreal species, mostly of Atlantic origin. In Baydaratskaya Bay (Denisenko et al. 2003), the number of Arctic species was approximately 17%, while boreal taxa constituted 3% (while such species were absent in our data). Thus, the fauna of this area keeps the pristine arctic features, with no clear sign of borealization.
Our data revealed a distinct depth-related zonation in the spatial distribution of the macrofauna. Three communities were clearly distinguished by their species richness, taxonomic, and functional structure, although they are rather variable in space and have many common species. The first community occupies sandy sediments in the shallowest coastal areas; it has relatively low diversity and biomass values and is dominated by polychaetes (mainly N. longosetosa) and small bivalves (juvenile Astarte borealis, Liocyma fluctuosa, Macoma spp.). The low biomass and lack of large bivalves and echinoderms are possibly related to regular physical disturbances due to the abrasive effects of land-fast ice and ice hummocks. Such features are typical for the Arctic benthos in shallow-water areas subject to ice-scouring disturbances (Conlan et al. 1998; Steffens et al. 2006). In the East Siberian Sea, for example, the upper depth limit of permanent macrofauna is reported to be several meters below the zone of ice scouring (Golikov et al. 1994).
A quantitatively rich community of large bivalves (Serripes groenlandicus, Ciliatocardium ciliatum, A. borealis) occupies the silty sands and sandy silts at intermediate depths (10–18 m), where the upper layer of summer warming water reaches the bottom. The richest community of A. borealis–C. ciliatum–Micronephthys minuta is situated deeper than 20 m below the depth of the summer thermocline layer on silty sediments in the central part of the bay. With respect to function, motile vermiform deposit feeders prevail in the shallowest part, but larger suspension feeders prevail in the deeper parts. These three main types of communities in Baydaratskaya Bay have been described with minor variations in previous publications (Denisenko et al. 1993; Jørgensen et al. 1999; Stepanova 2000; Kozlovskiy et al. 2011; Kokarev et al. 2015). Thus, the spatial pattern is confined to, and is most likely determined by, the hydrological conditions and sediment properties.
The contribution of low-thermal (Arctic and high boreal–Arctic) species to total community respiration was minimal in the shallow and warm waters but increased with depth. The contribution of widespread (and therefore eurythermic) species showed the opposite trend. Thus, species of different origins were segregated in their ecological preferences, even though the range of depths in our study was quite narrow. This pattern is unlikely to be related to bathymetric preferences because most species are known to occur in much wider depth ranges. Differences in their thermopreferenda are a more probable cause. The contribution of species with benthic reproduction modes is also higher at deep-water stations, which agrees with previous findings that species with Arctic distributions tend to lack planktonic larvae (Fetzer and Arntz 2008).
Long-term dynamics
The data considered in this study covered approximately seven decades, and we did not detect any convincing evidence of directional changes or climate-driven regime shifts in benthic communities until 2013. The total biomass and species diversity varied widely in time (among surveys) and space (among stations) but did not show any long-term trends; the majority of correlations between community properties and climate indices are weak. The biogeographic composition of the fauna also remained constant over time, with a prevalence of boreal–Arctic species. Ecological status, assessed by two different approaches (W statistics and AMBI and M-AMBI indices), was evaluated as stable and “undisturbed”.
The pronounced depth zonation described above persisted throughout the study period without any long-term trends in the taxonomic or functional structure of the assemblages. A few temporal variations in community structure, detected by the multivariable analysis, mainly consisted in the rearrangement of dominating bivalve species (Ciliatocardiumciliatum, Yoldia hyperborea, and Astarte spp.). However, these variations at different depths were non-synchronous, and thus formally correlated with different climate indices (Table 7). The first fluctuation occurred at intermediate depths (10–20 m) in the nineties and was related to the drop in the abundance of the large bivalves, hairy cockle C. ciliatum and Y. hyperborea. Notably, Jørgensen et al. (1999) and Denisenko et al. (2003), analyzing the independent datasets collected in the bay in 1993, also reported the dominance of Serripes groenlandicus, Astarte borealis, and polychaetes at the shallow coastal sandy-mud stations, while C.ciliatum was not mentioned among the common species. Community-level characteristics (total biomass, diversity, W-index, and biomass of other main groups, i.e., polychaetes, gastropods, and crustaceans), however, showed no noticeable deviations from their typical values in that period. The next survey in 2007 showed that the Ciliatocardium and Yoldia populations had completely recovered, and the community had returned to the previous state. Moreover, the analysis of the average individual weight values revealed that in the 2007 samples, mature mollusks already prevailed, and therefore, active settlement and recovery had begun at least several years prior. Deeper, the abundance of Astarte clams decreased while abundance of C. ciliatum increased from the forties to the beginning of the twenty-first century. All these bivalves are widespread boreal–Arctic species with similar ecological properties, both prefer silty sediments at wide range of depths, and are common components of many Arctic and boreal seas (Naumov 2006). Long-term monitoring of A. borealis (Skazina et al. 2013) suggests that in high latitudes, this species may have a very long lifespan (up to 48 years) and a prolonged recruitment failure, which could be caused by scarcity of hard substrates needed for egg attachment in a silted environment. Similar quasi-cyclic fluctuations with several years' periodicity are described for other large bivalves (Naumov 2006). It is doubtful that the climatic forces would induce the opposite changes in same population depending on depth. Therefore, the revealed changes are more likely the result of natural fluctuations in local populations due to local recruitment and larval settlement, rather than response to environmental variations associated with decadal climate oscillations. These results indicate that the overall benthic ecosystem remained generally stable over a long period until 2013, when abrupt changes occurred in the deep-water area. These changes are worthy of special attention and are discussed below.
The 2013 crisis: symptoms and possible causes
The samples collected in 2013 indicated rapid and extensive structural changes in the deepest ( > 20 m) part of the bay. In comparison to the previous year, the benthic fauna at these stations were characterized by noticeable decreases in total biomass and species diversity; moreover, at one station in this area, the macrofauna were completely absent. The average total biomass dropped sixfold, while the total abundance increased two and a half times. The most striking component of the benthic reorganization was the abrupt change in species structure. Large bivalves almost completely disappeared or were represented by juveniles only; the abundance of gastropods dropped one 100-fold, and the abundance of amphipods dropped sixfold. At the same time, the abundance of few species, mainly small polychaetes, increased abruptly. Notably, all of the species that increased are assigned to the “indifferent to disturbance”, “tolerant to disturbance”, or “opportunistic” ecological groups according to the AZTI classification. Consequently, these changes resulted in negative W-index values and a deterioration of both AMBI and M-AMBI ecological quality values (Figs. 8, 9). The functional structure shifted toward the prevalence of small, vermiform, mobile subsurface deposit-feeding, and omnivore forms (Fig. 7b). Thus, there was a distinct shift in the structure and function of the macrofauna at the deepest stations, indicating habitat quality degradation, whereas the faunal associations in the shallower parts of the bay remained essentially unaffected.
The most likely reason for such catastrophic changes was the direct impact of the burial of the pipeline by a trencher that began in late 2011. Intensive dredging and dumping of sediments continued from 2012 to 2013. During this period, a total of 2.5 million tons of sediment was resuspended. The actual data on water turbidity and sedimentation are not available, but computer modeling (Fillipov 2008; Mironyuk 2014) showed that the area of high turbidity (with a suspended matter content of 100 mg L−1 over the background level) should be at least 5–7 km2, while the area of the less-turbid zone (approximately 25–50 mg L−1 over the background suspension content) should be 12 km2. As a result of dredging and resedimentation, approximately 45 km2 of sea bottom would be covered by a 5-mm thick layer of silty deposits; in at least one-third of this area, the deposit layer was predicted to be thicker than 1 cm. The most intensive sedimentation was likely to occur in narrow (1–2 km wide) areas in the central part of the bay south of the pipeline track. The dumping area for dredged sediment disposal was also located in this zone (see the map in Fig. 1b). All five deep-sea stations in 2013 (including one lacking macrofauna) were located inside of this area. The stations in 2012 were located north of the track; thus, the macrobenthos there was apparently less affected by dredging.
Increased concentrations of suspended matter, particularly fine fractions, may potentially affect the benthic macrofauna. The available experimental data (reviewed in Wilber and Clarke 2001; Berry et al. 2003), however, indicate that suspension contents up to 100 mg L−1 lead to reduced feeding and growth rates of filter-feeding benthos (crustaceans and bivalves) but do not affect the survival of adults, larvae, or juveniles. Much higher suspended matter concentrations are required to elicit mortality or serious sublethal effects. In view of the model-estimated suspension levels in the bay, a noticeable impact of this short-term factor on the benthos seems unlikely.
The subsequent intensive sedimentation could affect the benthic organisms more seriously. In an extensive review, Essink (1999) suggested that for sessile benthic organisms, such as bivalves, the fatal depth of mud deposition is only 0.5–2 cm, while motile organisms, particularly polychaetes, can survive much more intensive sediment deposition. At lower temperatures, however, benthic crawling activity, and therefore the chance of escaping potential burial, is smaller. Thrush et al. (2004) showed that as little as 3 mm of rapidly deposited sediment is sufficient to alter the macrobenthic community structure. Ten days after the application of 7 mm of sediment, experimental plots had lost approximately 50% of their individuals and species. Smith and Kukert (1996) recorded low macrobenthic diversity, small mean body size, low biomass, but high abundance of very small deposit-feeding polychaetes in response to high rates of sedimentation. A significant increase in the abundance of polychaete families with opportunistic lifestyles (e.g., Cossuridae, Capitellidae, Cirratulidae, and Paranoidae) was described shortly after the dumping of dredged sediments in Québec, Eastern Canada (Harvey et al. 1998). This is exactly what we observed in 2013 at the stations inside the dumping area. The decreased diversity and prevalence of small opportunistic species may also be caused by organic enrichment due to the redeposition of organic matter buried in deeper layers. We observed a high amount of plant debris in some of the 2013 samples.
The recovery of the benthos at a dump site may be considerably long. More than 2 years was required for the disturbed areas in Québec to re-establish community structure (Harvey et al. 1998). In the Arctic, this process appears to be much slower due to the low rates of recruitment and growth. According to some estimates, it can take up to 12 years for the community to be restored (De Groot 1979). For instance, the macrofauna of sites in the Barrow Strait that were exposed to ice scour did not completely recover until after 9 years of observation (Conlan and Kvitek 2005). Unfortunately, more recent data from the bay are not available.
Performance of the AZTI Marine Biotic Indices
The AZTI Marine Biotic Indices are generally recognized as the meaningful indicators of the habitat status of soft marine sediment habitats. However, there are few examples of the application of the AMBI and no examples of the application of M-AMBI in Arctic waters (Borja et al. 2015). Thus, we believe it is appropriate to briefly comment on their performance in our case.
Among the 219 unique taxa in our combined list, 33 lacked classifications in the AMBI. However, these were less important species that together represented 0.4% to 6.9% of the individuals at a station and only 1% of the whole dataset. This is far less than the 20% limit of “not assigned” taxa recommended by Borja and Muxika (2005); the available AZTI species list is therefore sufficient for obtaining a reliable evaluation of the Arctic macrofauna. Both AMBI and M-AMBI values appeared to be highly coherent with all other metrics used (diversity, biomass, community structure, and W statistic). Thus, our results support the application of these indices of community integrity to detect the environmental impacts in Arctic marine ecosystems and show that the faunal response rapidly follows a local disturbance. At the same time, the annual values varied in a relatively narrow range (0.52–1.9 for AMBI and 0.65–0.93 for M-AMBI) because we averaged the station-based estimates over all the depths without making the distinction between intact and impacted areas. This was done to achieve the comparability of the results based on unequal, and sometimes very limited, data. Further work is necessary to optimize this approach for the Arctic region. It requires (i) improving the ecological knowledge of some species that widely occur in the Arctic but are still lacking from the AMBI EG list and (ii) calculating regionalized reference conditions, considering the differences between geographic regions, depth zones, and sediment types.
Conclusions
In summary, we conclude that the benthic macrofauna in the Baydaratskaya Bay remained relatively stable over the last several decades. The structure and distribution of communities are mainly driven by depth and hydrology and do not show any long-term trends. At the same time, there were some local but evident disturbance effects that were evidently caused by recent human activities (the dumping of dredged sediments). These negative effects appear to be rather strong and rapidly manifested, indicating the high sensitivity and vulnerability of this ecosystem. The presented data could be used as the baseline assessment for the standard level of natural variation in this benthic ecosystem, which could further be used as a reference point when monitoring future changes in the ecosystem and the possible effects of human or natural pressures.
References
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E ltd, Plymouth
Antipova TV, Semenov VN (1989) Benthos of the Kara Sea. Biocoenoses of zoobenthos of the southern-western parts of the Kara Sea. In: Matishov GG (ed) Ecology and bioresources of the Kara Sea. Kola Science Centre of the Russian Academy of Sciences, Apatity, pp 120–150 (in Russian)
Azovsky AI (2018) Analysis of long-term biological data series: methodological problems and possible solutions. Biol Bull Rev 79:329–341 (in Russian, with English summary)
Azovsky AI, Chertoprood MV, Kucheruk NV, Rybnikov PV, Sapozhnikov FV (2000) Fractal properties of spatial distribution of intertidal benthic communities. Mar Biol 136:581–590. https://doi.org/10.1007/s002270050718
Benjamini Y, Yekutieli D (2001) The control of the false discovery rate in multiple testing under dependency. Ann Stat 29:1165–1188
Berry W, Rubinstein N, Melzian B, Hill B (2003) The biological effects of suspended and bedded sediment (SABS) in aquatic systems: a review. United States Environmental Protection Agency, Duluth
Borja Á, Muxika I (2005) Guidelines for the use of AMBI (AZTI’s Marine Biotic Index) in the assessment of the benthic ecological quality. Mar Poll Bull 50:787–789. https://doi.org/10.1016/j.marpolbul.2005.04.040
Borja Á, Franco J, Pérez V (2000) A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Mar Poll Bull 40:1100–1114. https://doi.org/10.1016/S0025-326X(00)00061-8
Borja Á, Marín SL, Muxika I, Pino L, Rodríguez JG (2015) Is there a possibility of ranking benthic quality assessment indices to select the most responsive to different human pressures? Mar Poll Bull 97:85–94. https://doi.org/10.1016/j.marpolbul.2015.06.030
Bowler DE, Hof C, Haase P et al (2017) Cross-realm assessment of climate change impacts on species’ abundance trends. Nat Ecol Evol 1:0067. https://doi.org/10.1038/s41559-016-0067
Clarke KR (1990) Comparisons of dominance curves. J Exp Mar Biol Ecol 138:143–157. https://doi.org/10.1016/0022-0981(90)90181-B
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E, Plymouth
Conlan KE, Kvitek RG (2005) Recolonization of soft-sediment ice scours on an exposed Arctic coast. Mar Ecol Progr Ser 286:21–42. https://doi.org/10.3354/meps286021
Conlan KE, Lenihan HS, Kvitek RG, Oliver JS (1998) Ice scour disturbance to benthic communities in the Canadian high Arctic. Mar Ecol Prog Ser 166:1–16
De Groot SJ (1979) An assessment of the potential environmental impact of large-scale sand-dredging for the building of artificial islands in the North Sea. Ocean Manag 5:211–232. https://doi.org/10.1016/0302-184X(79)90002-7
Degen R, Aune M, Bluhm BA, Cassidy C, Kędra M, Kraan C, Vandepitte L, Włodarska-Kowalczuk M, Zhulay I, Albano PG, Bremner J (2018) Trait-based approaches in rapidly changing ecosystems: A roadmap to the future polar oceans. Ecol Ind 91:722–736. https://doi.org/10.1016/j.ecolind.2018.04.050
Denisenko SG, Anisimova NA, Denisenko NV, Zhukov EI, Polyanskyi VA, Semenov VN (1993) Distribution, structural and functional organization of zoobenthos. In: Chinarina AD (ed) Hydrobiology research of the Baydaratskaya Bay (the Kara Sea) 1991–1992. KSC RAS, Apatity, pp 1–64 (in Russian)
Denisenko NV, Rachor E, Denisenko SG (2003) Benthic fauna of the southern Kara Sea. Proc Mar Sci 6:213–236
Ecological Survey (Marine Areas), Part 2 (2006) Consolidated technical report no. 31/05(2)-01-PП-ИЭИ-0406-C1. Peter Gaz LLC, Moscow (in Russian)
Ermakova LA, Novikhin AE (2011) Some data on hydrology of the near bottom layer of the Kara Sea using the materials of expeditions of R/Vs Barkalav-2007 and Barkalav-2008. Probl Arkt Antarkt 3:89–100 (in Russian)
Essink K (1999) Ecological effects of dumping of dredged sediments: options for management. J Coast Conserv 5:69–80. https://doi.org/10.1007/BF02802741
Fetzer I, Arntz WE (2008) Reproductive strategies of benthic invertebrates in the Kara Sea (Russian Arctic): adaptation of reproduction modes to cold water. Mar Ecol Prog Ser 356:189–202. https://doi.org/10.3354/meps07271
Filatova ZA, Zenkevich LA (1957) Quantitative distribution of benthic fauna of the Kara Sea. Trudy Vsesoyuznogo Gidrobiologicheskogo Obshchestva 8:3–62 (in Russian)
Filippov YuG (2008) The use of mathematical modeling for the evaluation of possible ecological loss at the construction of underwater pipelines over the Bay of Baidara and the Gulf of Ob. Trudy Gosudarstvennogo Okeanograficheskogo Instituta 211:340–348 (in Russian, with English summary)
Frid CLJ, Caswell BA (2015) Is long-term ecological functioning stable: the case of the marine benthos? J Sea Res 98:15–23. https://doi.org/10.1016/j.seares.2014.08.003
Geptner AP (Ed) (1997) Natural environment of Baydaratskaya Bay. Principal results of the survey for the underwater passage of the Yamal–Center gas pipeline construction. GEOS, Moscow (in Russian)
Golikov AN, Gagaev SYu, Galtsova VV et al (1994) Ecosystems of the Chaun Bay. East Siberian Sea. Explor Fauna Seas 47(55):4–112 (in Russian)
Harvey M, Gauthier D, Munro J (1998) Temporal changes in the composition and abundance of the macro-benthic invertebrate communities at dredged material disposal sites in the Anse á Beaufils, Baie des Chaleurs, Eastern Canada. Mar Poll Bull 36:41–55. https://doi.org/10.1016/S0025-326X(98)90031-5
Huntington HP, Boyle M, Flowers GE, Weatherly JW, Hamilton LC, Hinzman L, Gerlach C, Zulueta R, Nicolson C, Overpeck J (2007) The influence of human activity in the Arctic on climate and climate impacts. Clim Change 82:77–92. https://doi.org/10.1007/s10584-006-9162-y
Josefson AB, Mokievsky V, Bergmann M, Blicher ME, Bluhm B, Cochrane S, Denisenko NV, Hasemann C, Jørgensen LL, Klages M, Schewe I (2013) CAFF Arctic biodiversity assessment-marine invertebrates. In: CAFF status report, pp 224–257
Jørgensen LL, Pearson TH, Anisimova NA, Gulliksen B, Dahle S, Denisenko SG, Matishov GG (1999) Environmental influences on benthic associations of the Kara Sea (Arctic, Russia). Polar Biol 22:395–416. https://doi.org/10.1007/s003000050435
Jørgensen LL, Archambault P, Blicher M, Denisenko N, Gudmundsson G, Iken K et al (2017) Benthos. In: Barry T, Price C, Olsen M, Christensen T, Frederiksen M (eds) State of the Arctic marine biodiversity report. Conservation of Arctic Flora and Fauna International Secretariat, Akureyri, pp 85–107
Kokarev VN, Kozlovsky VV, Azovsky AI (2015) Current state of macrobenthic communities in Baydaratskaya Bay (Kara Sea). Oceanology 55:724–729. https://doi.org/10.1134/S0001437015050069
Kokarev VN, Vedenin AA, Basin AB, Azovsky AI (2017) Taxonomic and functional patterns of macrobenthic communities on a high-Arctic shelf: a case study from the Laptev Sea. J Sea Res 129:61–69. https://doi.org/10.1016/j.seares.2017.08.011
Kortsch S, Primicerio R, Beuchel F, Renaud PE, Rodrigues J, Lønne OJ, Gulliksen B (2012) Climate-driven regime shifts in Arctic marine benthos. Proc Natl Acad Sci 109(35):14052–14057. https://doi.org/10.1073/pnas.1207509109
Kozlovskiy VV, Chikina MV, Kucheruk NV, Basin AB (2011) Structure of the macrozoobenthic communities in the southwestern Kara Sea. Oceanology 51:1012–1020. https://doi.org/10.1134/S0001437011060087
Kucheruk NV, Mokievskiy VO, Denisov NE (1998) Macrobenthos of the coastal shallow zone in the southwestern part of the Kara Sea. Oceanology 38:86–95
Mironyuk SG (2014) Assessment of environmental consequences and use of submerged transition of the gas main pipeline across Baydaratskaya Bay (Kara Sea). Arkt Ekol Ekon 3:72–78 (in Russian)
Motychko VV, Opekunov AYu, Konstantinov VM, Sokolov GN (2013) Morpholithogenesis and composition of bottom sediments of Baydaratskaya Bay. Vestnik of St-Petersburgb Gos. Universitet 1:65–77 (in Russian)
Muxika I, Borja A, Bald J (2007) Using historical data, expert judgement and multivariate analysis in assessing reference conditions and benthic ecological status, according to the European Water Framework Directive. Mar Poll Bull 55:16–29. https://doi.org/10.1016/j.marpolbul.2006.05.025
Naumov AD (2006) Clams of the White Sea. Explor Fauna Seas 59(67):367 (in Russian)
Piepenburg D (2005) Recent research on Arctic benthos: common notions need to be revised. Polar Biol 28:733–755. https://doi.org/10.1007/s00300-005-0013-5
Renaud PE, Włodarska-Kowalczuk M, Trannum H, Holte B, Węsławski JM, Cochrane S, Dahle S, Gulliksen B (2007) Multidecadal stability of benthic community structure in a high-Arctic glacial fjord (van Mijenfjord, Spitsbergen). Polar Biol 30:295–305. https://doi.org/10.1007/s00300-006-0183-9
Renaud PE, Sejr MK, Bluhm BA, Sirenko B, Ellingsen IH (2015) The future of Arctic benthos: expansion, invasion, and biodiversity. Prog Oceanogr 139:244–257
Skazina M, Sofronova E, Khaitov V (2013) Paving the way for the new generations: Astarte borealis population dynamics in the White Sea. Hydrobiologia 706:35–49
Smith CR, Kukert H (1996) Macrobenthic community structure, secondary production, and rates of bioturbation and sedimentation at the Kane’ohe Bay Lagoon Floor. Pac Sci 50:211–229
Solyanko K, Spiridonov V, Naumov A (2011) Biomass, commonly occurring and dominant species of macrobenthos in Onega Bay (White Sea, Russia): data from three different decades. Mar Ecol 32:36–48. https://doi.org/10.1111/j.1439-0485.2011.00438.x
Steffens M, Piepenburg D, Schmid MK (2006) Distribution and structure of macrobenthic fauna in the Eastern Laptev Sea in relation to environmental factors. Polar Biol 29:837–848. https://doi.org/10.1007/s00300-006-0122-9
Stepanova VB (2000) The data on zoobenthos in Baydaratskaya Bay. Vestn Ekol Lesovedeniya Landshaftovedeniya 1. https://www.ipdn.ru/rics/ve2/index.htm. Accessed 26 June 2018 (in Russian)
Thrush SF, Hewitt JE, Cummings VJ, Ellis JI, Hatton C, Lohrer A, Norkko A (2004) Muddy waters: elevating sediment input to coastal and estuarine habitats. Front Ecol Envir 2:299–306. https://doi.org/10.1890/1540-9295(2004)002[0299:MWESIT]2.0.CO;2
Vozzhinskaya VB, Bel'kovich VM, Gorelova TA, Kuzin VS, Kucheruk NV, Mokievskii VO, Vinogradov GM (1997) Hydrobiological and ecological studies in the Arctic: Sea biota of the Kara Sea southwestern coast (Baidaratskaya Bay). Biol Bull 24:583–593
Warwick RM (1986) A new method for detecting pollution effects on marine macrobenthic communities. Mar Biol 92:557–562. https://doi.org/10.1007/BF00392515
Wassmann P, Duarte CM, Agustì S, Sejr MK (2011) Footprints of climate change in the Arctic marine ecosystem. Glob Change Biol 17:1235–1249. https://doi.org/10.1111/j.1365-2486.2010.02311.x
Wilber DH, Clarke DG (2001) Biological effects of suspended sediments: a review of suspended sediment impacts on fish and shellfish with relation to dredging activities in estuaries. N Am J Fish Manag 21:855–875. https://doi.org/10.1577/1548-8675(2001)021%3c0855:BEOSSA%3e2.0.CO;2
Acknowledgements
We thank E.M. Krylova, A.B. Basin, and I.A. Jirkov for consulting about the identification and taxonomical problems. This study was supported by the Russian Scientific Foundation (Grants No. 14–50-00029, database design, and No. 14–50-00095, field research) and the Russian Foundation for Basic Research (Grant No. 18–04-00206, collections treatment, and data analysis).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Azovsky, A.I., Kokarev, V.N. Stable but fragile: long-term dynamics of arctic benthic macrofauna in Baydaratskaya Bay (the Kara Sea). Polar Biol 42, 1307–1322 (2019). https://doi.org/10.1007/s00300-019-02519-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-019-02519-y