Abstract
The impact of penguins on vegetation cover is a frequently documented but not fully explained phenomenon, especially over geological time scales. To address this we collected two ornithogenic sediment cores from Ardley Island, Antarctica, and reconstructed historic variations of penguin population and different vegetations by using biomarkers fecal sterols and n-alkanols in the cores. The three major types of vegetations in the colony, namely coprophilic algae, moss and lichen, exhibited quite different trends, in concert with fluctuations in the penguin populations: a moderate penguin population is favorable for coprophilic algae and mosses, while lichen abundances decreased whenever penguin population increased, and vice versa. Cholestanol and alkanols biomarkers, in combination with bioelements, provided integrated information about the 2,400-year evolution of the penguin colony: historical penguins and vegetation populations, their fluctuations, and their interactions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Penguin colonies are a common and important biota in the maritime Antarctic terrestrial ecosystems (Tatur and Myrcha 1989; Tatur et al. 1997), and co-occur with vegetation dominated by algae, mosses and lichens (Tatur et al. 1997; Sun et al. 2004a; Vidal et al. 2003). Penguins, as typical “guano birds”, i.e. densely nesting, breeding in large colonies, and depositing large amount of excreta at the nests, may profoundly impact vegetation near their colonies (Hutchinson 1950; Ellis 2005). Penguins manure the surrounding vegetation through their effect on the soils, and at the same time they cause considerable physical disturbances such as trampling and uprooting of vegetation.
Most of the studies of the impact of seabirds on vegetation (Ellis 2005) are based on field observations of the spatial distribution of bioelements and/or isotope data of soil and plants (Hovenden and Seppelt 1995; Cocks et al. 1998; Vidal et al. 2003; Michel et al. 2006); and long-term, continuous and quantitative data of penguin and vegetations remain very limited. Tatur et al. (1997) studied the plant colonization processes at several abandoned penguin rookeries. Sun et al. (2000) applied nine bioelements (such as F, P and Cu), which are highly enriched in the sediments influenced by penguin droppings, and reconstructed the historic penguin populations (Sun et al. 2000).
In our previous study (Sun et al. 2004), we reported a general, seesaw-like relationship between penguin colonies and tundra vegetation using the bioelements and X-ray photographs of the sediments on Ardley Island and Barton Peninsula. However, plants near seabird colonies may have elemental and even isotopic compositions similar to those of seabirds (e.g., Cocks et al. 1998; Xie and Sun 2003; Liu et al. 2006); and the element profiles may not be sufficiently specific for the study of historic variation of penguins or vegetation since bioelements in the sediments might come from both penguins and vegetation.
In the present study, we used more specific organic molecular markers to reconstruct the historical abundances of penguins and vegetation and examined historic interactions between penguins and vegetation.
Materials and methods
Sampling site
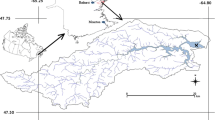
Ardley Island (62°13′S, 58°56′W) (maritime Antarctica) (Fig. 1), 2.0 km in length, 1.5 km in width, is defined as a site of special scientific interest by Scientific Committee on Antarctica Research (SCAR). There were about 10,000 penguins on the island in the breeding season of 1998; 70–80% of the island was covered by three major vegetation types: algae, mosses and lichens, around the penguin living area.
Map of location of Y2 Lake on Ardley Island. The bottom figure is the enlargement of Ardley Island and the black areas showed in the bottom figure are lakes. (From Liu et al. 2006)
We collected two sediment cores Y2 (67.5 cm in length) and its backup Y2-1 (60 cm in length) from a shallow lake Y2 on the island, and both cores had a strong pungent odor from penguin droppings. Y2 sediment core have been shown to be ornithogenic as inferred by its profile of enriched bioelements (Sun et al. 2000), isotopes (Liu et al. 2006), HCl-soluble 86Sr/87Sr (Sun et al. 2005), and chitinase genes (Xiao et al. 2005).
In this study, 14 samples of Y2-1 from depths of 0–1, 8–9, 14–15, 19–20, 20–21, 24–25, 30–31, 35–36, 41–42, 44–45, 45–46, 52–53, 55–56, and 59–60 cm were analyzed for TOC, biomarkers and bioelements. As shown in our previous studies on the core Y2, bioelements have significant changes at these depths.
Sample analysis
Freeze-dried samples were Soxhlet extracted for 72 h with 2:1 dichloromethane/methanol. The extracts were concentrated by rotary evaporation and then saponified using 0.5 M KOH/MeOH. Neutral lipids were partitioned out of the basic solution with hexane. The pH of the saponified extract was then brought to 2 with 6 N HCl and acidic lipids were extracted with 20% methylene chloride in hexane. Acidic lipids were allowed to sit in the presence of anhydrous Na2SO4 overnight in order to remove traces of water. Neutral lipids were further separated on 5% deactivated silica gel column chromatography using solvents of increasing polarity from hexane through methylene chloride. The fractions containing hydrocarbons (eluted with hexane) and n-alkanols/sterols (eluted with methylene chloride) were collected separately. The alcohols and acids fraction was treated with BSTFA (N,O-bis-trimethylsilyltrifluoroacetamide) to form trimethylsilyl (TMS)-ether derivatives.
The derivatives of alcohol fraction (including n-alkanols and sterols derivates) were analyzed on an HP 5890 gas chromatograph–mass spectrometer with a DB5 (50 m × 0.32 mm × 0.25 μm) capillary column (J&W). Helium was used as the carrier gas. The mass spectrometer was operated in EI mode at 70 eV. The GC oven was programmed as follows: hold 2 min at 60°C, increase to 200°C at 10°C/min, increase to 280°C at 3.5°C/min, hold 15 min at 280°C, increase to 300°C at 1.5°C/min, and hold 30 min at 300°C. Analysis was done in SCAN mode.
Gas chromatograph was conducted on an HP 5890 with a splitless/split injector and a flame ionization detector (FID). The same column and temperature program as the GC performance were used. Hydrogen was used as the carrier gas with a flow rate of 2 ml/min. Organic biomarkers were analyzed in State Key Laboratory of Organic Geochemistry, Guangzhou Institute of Geochemistry, Chinese Academy of Science (CAS).
Dating
The chronology of the sediment core Y2 was determined by conventional radiocarbon dating and cosmogenic radioisotope dating methods in Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences (CAS). After treatment with diluted HCl to remove possible carbonate content, 14C measurement was conducted on organic carbon components by using a Quantalas-1220 liquid scintillometer. The radiocarbon ages were reported as year bp (years before 1950). The quoted errors in the dates are based on the reproducibility of measurement. The chronology was firstly reported by Sun et al. (2000), and the method was detailed by Sun et al (2001). The chronologies of Y2-1 and Y2 were assumed to be the same: the distance between them was less than 0.5 m and their elemental profiles are similar.
Results
The concentration-versus-depth profiles of TOC and bioelements in the sediment core Y2-1 are shown in Fig. 2. The levels of bioelements and TOC are significantly correlated with each other, and they are consistent with those of Y2 (Sun et al. 2000; Sun and Xie 2001). This was expected as Y2-1 was essentially the replicate of Y2 and indicated that Y2-1 was also significantly impacted by penguin droppings.
Key ion m/z 215 was selected to analyzed sterols and m/z 103 for n-alkanols. The alcohols in Y2-1 were mainly cholestanol (5α-cholestan-3β-ol), phytol and cholesterol (cholest-5-en-3β-ol). Epicoprostanol (5β-cholestan-3α-ol), coprostanol (5β-cholestan-3β-ol), and even carbon number predominant alkanols were also detected but in relatively lower levels (Fig. 3).
Molecular markers for penguin feces
Cholesterol, cholestanol, coprostanol and epicoprostanol are the common fecal sterols in animal and birds’ feces, and they have been used as biomarkers of fecal contamination (Murtaugh and Bunch 1967; Leeming et al. 1996; Bull et al. 2002). Cholesterol made up 98% of the total sterols in fresh penguin guano (Venkatesan 1989), and cholestanol is the main product of microbially mediated reduction of cholesterol in sediments (Murtaugh and Bunch 1967; Hatcher and McGillivary 1979). Normally the concentrations of cholestanol were very low in sediments, and much lower than those of cholesterol (e.g., Fernandes et al. 1999; Bull et al. 1999).
In Y2-1, the concentrations of cholestanol (up to 260 μg/g) were very high and many times of those of cholesterol, and their profiles were significantly correlated (R 2 = 0.956, P < 0.001). Such striking similarity indicates that cholesterol and cholestanol were from the same sources.
In Antarctica organic matters in sediments tend to be well preserved due to very low temperature and weak sunlight (Beyer et al. 1995); furthermore, sterols usually have a long geological record for their comparative stable structures (Volkman 1986). In Core Y2-1 only one sterane, the decomposition product of sterols, cholest-2-ene (examined by M/Z 213, 215 and 217), was detected in the hydrocarbon fraction, and its highest concentration was only 0.5 μg/g, negligible as compared with the concentrations of cholestanol (maximum 260 μg/g), so we think the decomposition of the sterols can be ignored here.
Considering that Y2-1 was significantly impacted by penguin droppings, cholestanol in Y2-1 was most likely derived from penguin droppings via the hydrogenation of cholesterol; and cholestanol and cholesterol could be good biomarkers for penguins’ fecal influence or penguin population.
Molecular markers for different vegetations
As shown in Fig. 4, the profiles of even carbon numbered alkanols (C16–C30) in Y2-1 have three distinct patterns: the first pattern is represented by C16, C18 and C20, the second by C22, C24, and C26, and the third by C28 and C30. Fatty alkanols come from the epicuticular waxes of vegetation, and they have been used to distinguish different types of vegetation. In general, algae have n-alkanol distributions dominated by C16–C22, mosses by C22–C26, and terrestrial plants by C26, C28 and C30 (Meyers 2003).
Profiles of n-alkanols in Y2-1 (μg/g). The profiles form three distinct patterns: 1 C16, C18 and C20 alkanol; 2 C22, C24 and C26 n-alkanol; 3 C28, and C30 n -alkanol. According to these patterns and characteristics of the vegetation on Ardley Island, we suggested that these three distinct patterns represent coprophilic algae, mosses and lichens, respectively
On Ardley Island, coprophilic algae, mosses and lichens are three major plants, and their abundances are consistent with their characteristics as summarized in Table 1 and correlated with the levels of corresponding fatty alkanols. Coprophilic algae are the first thin, green algae growing spontaneously on the uncovered guano surface (Tatur et al. 1997). As shown in Fig. 5, the abundance of nC16-ol and nC18-ol varied closely with that of cholestanol and cholesterol and thus penguin population. Mosses are the dominant plants in the surface sediments around Lake Y2; and in the core Y2-1, nC24-ol and nC26-ol are the only increasing alkanols in the surface sediments (Fig. 4). On Fildes Peninsula, the peninsula next to Ardley Island (Fig. 1), C24 and C26 n-alkanols have been used as biomarkers for mosses (Zhang et al. 2000). Lichens can live with minimum amount of nutrition (Friedmann 1982, Friedmann and Weed 1987); and more nutrition is unfavorable for its growth due to physical disturbances from larger penguin population. At the depth 36 cm, where fecal sterols decreased to an undetectable level and the climate seemed not favorable for penguins, only nC28-ol and nC30-ol increased.
Based upon the characteristics of coprophilic algae, mosses, lichens, and the distinct profiles of alkanols in Y2-1, C16–C20, C22–C26 and C28–C30 n-alkanols were used in this study as biomarkers for coprophilic algae, mosses and lichens, respectively.
Discussion
Historic changes of penguins and vegetations
The profiles of fecal sterols (cholestanol and cholesterol), n-alkanols, TOC and F in Y2-1 are given in Fig. 5. They provide a historic record of both penguins and plants, over the past 2,400 years. For convenience of discussion, we divided the past 2,400 years into five periods mainly according to the fluctuations of biomarkers and bioelements.
Period I (2,340–1,800 y bp): corresponding to the Neoglaciation Age (Ingolfsson et al. 1998; Yoon et al. 2002). Concentrations of F, TOC and all biomarkers were all low, indicating a low penguin population and poor vegetation.
Period II (1,800–1,360 y bp): penguin population increased to a high level, and vegetation stayed at low levels in spite of short-term fluctuations in penguin population. The short-term fluctuation in penguin population seemed to have no material effect on vegetation abundance.
Period III (1,360–700 y bp): at first along with the decline of penguin population, coprophilic algae decreased, mosses increased, and lichens reached a peak level. The increase of penguin population after that promoted the growth of coprophilic algae and mosses, but further increase seemed to have negative effect on both vegetations. Lichens decreased sharply with the increase of penguin population.
Period IV (700–400 y bp): the reduction in the penguin population caused the decrease of coprophilic algae and mosses, but lichens increased.
Period V (400 y bp-present): corresponding to the surface sediment, except TOC and C26 n-alkanol, the levels of all other bioelements and biomarkers decreased. This is consistent with the observations of the present Y2 Lake: mass mosses are abundant and there are no penguins living there.
Comparison between biomarkers and bioelements
Elemental analysis of lake sediments usually focuses on inorganic and lithological characters such as mineral composition and grain size. Bioelements are different; they come from bioaccumulation by biota, animals or vegetation; and they have been applied as proxies for historical population of penguins (Sun et al. 2000), seals (Sun et al. 2004b), and other seabirds (Liu et al. 2006). Sea animals and seabirds, including penguins, are more capable of accumulating bioelements than vegetations. Penguins live very densely in their colonies, and are the dominant sources of bioelements.
The main advantage of bioelements as proxies is their stability after deposition. Bioelements, however, do have a number of drawbacks. First, some bioelements, such as Ca and Ba, could be from local soil and rock. Second, bioelements, could come from both animals and vegetation. For example, F content is very high at 14,725 and 2,285 μg/g (Xie and Sun 2003), respectively, in both penguin guano and mosses on Ardley Island. Thus reconstruction of historical sea animal populations by bioelements alone could be inaccurate.
Molecular markers, or biomarkers, are quite distinct from inorganic bioelements. In the sediment core Y2-1, as shown in Fig. 5, the profiles of biomarkers showed some significant differences from those of bioelements. First, biomarkers, particularly cholestanol, after peaking in period II, dropped back to and rose from a near-zero value several times, whereas TOC and bioelements, after peaking in period II, fluctuated and stayed at a relatively high level. For example, at 36 cm (ca. 1,220 y bp) of Y2-1, the concentration of cholestanol was undetectable, the same as in period I (2,300–1,800 y bp); but the concentration of F was 4,280 μg/g, far above that in the bottom of period I (60 cm 604 μg/g).
Second, the penguin populations as indicated by sterols fluctuated more frequently and intensively than those by bioelements (for example, periods II and IV), and they are consistent with recent field observations of penguin populations in Antarctica. For example, the number of emperor penguins at Pointe Géologie archipelago, Terre Adélie, declined by 50% because of a decrease in adult survival during the late 1970s; and the biggest decline occurred between 1975 and 1982 with the number of penguins dropped from about 6,000 pairs (1975) to 3,000 pairs (1982) (Barbraud and Weimerskirch 2001). The breeding population of Adélie penguin in the Ross Sea increased from about 1,000 pairs (1979) to 4,000 pairs (1989) (Croxall et al. 2002).
Third, most of the differences between the profiles of bioelements and biomarkers occurred when the penguin population was not high and vegetation was abundant (Fig. 5). This again indicates that bioelements, unlike biomarkers, come from both vegetation and penguins.
Fecal sterols (cholestanol and cholesterol), as molecular markers or biomarkers, have a single and specific source of penguin droppings, and they provide more accurate, more sensitive and higher resolution information about historical penguins populations than bioelements.
Evolution of the ecosystem: penguins and vegetations
The relationship between animals and vegetation is critical for studying an ecological system and its evolution. Antarctic ecosystems are simpler than those on other continents, and a good understanding of the relationship between penguins and vegetation in the simple penguin colony will be helpful for understanding other and more complex ecosystems.
The long temporal and quantitative record of penguin population, vegetation abundance, and their relationship, as recorded in the Y2-1 sediment core, is consistent with the common distribution pattern of vegetation around active penguin rookeries and with the generally observed sequence of vegetation at abandoned penguin rookeries: beginning with ornithocoprophilic or nitrophilic species and ending with nitro-neutral communities (Tatur et al. 1997).
To a large degree the consistencies were controlled by the balance between the nutrient requirements of the vegetation and the availability of soil nutrient provided by penguins.
-
1.
Coprophilic algae depended more upon nutrient input from penguin guano than mosses; they are found nearest to penguin rookeries among the three major vegetation types. Hence their abundances varied almost immediately with penguin population changes.
-
2.
Moss depended less on soil nutrition, so its abundance around Y2 Lake was less sensitive to penguin population changes.
Moderate penguin population seemed to be favorable for both coprophilic algae and mosses since they supply sufficient nutrients from penguin without too much physical disturbance like tramping.
-
3.
In contrast, lichens can live in the most arid soil or even on stones in Antarctica (Friedmann 1982, Friedmann and Weed 1987), and manuring would not benefit their growth. The decrease of penguin population coming with the cooling climate (like the time corresponding to 36 and 15 cm in the cores) might result in less physical disturbances from penguins and more living spaces for lichens. So the relationship between lichens and penguins was mainly seesaw like: lichen abundance decreased when penguin population increased, and vice versa.
By using fecal sterols and three distinct alkanol biomarkers, we reconstructed the historical penguins and vegetations abundances in a penguin colony of Antarctic for the past 2,400 years. The long temporal relationship between penguin population and vegetation abundance is consistent with the spatial distribution pattern of vegetation around active penguin rookeries and the generally observed time sequence of vegetations at abandoned penguin rookeries. Penguins play a dominant role in this simple ecosystem, and the abundance of different vegetations is mainly controlled by their dependence upon the nutrition provided by penguins.
References
Barbraud C, Weimerskirch H (2001) Emperor penguins and climate change. Nature 411:183–186
Beyer L, Sorge C, Blume H-P, Schulten H-R (1995) Soil organic matter composition and transformation in gelic Histosols of coastal continental Antarctica. Soil Biol Biochem 27:1279–1288
Bull ID, Simpson IA, Dockrill ST, Evershed RP (1999) Organic geochemical evidence for the origin of ancient anthropogenic soil deposits at Tofts Ness, Sanday, Orkney. Org Geochem 30:535–556
Bull ID, Lockheart MJ, Elhmmali MM, Roberts DJ, Evershed RP (2002) The origin of faeces by means of biomarker detection. Environ Int 27:647–654
Cocks MP, Balfour DA, Stock WD (1998) On the uptake of ornithogenic products by plants on the inland mountains of Dronning Maud Land, Antarctica, using stable isotopes. Polar Biol 20:107–111
Croxall JP, Trathan PN, Murphy EJ (2002) Environmental change and Antarctic seabird populations. Science 297:1510–1514
Ellis JC (2005) Marine birds on land: a review of plant biomass, species richness, and community composition in seabird colonies. Plant Ecol 181:227–241
Fernandes MB, Sicre M-A, Cardoso JN, Macedo SJ (1999) Sedimentary 4-desmethyl sterols and n-alkanols in an eutrophic urban estuary, Capibaribe River, Brazil. Sci Total Environ 231:1–16
Friedmann EI (1982) Endolithic microorganisms in the Antarctic cold desert. Science 215:1045–1053
Friedmann EI, Weed R (1987) Microbial trace-fossil formation, biogenous, and abiotic weathering in the Antarctic cold desert. Science 236:703–705
Hatcher PG, McGillivary PA (1979) Sewage contamination in the New York Bight—Coprostanol as an indicator. Environ Sci Technol 13:1225–1229
Hovenden MJ, Seppelt RD (1995) Exposure and nutrients as delimiters of lichen communities in continental Antarctica. Lichenologist 27:505–516
Hutchinson GE (1950) Survey of existing knowledge of biogeochemistry: 3. The biogeochemistry of vertebrate excretion. Bull Am Mus Nat Hist 96:1–544
Ingolfsson O, Hjort C, Berkman PA, Björck S, Colhoum E, Goodwin ID, Hall B, Hcrakawa K, Melles M, Moller P, Prentice ML (1998) Antarctic glacial history since the Last Glacial Maximum: an overview of the record on land. Antarct Sci 10:326–344
Leeming R, Ball A, Ashbolt N, Nichols P (1996) Using faecal sterols from humans and animals to distinguish faecal pollution in receiving waters. Water Res 30:2893–2900
Liu XD, Li HC, Sun LG, Yin XB, Zhao SP, Wang YH (2006) δ13C and δ15N in the ornithogenic sediments from the Antarctic maritime as paleoecological proxies during the past 2,000 y. Earth Planet Sci Lett 243:424–438
Meyers PA (2003) Applications of organic geochemistry to paleolimnological reconstructions, a summary of examples from the Laurentian Great Lakes. Org Geochem 34:261–289
Michel RFM, Schaefer CEGR, Dias LE, Simas FNB, Benites VD, Mendonca ED (2006) Ornithogenic gelisols (cryosols) from Maritime Antarctica: pedogenesis, vegetation, and carbon studies. Soil Sci Soc Am J 70:1370–1376
Murtaugh JJ, Bunch RL (1967) Sterols as a measure of fecal pollution. J Water Pollut Contr 39:404–409
Sun LG, Xie ZQ (2001) Changes in lead concentration in Antarctic penguin droppings in the past 3,000 years. Environ Geol 40:1025–1208
Sun LG, Xie ZQ, Zhao JL (2000) A 3,000-year record of penguin populations. Nature 407:858
Sun LG, Xie ZQ, Zhao JL (2001) The sediments of lake on the Ardley Island, Antarctica: identification of penguin-dropping soil. Chinese J Polar Sci 12:1–8
Sun LG, Zhu RB, Yin XB, Liu XD, Xie ZQ, Wang YH (2004a) A geochemical method for reconstruction of the occupation history of penguin colony in the maritime Antarctic. Polar Biol 27:670–678
Sun LG, Liu XD, Yin XB, Zhu RB, Xie ZQ, Wang YH (2004b) A 1,500-year record of Antarctic seal populations in response to climate change. Polar Biol 27:495–501
Sun LG, Zhu RB, Liu XD, Xie ZQ, Yin XB, Zhao SP, Wang YH (2005) HCl-soluble 87Sr/86Sr ratio in the sediments impacted by penguin or seal excreta as a proxy for the size of historical population in the maritime Antarctic. Mar Ecol Prog Ser 303:43–50
Tatur A, Myrcha A (1989) Soils and vegetation in abandoned penguin rookeries (maritime Antarctic). Proc NIPR Symp Polar Biol 2:181–189
Tatur A, Myrcha A, Niegodzisz J (1997) Formation of abandoned penguin rookery ecosystems in the maritime Antarctic. Polar Biol 17:405–417
Venkatesan MI, Ruth E, Kaplan IR (1989) Sterols in ocean sediment, novel tracers to examine habitats of cetaceans, pinnipeds and humans. Mar Biol 102:431–437
Vidal E, Jouventin P, Frenot Y (2003) Contribution of alien and indigenous species to plant-community assemblages near penguin rookeries at Crozet archipelago. Polar Biol 26:432–437
Volkman JK (1986) A review of sterol markers for marine and terrigenous organic matter. Org Geochem 9:83–99
Xiao X, Yin XB, Lin J, Sun LG, You ZY, Wang P, Wang FP (2005) Chitinase genes in lake sediments of Ardley Island, Antarctica. Appl Environ Microb 71:7904–7909
Xie ZQ, Sun LG (2003) Fluorine content in bones of Adelie penguins and environmental media in Antarctica. Environ Geochem Health 25:483–490
Yoon HI, Park BK, Kim Y, Kang CY (2002) Glaciomarine sedimentation and its paleoclimatic implications on the Antarctic Peninsula shelf over the last 15,000 years. Palaeogeogr Palaeoclimatol Palaeoecol 185:235–254
Zhang G, Sheng GY, Peng PA, Hong HA, Zhou SC (2000) Organic geochemistry of lake sediments in Fildes Peninsula, King George Island, Antarctica. Chin Sci Bull 45:2758–2762
Acknowledgments
We thank Dr. John K. Volkman and Dr. Rhys Leeming for their help in identifying the fecal sterols; we also thank Prof. Liqi Chen, Prof. Guoying Sheng and Dr. Jianfang Hu for providing valuable comments and suggestion to improve the manuscript. This research was supported by the Key Project of National Natural Science Foundation of China (No. 40476001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Wang, Y., Wang, X. et al. Penguins and vegetations on Ardley Island, Antarctica: evolution in the past 2,400 years. Polar Biol 30, 1475–1481 (2007). https://doi.org/10.1007/s00300-007-0308-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-007-0308-9