Abstract
The purpose of this work is to characterize several potential sources of organic matter (OM) in the Marquesas Islands, French Polynesia, and to understand how these sources contribute to OM pools. Stable isotope (δ13C and δ15N) and fatty acid (FA) compositions of OM potential sources (algal turf, benthic macroalgae, detrital terrestrial plants (DTP), phytoplankton) and OM pools (sediment (SOM) and particulate organic matter (POM)) were studied in coastal areas in Nuku Hiva Island. Isotope compositions of marine POM (δ13C = − 22.5 ± 0.8 ‰; δ15N = 12.1 ± 1.1 ‰) and SOM (δ13C = − 19.1 ± 0.9 ‰; δ15N = 14.4 ± 0.5 ‰) highlighted that OM sources at the base of the local food web had unusually high δ15N values. Potential FA markers of diatoms and/or cyanobacteria (20:5ω3 and 16:1ω7) were found in these two OM pools. In addition, coastal SOM also displayed FA markers of bacteria, macroalgae and terrestrial plants. For marine POM, potential macroalgae FA markers were recorded (18:2ω6 and 16:2ω4). The stable isotope mixing model highlighted the major contribution of phytoplankton to the coastal SOM (62%), followed by benthic macroalgae (32%), whereas marine POM was mainly composed of a mixture of algal turf and phytoplankton in similar averaged proportions (38%) but presenting wide variations. Our results as a whole strongly suggest that pelagic-benthic coupled processes drive the characteristics and properties of OM sources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ever-increasing pace of anthropogenic and climatic stressors has motivated a widespread interest in understanding the contribution of species to ecosystem functioning. This stems from the concern that a loss of species may correspond to a net loss of ecosystem productivity. However, understanding the mechanisms that maintain ecosystem productivity also require a definition of the major sources of energy and nutrients that fuel the entire ecosystem (Bierwagen et al. 2018).
In coastal ecosystems, the high diversity of sources available to primary consumers complicates the characterization of the food web and the understanding of energy flows (Mann 1988). Carbon sources may be of continental or marine origin. Moreover, they can come from coastal, pelagic or deep waters, and from living organisms or detrital matter (Gazeau et al. 2004; Bode et al. 2006). Food webs in coastal areas are often supported by phytoplankton (Bode et al. 2006). However, in several ecosystems, benthic production or the inputs from the terrestrial realm can also be major contributors to the organic matter (hereafter OM) pools (Riera and Richard 1996; Briand et al. 2015). Changes in the major source of nutrient may influence the primary production of coastal system with potential implications for the functioning of the ecosystem (Liénart et al. 2018). The pool of nutrient can be altered by several drivers of natural and anthropogenic origin. For example, nutrients can be altered by variation in the anthropogenic inputs or changes of the water mixing regime (D’Angelo and Wiedenmann 2014). Global climate change may also have an important effect by modifying the exchanges of organic matter at the interface between ocean and land (Liénart et al. 2018). For example, the input of the Particulate Organic Matter (hereafter POM) carried by rivers may dramatically change if water flow or seasonality are affected. Therefore, defining the vulnerability of coastal ecosystems requires an understanding of the basal organic matter that fuel the ecosystem.
Among coastal ecosystems, coral reefs are complex semi-open ecosystems. This complexity arises from the high species diversity and the large biomass typical of these systems (Choat et al. 2004). High diversity corresponds to myriads of potential trophic interaction (Hubert et al. 2011), which in turn makes it complex to describe the topology and functioning of coral reef food webs (Frisch et al. 2014). Benthic primary producers may be important carbon sources for coral reef food webs, although numerous species also rely on oceanic production (plankton) (Wyatt et al. 2013; Le Bourg et al. 2017). The type of resource that mainly contribute to the productivity of coral reefs partly depends on environmental (e.g. sea surface temperature, nutrient concentrations) and hydrodynamic conditions. These benthic and pelagic potential food sources are moreover available as mixtures of OM pools, their proportions depending on their abundance and their export capacity (degradation, consumption and rejection by feces, etc.). Indeed, POM is a mixture made of both living (e.g. bacteria, phytoplankton) and non-living matter (e.g. detritus, fecal pellets, clays) (Volkman and Tanoue 2002; Briand et al. 2015). This POM contributes significantly to coral ecosystem nutrient budgets and plays an important role in the transfer of organic matter between the land and the ocean (Sorokin and Sorokin 2010; Wyatt et al. 2010). Sedimentary organic matter (SOM) is another OM pool potentially composed of the same sources as POM, with the addition of macroalgae fragments, meiofauna and/or microphytobenthos (Wyatt et al. 2013; Briand et al. 2015). These differences of composition between SOM and POM, as well as a different bacterial activity, may lead to different signature of carbon and nitrogen stable isotopes.
The Marquesas Islands are a unique case among tropical coral ecosystems. The archipelago almost devoid of coral reefs. Coral communities may be locally abundant, but they are in the form of scattered colonies with low diversity (Benzoni and Pichon 2016). The surface waters around the Marquesas have high concentrations of nutrients; those of nitrate (NO3−) and phosphate (PO4−) are higher than 1 μM and 0.3 μM, respectively (Martinez et al. 2016). These concentrations are one hundred times higher than those measured in the South Pacific subtropical gyre (Martinez et al. 2016). This nutrient richness promotes the development of a significant phytoplankton biomass around the Marquesan coasts, with an average of 0.2 μg l− 1 of Chl-a over the year (Martinez et al. 2016). The richness of the Marquesan marine waters seems to be linked to a particular local enrichment mechanism, likely due to current disturbance related to the island topography (the so called “island effect”; Raapoto et al. 2018). These characteristics may imply a particular model of coastal trophic functioning, probably supported mainly by pelagic primary production.
Trophic markers such as stable isotopes (SI), fatty acids (FA) are useful tools to determine the flows of carbon in coastal ecosystems. In addition, C:N ratio provides information about the quality of the OM. Carbon (C) and nitrogen (N) isotope compositions are commonly used to describe the organic matter and to determine the flows of energy in coastal food webs (Peterson 1999; Letourneur et al. 2013; Wyatt et al. 2013; Briand et al. 2016). For example, the δ13C values may distinguish benthic and pelagic carbon sources (Peterson 1999). Nitrogen isotope compositions are often used to estimate the consumers’ trophic positions (Post 2002); however, in some cases the detection of 15N-enriched components may also provide information on the possible occurrence of upwelling (Liu and Kaplan 1989). Isotope mixing models enable estimation of the relative contribution of isotopically different OM sources in a mixture (e.g. consumer tissue, OM pools; Parnell et al. 2010). Fatty acid analysis can also be a reliable method to trace food sources in food webs. FAs are carbon-rich compounds that are ubiquitous in all organisms, but some FAs are biosynthesized de novo only by specific organisms at the base of the food chain (e.g. bacteria, diatoms, macroalgae, vascular plants; Koussoroplis et al. 2010), and are poorly metabolized by the consumers, making these FAs good trophic markers. As a result, FAs may help to characterize and to trace the organic matter within food webs (Koussoroplis et al. 2010). Organic matter sources with high nutritional quality have an important functional role. For example, compounds such as essential fatty acids (EFAs) are known to be essential for the achievement of many physiological processes (Arts et al. 2001; Koussoroplis et al. 2010). The ratio of polyunsaturated to saturated fatty acids (PUFAs/SFAs) may also be an indicator of the quality of potential food sources (Dalsgaard et al. 2003; Meziane et al. 2006) and provides information on the relative quantity of fresh natural OM such as phytoplankton compared to detritus (Biandolino et al. 2008). A low ratio indicates a poor quality of the resource (Moynihan et al. 2016). The quantity of EFAs such as 20:4ω6, 20:5ω3 and 22:6ω3 (i.e. arachidonic, eicosapentaenoic and docosahexaenoic acids, respectively) may also be used to assess the nutritional quality of the lipid fraction. Finally, the C:N ratio also provides information about the quality of the organic matter and is thus an essential metric to understand the use of different OM sources by primary consumers. For marine OM, ratios ~ 5–7 indicate high nutritional quality (i.e. fresh material), due to the presence of a significant amount of amino acids (Danovaro et al. 2001). Higher C:N ratios reflect the degradation of compounds rich in nitrogen, so ratios higher than 12 characterize refractory organic matter (Hedges et al. 1986). Primary consumers can preferentially select sources with a higher nitrogen content, providing better nutritional quality (Jacquin et al. 2006; Schaal et al. 2009) and likely assimilate these food sources in a much easier way.
This multi-trophic markers approach therefore enables discrimination between the different food sources fueling a food web. Using this approach, we have tested the hypothesis that, in this region characterized by high nutrient concentrations and plankton biomass, organic matter of pelagic origin provides significant support for the coastal ecosystems. Thus, the first aim of this study is to characterize the main organic matter sources at the base of a Marquesan coastal ecosystem by investigating the carbon and nitrogen stable isotope compositions, the C:N ratio and FA compositions in macroalgae, algal turf, detrital terrestrial plant (DTP) material, phytoplankton, sediment and particulate OM. Our second aim is to quantify the relative contributions of the different primary producers to the particulate and sedimentary organic matter pools.
Material and methods
Studied site
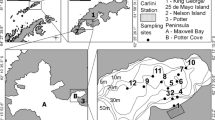
The study was carried out in August 2016 (cold, dry season) and in March 2017 (warm, wet season) in the southeast part of Nuku Hiva, Marquesas Archipelago (7°50′-10°35′S, 138°25′-140°50′O), French Polynesia (Fig. 1).
Rainfall, although unstable, is usually more regular and abundant from January to August (Laurent 2016). The intensification of the trade winds during the austral winter accentuates the surface currents. This phenomenon, combined with the slight decrease in surface water temperatures, results in a seasonal increase in vertical mixing and thus nutrient enrichment of the surface water layers (Raapoto et al. 2018). During the sampling, the mean water temperatures were 27.6 ℃ in August and 29.2 ℃ in March.
The marine seafloor is dominated by rocky habitats, characterized mainly by steep scree slopes of volcanic rock, but also by soft-bottom habitats, algae groves, coral habitats and caves. The benthic community is composed mainly of algal turf, macroalgae (mostly calcified and encrusting), scattered coral colonies and sponges. This assemblage of organisms reflects an environment with strong hydrodynamic conditions (Payri et al. 2016).
Sampling
Organic matter sources were sampled at five stations in shallow waters (from 0.5 to 20 m): three coastal sites (C1, C2 and C3), a river mouth site (R) and an ‘oceanic’ site (P). Only POM and phytoplankton were collected at station P due to high depth, ~ 150–200 m) (Fig. 1). The river has a low flow, from ~ 0.5 to ~ 3.0 m3 s− 1, depending on rainfall, and low nutrient concentrations (0.5–1.0 µmol l−1 NO3− and 0.4–1.0 µmol l−1 NH4+ for instance; Fey et al. unpublished data). Sampling was performed by targeting the OM sources that potentially constitute the food web basis, including the OM pools (i.e. POM and SOM). More specifically, eleven different macrophytes (algal turf and macroalgae, n = 73) and DTP material (i.e. tree leaves) transported by the river (n = 16) were collected by hand and placed in hermetic bags. Algal turfs and macroalgae were collected on the pebbles found at each site. They were scraped carefully from the substratum and sorted by taxonomical groups: turf, Rhodophyta, Ochrophyta and Chlorophyta. Algal turfs are a complex assemblage dominated by filamentous algal species and may represent a major OM source on coral reefs (Vermeij 2010; Letourneur et al. 2013; Briand et al. 2015). Among the macroalgae, four taxa were sampled for Rhodophyta, two for Ochrophyta and two others for Chlorophyta (see “Results”). Macroalgae were cleared of their epiphytes to avoid potential confusion in the results.
The POM of the subsurface water (< 1 m, n = 49) were also sampled: 1–4 l of water were vacuum-filtered through a pre-combusted (500 ℃, 6 h) and pre-weighed glass fiber filter (0.7 µm porosity GF/F, Whatman®). Surface POM isotope compositions are often used as a proxy of those of phytoplankton in oceanic ecosystems. However, in an area subjected to river inputs, surface water POM may be influenced by OM of terrestrial or detrital origin, and there may be a significant difference between POM and phytoplankton isotope compositions (Harmelin-Vivien et al. 2008). We thus analyzed POM (i.e. phytoplankton, plus various kind of organic particles) and phytoplankton sensu stricto. In this paper, the term ‘phytoplankton’ refers to the categories of nano- (2–20 µm), and micro-plankton (20–63, and 63–200 µm) fractions that were separated through sieving, thus having a larger size range than in most comparable studies (usually 63–180 μm) and taking into account the abundance of nano-plankton in Marquesan waters (Dolan et al. 2007). During POM collection, micro-zooplankton (100–300 µm) may be captured (Wyatt et al. 2013). Although its contribution to the POM composition is usually relatively low (Sorokin and Sorokin 2010), an effort was made to remove all detected organisms in addition to filtering water with a 200 μm-mesh sieve.
Sediment organic matter (SOM) was sampled at the bottom substrate (< 5 cm, n = 47). Large particles such as stones, shells, etc. were removed from these samples. All samples were kept in ice chests during sampling and (except for POM) immediately stored at − 20 ℃ until further analyses.
Biochemical analyses
Stables isotopes
All samples except filters were freeze-dried and ground to fine powder with an agate mortar and pestle. The POM and phytoplankton collected on filters were scraped off with a scalpel. Inorganic carbonates (shells, spikes, etc.) have higher δ13C values than organic carbon. As a result, a contamination of samples by carbonates can bias the value of the isotope composition of a food source or a consumer (Pinnegar and Polunin 1999). Therefore, samples potentially containing carbonates (i.e. POM, SOM, encrusting algae and phytoplankton) were acidified prior to measurement of δ13C values. Filters were acidified using HCl fumes for 4 h under vacuum. The other samples were acidified in glass vials by stepwise addition of 0.5 mol l− 1 or 2 mol l− 1 HCl (for algae and SOM, respectively), until cessation of bubbling. These samples were then dried at 60 ℃ using a dry block heater, homogenized with ultrapure water, freeze-dried and ground again. Measurements of δ15N values were carried out on raw samples. Approximately 1 mg of powder was weighed into a tin cup (8 × 5 mm) for vegetal/animal samples, ~ 15 mg for SOM and ~ 3 mg for POM, using a precision balance (ADAM PW124®, d = 0.0001 g). Carbon and nitrogen isotope compositions and C:N ratios of all samples were determined using an elemental analyzer (Flash EA 2000, Thermo Scientific®, Milan, Italy) coupled with a continuous-flow isotope ratio mass spectrometer (Delta V Plus, Thermo Scientific®, Bremen, Germany) via a Conflo IV interface. Analyses were carried out at the LIENSs stable isotope facility of the University of La Rochelle, France. Isotope compositions are expressed in the δ notation as deviations from the standard reference materials (N2 in air for δ15N and Vienna Pee Dee Belemnite for δ13C, according to the formula:
where X is 13C or 15 N, R is the ratio corresponding to 13C:12C or 15 N:14 N (proportion of heavy to light isotope). Calibration was done using reference materials (USGS-24, -61, -62, IAEA-CH6, -600 for carbon; USGS-61, -62, IAEA-N2, –NO–3, − 600 for nitrogen). The analytical precision of the measurements was < 0.15‰ for carbon and nitrogen based on analyses of USGS-61 and USGS-62 used as laboratory internal standards. Overall, this represents 239 stable isotope measures (96 samples of OM pools and 143 primary producers).
Fatty acids
Fatty acid analyses were conducted for both seasons on subsamples of SOM, river and marine POM, terrestrial detritus, three Rhodophyta species (Ceratodictyon scoparium, Pterocladiella caerulescens and Peyssonnelia spp.), algal turf and phytoplankton. Four samples were analyzed for each OM source, except phytoplankton for which the available material was not sufficient (n = 2).
Lipids were extracted following Meziane et al. (2007), and using 30 mg of freeze dried material for tree leaves, algae and phytoplankton, 1 g for SOM and 5 to 20 mg for POM. An internal standard, tricosanoic acid (23:0), was added in each sample before extraction to calculate the final concentrations of fatty acid methyl esters (FAME) following their separation by gas chromatography analysis (Varian 3800-GC), using a flame ionization detector. Identification of each FAME was done by comparing retention times with those of a commercial standard (Supelco®) and confirmed using a mass spectrometer coupled to a gas chromatograph (Varian 450-GC; Varian 220-MS). Fatty acid concentrations are expressed as percentages (%) of total FA or absolute concentrations (mg g− 1).
The FA were classified according to their degree and type of unsaturation i.e. saturated fatty acids (SFAs; e.g. 14:0, 16:0 and 18:0), monounsaturated fatty acids (MUFAs; e.g. 16:1ω7 and 18:1ω9), polyunsaturated fatty acids (PUFAs; e.g. 20:4ω6, 20:5ω3 and 22:6ω3; having a terminal end omega-3 (ω3) or omega-6 (ω6) indicates essential fatty acids: EFAs) and branched fatty acids (BrFAs; e.g. iso-15:0 and anteiso-15:0).
Quality indicators
C:N ratios were calculated for all OM sources sampled. For fatty acids, the ratios of polyunsaturated to saturated fatty acids (PUFAs/SFAs) were also calculated (Biandolino et al. 2008; Moynihan et al. 2016).
Statistical analyses and models
Statistical analysis
Differences in mean δ13C values, δ15N values, C:N ratios and fatty acid functional groups (e.g., MUFAs, PUFAs) according to the potential OM sources (e.g. POM, phytoplankton, etc.) were tested using two-way analyses of variance (ANOVA; F) after assumption of normality. Data homogeneity was tested using the Levene test. A Kruskal–Wallis test (KW) was performed when the variances were heterogeneous. Significant results were tested with a post-hoc Tukey test for ANOVA or a Mann–Whitney pairwise permutation test for the Kruskal–Wallis test. A Student test (t) or Wilcoxon test (W) was used when comparison between two means was necessary. All tests were considered significant when p-value < 0.05.
A principal component analysis (PCA) was performed to explore the links between the different fatty acid percentages and the samples of OM pools. This PCA was performed using the 25 individual FAs measured displaying an average proportion greater than 1% within at least one group of samples. The statistics and graphical representations were performed using R version 3.4.4 (R Core Team 2018).
Bayesian mixing model
The isotope composition of an organic matter pool can be considered as the result of the mixture of the isotope composition of the different sources (Phillips 2001; Phillips and Gregg 2003). The contributions of the different sources to the pools of OM (i.e. POM, SOM) were determined using the Bayesian mixing model SIAR (Parnell et al. 2010) using the isotope compositions of the two elements (C and N). Models were run for 200,000 iterations, the burn-in was set up at 50,000 iterations and a one-fifteenth thinning was applied. This model provides ranges of solutions of the different proportions of sources to explain the isotope composition of a mixture, here the POM or the SOM. The trophic fractionation factor (TFF) can significantly influence the model’s output (Bond and Diamond 2011). However, in this study, the TFF was set at 0 because no consumption process is involved in OM pools, as only the mixture of the different OM sources was considered (Briand et al. 2015).
Results
Stable isotope analyses
Seasonal and spatial variations of δ 13 C and δ 15 N values
The detrital terrestrial plant material samples had similar carbon and nitrogen isotope compositions between warm and cold seasons (Figure S1, p = 0.320). δ15N values of algal turf were similar between sites and seasons (p = 0.067), but their δ13C values displayed spatio-temporal variations (i.e. site C3 in warm season; p < 0.05; Figure S1). No significant differences were observed between sites for the δ13C of macroalgae (p > 0.05) while δ15N differed between sites (C1 vs. C2; p < 0.01) and between seasons (C2; p < 0.001) (Figure S1). Isotope composition of phytoplankton was more variable: we detected seasonal difference for δ15N in both coastal and oceanic samples (higher δ15N values of ~ 2.5–3.0 ‰ during the warm season, Figure S1). During the cold/dry season, phytoplankton had similar δ13C and δ15N between coastal sites (C1-3) (p > 0.05, and all samples from coastal sites were significantly more enriched in 13C and 15 N than oceanic phytoplankton (p < 0.001). During the warm/wet season, oceanic phytoplankton had a lower δ15N value than coastal phytoplankton from site C1 and a higher δ13C value than coastal phytoplankton from sites C2-C3 (p < 0.001).
Even if some seasonal and spatial patterns were observed, primary producers had a rather clustered isotopic composition that was clearly distinguishable from that of phytoplankton (Fig. 2; Figure S1). Thus, to facilitate the subsequent analysis, primary producer samples from the two seasons were pooled. In this case, coastal and oceanic phytoplankton had similar isotope compositions (δ13C and δ15N; p > 0.05), these were therefore pooled under the common name ‘Phytoplankton’ (Phyto; Fig. 2, Table 1).
δ13C vs δ15N mean values of OM sources, both seasons pooled. Grey circles represent primary producers and triangles represent river OM pools. Black squares represent marine POM and coastal SOM. Error bars indicate standard deviations. Codes are detailed in Table 1
Pools of organic matter (POM and SOM) exhibited strong differences in carbon and nitrogen isotope compositions between marine and river mouth sites (p < 0.01), for both seasons (Figure S1). For SOM, river and coastal samples both had similar isotope compositions from one season to another (p > 0.05). Among POM, river POM displayed seasonal differences both in δ13C and δ15N values (p < 0.001 and < 0.01, respectively). Oceanic POM had slightly different δ15N values between the two seasons (p < 0.001), but no difference was observed for δ13C values (p = 0.990). Coastal POM samples had similar isotope composition between sites and seasons (p > 0.05) (Figure S1). Thus, coastal and oceanic POM samples were pooled into ‘marine POM’ (M-POM) in the rest of the study.
Characteristics of organic matter sources based on their isotope compositions
Terrestrial sources (river POM, river SOM and DTP) were both depleted in 13C and 15 N; on average δ13C values varied from − 29.5 to − 24.3 ‰ and δ15N values from 1.9 to 6.2 ‰ (Table 1; Fig. 2). Isotope compositions of marine OM sources (OM pools and primary producers) were more enriched in 13C and 15 N, and ranged from − 23.9 to − 12.7 ‰ for δ13C values, and 10.9 to 15.0 ‰ for δ15N values (Table 1). Coastal SOM presented higher δ13C and δ15N values (− 19.1 ± 0.9 ‰ and 14.4 ± 0.5 ‰, respectively) than M-POM (− 22.5 ± 0.8 ‰ and 12.1 ± 1.1 ‰, respectively; p < 0.001; Table 1). Among marine primary producers, algal turf was the most 13C-depleted (− 23.9 ± 1.7 ‰), and macroalgae were the most 13C-enriched (− 16.4 ± 2.0 ‰), especially Cladophoropsis sudanensis (Table 1; Fig. 2). For δ15N values, phytoplankton had the highest values (15.0 ± 1.8 ‰) and macroalgae the lowest (11.6 ± 0.9 ‰). By taking into account both primary producers and OM pools, some similarities of isotope compositions could be highlighted. Phytoplankton and coastal SOM had similar δ15N values (p = 0.270), as well as macroalgae and M-POM (p = 0.07). Regarding the δ13C values, all food sources had different carbon isotope compositions (p < 0.001), except algal turf and R-SOM (p = 0.25; Fig. 2).
Among OM pools, C:N ratios were lower overall for SOM compared to POM, but only significantly different between coastal SOM and marine POM, with 5.3 ± 2.9 and 7.1 ± 1.3, respectively (p < 0.001; Table 1). For primary producers, DTP and algal turf exhibited the highest and most variable values, with 15.5 ± 33.6 and 18.1 ± 16.0, respectively. Generally, macroalgae exhibited C:N values that oscillated around 10 (Table 1) and that were similar between species (p > 0.05). Finally, phytoplankton had the lowest mean ratio among the primary producers, with 5.6 ± 1.6 (p < 0.001). This C:N ratio was similar to that obtained for the coastal SOM (p = 0.056).
Fatty acids
In this study, thirty-three fatty acids were detected at concentrations > 1% of the total FA identified (57 FAs) in at least one of the OM sources considered (Table 2). Saturated fatty acid (SFAs) contributions were dominant in all samples, ranging from ~ 43% to ~ 65% (Table 2). The monounsaturated fatty acid (MUFAs) percentages were highly variable and relatively close from one sample to another (p = 0.180), extremum being ~ 15% and ~ 24% for algal turf and coastal SOM, respectively. Overall, branched fatty acids (BrFAs) represented the lowest contribution to the total FAs (less than 5%), except for river and coastal SOM with ~ 10% in both cases (Table 2). Conversely, phytoplankton showed the lowest average value for this fatty acid category, with ~ 1.3%. Nevertheless, primary producers had significantly lower BrFA percentages than OM pools (p < 0.001; Table 2). PUFAs had a wide range of percentages, the highest mean contribution being for phytoplankton (~ 39%) and the lowest for the river SOM (~ 9%). However, no clear trend was observed between primary producers and OM pools, only detrital terrestrial plant material displayed a significantly higher PUFA contribution than R-SOM, C-SOM and M-POM (p < 0.05; Table 2).
Among SFAs, the 16:0 fatty acid contributed the most to the overall percentage, ranging from ~ 19% (DTP) to ~ 53% (Macroalgae; Table 2). The predominant monounsaturated fatty acid (MUFAs) was the 16:1ω7 in phytoplankton, coastal SOM and river POM (Table 2). Fatty acid 18:1ω9 exhibited a higher contribution in all other samples (Table 2). The PUFAs 18:3ω3 and 18:2ω6 contributed the most to the total FAs in the DTP samples (Table 2). Amongst essential fatty acids (EFAs), 20:4ω6 exhibited higher percentages in macroalgae species than in algal turf or phytoplankton (Table 2). In OM pools, relatives contributions of this EFA were maximum in coastal SOM (~ 3.2%) whereas contributions 20:5ω3 and 22:6ω3 were much higher in phytoplankton, (> 13% of total FAs; Table 2).Algal turf and macroalgae bear the highest relative contribution of 20:5ω3 following phytoplankton (~ 4.4% for (Table 2).
Phytoplankton samples were more concentrated in FA than the other samples, with > 40 mg g− 1. The lowest concentrations were measured in sediment samples both for river and coastal SOM (Table 2). The PCA ran on FA concentrations of the four primary producer categories (i.e. macroalgae, algal turf, phytoplankton and terrestrial detritus) well reflected these FA distributions within producers (Fig. 3a).
a Projection of variables (fatty acid trophic marker percentages) and individuals (primary producers) on the first two components resulting from the principal component analysis (PCA). Only FAs displaying an average proportion greater than 1% within at least one group of primary producer samples and with cos2 > 0. 4 are presented. b Projection of variables (fatty acid trophic marker percentages) and individuals grouped by category (C-SOM and marine POM) on the first two components resulting from the principal component analysis (PCA). Only FA displaying an average proportion greater than 1% within at least one group of samples and with cos2 > 0.2 are presented. Numbers in brackets indicate the percentage of the total variance explained by each axis. Eigenvalues histogram of the different components result from the PCA
Regarding the nutritional quality indices, phytoplankton displayed the best PUFAs/SFAs ratio and percentage of EFAs (20:5ω3, 22:6ω3 and 20:4ω6), with 0.90 ± 0.15 and 30.95 ± 3.57%, respectively (Table 2). The other categories of samples did not clearly stand out compared to the others, only DTP and river SOM had PUFAs/SFAs ratios that were significantly different, with 0.68 ± 0.27 and 0.16 ± 0.10, respectively (p < 0.05). The remaining groups had ratios close to 0.30.
The first two principal components of the PCA run on marine OM pools (i.e. M-POM and C-SOM) accounted for 46.4% of the total variation in the data set (Fig. 3b). 18:3ω3, 18:1ω7, 14:0, iso-15:0, anteiso-15:0 and 20:4ω6 fatty acids were the variables contributing the most to the first axis, while 24:0, 16:2ω4, 18:0, 17:0, 16:1ω7 and 20:5ω3 contributed more to the second axis. The PCA results highlighted three main groups of samples. Firstly, marine POM samples are linked to elevated percentages of 12:0, 14:0, 18:2ω6, 22:6ω3 and 16:2ω4 fatty acids (Fig. 3b). Then two other groups among coastal SOM samples stand out. One group of individuals is clearly linked to 24:0, 17:0 and 18:0 fatty acids, while the others are related to 18:3ω3, 18:1ω7, 20:4ω6, 16:1ω7 and 20:5ω3 fatty acids (Fig. 3b). The PCA highlighted the strong correlations between some FAs, such as 18:1ω7 and 20:4ω6, 16:1ω7 and 20:5ω3, 24:0 and 17:0, iso-16:0 and iso-17:0 (Fig. 3b).
Mixing model
Phytoplankton was clearly the most important source contributing to the mean composition of the coastal SOM, with ~ 62% (Fig. 4a). Macroalgae represented the second major source of carbon in the C-SOM, with ~ 32%. Mean contributions from other potential OM sources were almost negligible, with ~ 4% for algal turf and below 1% for sources of terrestrial origin (R-POM, R-SOM and DTP).
Relative importance of different OM sources in the composition of a coastal sedimentary organic matter (C-SOM) and b marine particulate organic matter (M-POM). DTP Detrital terrestrial plants material, Phyto Phytoplankton, R-POM river particulate organic matter. Violin plots represent the posterior distribution shape of the data (density estimation) in grey; Boxplots represent median, interquartile ranges (white box), credibility intervals (thin line) and outliers (black dots)
Algal turf and phytoplankton were both the main contributors to the marine POM, with ~ 38% on average in both cases (Fig. 4b). However, the violin plots bimodal distributions of these primary producers illustrates a ‘joint uncertainty’ regarding their relative mean contribution to the M-POM, which can vary from ~ 5–10 to ~ 65–70%. River POM, river SOM, DTP and benthic macroalgae had lower proportions, with mean contributions ranging from ~ 5 to ~ 7%.
Discussion
Isotope compositions
The carbon isotope compositions of the sources of OM is generally within the ranges commonly observed in the literature (Briand et al. 2015). However, some sources revealed a remarkable deviation from expected values. While benthic primary producers are usually more enriched in 13C than pelagic-primary producers (Peterson 1999), in our study algal turf has lower δ13C values than phytoplankton (− 23.9 ± 1.7 ‰ and − 20.5 ± 0.6 ‰, respectively). Algal turf is generally among the most 13C-depleted macrophytes, but generally has higher δ13C values than marine POM or phytoplankton (Letourneur et al. 2013; Briand et al. 2015, 2016). Despite a wide variability of δ13C values, the main groups of primary producers are clearly distinct from each other (Fig. 2). Nitrogen isotope compositions clearly distinguish land-based sources (δ15N values of 1.8 ± 2.3 ‰ and 6.2 ± 0.8‰, for DTP and river SOM, respectively) from marine sources (δ15N values of 11.6 ± 0.9 ‰ and 15.0 ± 1.8 ‰ for macroalgae and phytoplankton, respectively), as the latter are more enriched in 15 N. Our study highlights that primary producers had much higher δ15N values than those commonly observed in other coral ecosystems, even in the Pacific. Taking benthic primary producers as a reference, the δ15 N values recorded in the Marquesas Islands displayed differences of about 10 ‰ and 8 ‰ compared to New Caledonia and Moorea, respectively (Letourneur et al. 2013; Briand et al. 2015). These high δ15 N values are probably related to an enrichment in 15 N of the nitrogen nutrient pool (i.e. nitrites, nitrates and/or ammonium). Knowing that phytoplankton is abundant throughout the year in the Marquesas Islands (Martinez et al. 2016), nitrogen utilization by phytoplankton would probably lead to an increase in the δ15 N values in the residual nutrient pool (Savoye et al. 2003). Consequently, the nitrogen isotope compositions of primary producers incorporating these remaining nutrients would also rise. This assumption could be consistent if the nitrogen stock was not large enough to support the primary producers’ needs. However, the waters surrounding the Marquesas have relatively high nutrient concentrations (Martinez et al. 2016), which does not presuppose a lack of nutrients.
Fatty acids
The results obtained for marine OM pools (POM and SOM) are relatively similar to those found in other Pacific ocean studies (Liénart et al. 2016). These OM pools are characterized by a strong contribution of the SFAs (~ 62 and 50% TFAs), and a small contribution of the MUFAs (~ 17 and 24% TFAs) and branched fatty acids (~ 4 and 9% TFAs). PUFAs, however, have a higher contribution than in the literature, with ~ 16% for both POM and SOM (e.g. POM: ~ 9%-Liénart et al. 2016; SOM: ~ 7%-Liénart et al. 2018). Essential fatty acids represent ~ 4–6% of TFAs comprising PUFAs (Table 2). PCA (Fig. 3) separates OM pools into three groups, reflecting compositional differences between marine POM and two groups for coastal SOM. These results highlight contributions of various PUFAs such as 22:6ω3 (often found in dinoflagellate), 16:2ω4 (a marker for diatoms or algae), 18:2ω6 (suggesting OM derived from macroalgae or vascular plants) and some SFAs (12:0, 14:0 and 16:0) to marine POM (Budge and Parrish 1998; Arts et al. 2001). Thus, these FA markers indicate that the marine POM would consist of a mixture of different primary producers, both benthic and pelagic and potentially of terrestrial origin as well. For coastal SOM, one group exhibits contributions mainly from MUFAs and PUFAs. In the same way as for the POM, FAs markers found for this OM are likely to come from marine and terrestrial sources. 20:5ω3 and 16:1ω7 are indeed known to be markers of diatoms (Budge and Parrish 1998; Dalsgaard et al. 2003; Liénart et al. 2016), 20:4ω6 of Rhodophyta (Jaschinski et al. 2011) and 18:3ω3 of OM derived from macroalgae or vascular plants (Meziane et al. 2007). The FAs 18:1ω7 and anteiso-15:0 may indicate the presence of bacteria in this group (Dalsgaard et al. 2003). But the orientation of variables representing the branched fatty acids (iso-17:0, iso-16:0 and iso-15:0) in the PCA suggests the presence of bacteria within the two coastal SOM groups (Fig. 3). Marine heterotrophic bacteria are known to colonize settling particulate matter following plankton blooms, and can be abundant in sediments (Dalsgaard et al. 2003). However, the second group of coastal SOM samples had contributions of relatively more degraded organic material, as suggested by the high contributions of SFAs (24:0, 17:0 and 18:0; Wakeham et al. 1997). This result suggest a qualitative degradation of these samples with the help of a bacterial blooming related most likely to a storage problem of samples. A similar trend was observed in another study, with diatom markers 16:1ω7 and 20:5ω3 found in highest proportions in sediments rather than marine POM, and conversely for the dinoflagellates marker (22:6ω3; Richoux and Froneman 2008). The analysis of our primary producers gives results in agreement with designated FA markers in the literature, with major observations of fatty acids such as 20:4ω6 in benthic algae, 18:2ω6 and 18:3ω3 in detrital terrestrial plants, 16:1ω7, 20:5ω3 and 22:6ω3 in phytoplankton.
Indicators of OM quality
In this study, the sources with the highest quality were sedimentary OM (river and coastal), phytoplankton and marine POM. Their C:N ratios between 5 and 7 (Table 1) suggest the presence of a significant amount of amino acids (Danovaro et al. 2001). However, regarding quality indices obtained with FA analysis, phytoplankton is significantly of much higher quality (Table 2). The highest percentage of essential fatty acids was indeed measured in the latter (30.95 ± 5.57%), moreover its PUFAs/SFAs ratio indicates fresher OM than the other sources. Among OM pools, coastal SOM and POM have a similar quality based on their C/N ratios and FA quality indices, which is likely related to the fact that fresh phytoplankton represents a very important contribution to SOM composition (i.e. see mixing model data). This clearly indicates the important trapping of phytoplankton into the sediment and the importance of the benthic-pelagic coupling in the functioning of this ecosystem. The C:N ratios higher than 12 measured in algal turf and freshwater macrodetritus (DTP) indicates a lower nutritional quality of these sources, with a higher content of refractory organic matter (Hedges et al 1986). Benthic macroalgae OM also does not appear as a food resource of very good quality, based on its C:N ratios around 10. Thus, comparing all sources, the OM pools had lower C:N ratios than the benthic primary producers (macroalgae and algal turf), indicating that OM pools may be relatively more nutritious. Interestingly, although less important than for phytoplankton, the EFAs found in algal turf and macroalgae represent a high proportion of their PUFAs (Table 2). Therefore, despite their relatively poor quality in terms of nitrogen supply, marine macroalgae could be interesting food sources for consumers to supplement their needs in essential fatty acids (Arts et al. 2001). The marine POM and phytoplankton C:N ratios correspond to those obtained in other studies in temperate and tropical areas (~ 6.5 for POM, between 6 and 10 for phytoplankton; Savoye et al. 2003; Cresson et al. 2012).
Composition of OM pools
The Bayesian mixing model highlighted that coastal SOM is mostly made of phytoplankton, followed by macroalgae. Phytoplankton is known as a significant contributor to SOM through the sinking of dead phytoplankton (Cresson et al. 2012). Composition of marine POM is also influenced by phytoplankton, but with average contributions of smaller amplitude than for coastal SOM (~ 38%). Phytoplankton is not the major contributor in this case, and algal turf fragments also represent an important contribution to POM (~ 38%). However, it is likely erroneous to look only at these average contributions, due to the bimodal distributions displayed on the violin plots for algal turf and phytoplankton (Fig. 4). This bimodal distribution means that, if the algal turf contribution is at the top of its probability range, then the contribution of phytoplankton is likely to be at the bottom of its probability range, and vice versa. In contrast to other studies (Harmelin-Vivien et al. 2008; Letourneur et al. 2013), our results did not indicate any clear influence of river organic matter on marine POM. The greater macroalgae relative contribution observed for SOM (compared to POM) could be explained by a coarser fragmentation of macroalgae, these staying less easily in suspension than algal turf fragments. A lower degradation rate would probably favors the trapping of OM derived from benthic macroalgae in sediment over a longer period. It is also plausible that other organisms or compounds, which are difficult to collect, may influence the composition of OM pools. In our case, according to the results obtained with the fatty acids analyses (see above), bacteria probably influence the OM pools’ compositions. Additional analyses highlighted high abundances of cyanobacteria, mostly Synechococcus spp., during the warm season (results not shown). These organisms are very small (0.5 to 1.8 µm), therefore it is possible that they were not captured in a representative manner in the size class < 20 µm (taken into account in this study). Haas et al. (2011) estimated that reefs dominated by algal turf and macroalgae will support 2–3 times as much microbial abundance in the water column as coral or crustose algae dominated reefs.
Conclusions
Our findings suggest that the most important food sources available for primary consumers in this system consist of organic matter principally derived from phytoplankton, with complements of macroalgae and algal turf. OM sources of terrestrial origin had a very minor influence. In addition, phytoplankton was characterized by the best nutritional quality, with low C:N ratio and high essential fatty acid contributions to total FAs, which are important for physiological processes. These characteristics make this OM source particularly attractive for primary consumers. This suggests that the supply of labile organic matter in the system results from a pelagic-benthic coupling. Our study also highlights an atypical Marquesan baseline, marine primary producers having significantly higher δ15N values than those observed in other South Pacific areas. However, further research is needed to examine the processes leading to the enrichment in 15 N in primary producers. It would be interesting to measure δ15N values in nitrate pools and to follow the nutrient inputs over a long time period. A better knowledge of the bacterial processes occurring in Marquesan waters could also provide useful clarification.
Data Availability Statement
Data were avalaible on demand to the first and/or the corresponding author.
References
Arts MT, Ackman RG, Holub BJ (2001) “Essential fatty acids” in aquatic ecosystems: a crucial link between diet and human health and evolution. Can J Fish Aquat Sci 58:122–137
Benzoni F, Pichon M (2016) Les coraux peu profonds (Cnidaria, Anthozoa, Scleractinia) des Îles Marquises. In: Galzin R, Duron SD, Meyer JY (eds) Biodiversité terrestre et marine des îles Marquises, Polynésie française. Société Française d’Ichtyologie, Paris, p 526
Biandolino F, Prato E, Carappo C (2008) Preliminary investigation on the phytoplankton contribution to the mussel diet on the basis of fatty acids analysis. J Mar Biol Assoc UK 88:1009–1017
Bierwagen SL, Heupel MR, Chin A, Simpfendorfer CA (2018) Trophodynamics as a tool for understanding coral reef ecosystems. Front Mar Sci 5:24. https://doi.org/10.3389/fmars.2018.00024
Bode A, Alvarez-Ossorio MT, Varela M (2006) Phytoplankton and macrophytes contributions to littoral food webs in the Galician upwelling estimated from stable isotopes. Mar Ecol Progr Ser 318:89–102
Bond AL, Diamond AW (2011) Recent Bayesian stable-isotope mixing models are highly sensitive to variation in discrimination factors. Ecol Applic 21:1017–1023
Briand MJ, Bonnet X, Goiran C, Guillou G, Letourneur Y (2015) Major sources of organic matter in a complex coral reef lagoon: Identification from isotopic signatures (δ13C and δ15N). PLoS ONE 10(7):e0131555
Briand MJ, Bonnet X, Guillou G, Letourneur Y (2016) Complex food webs in highly diversified coral reefs: Insights from δ13C and δ15N stable isotopes. Food Webs 8:12–22
Budge SM, Parrish CC (1998) Lipid biogeochemistry of plankton, settling matter and sediments in Trinity Bay Newfoundland II Fatty acids. Org Geochem 29:1547–1559
Choat JH, Robbins WD, Clements KD (2004) The trophic status of herbivorous fishes on coral reefs-II. Food processing modes and trophodynamics. Mar Biol 145:445–454
Cresson P, Ruitton S, Fontaine MF, Harmelin-Vivien ML (2012) Spatio-temporal variation of suspended and sedimentary organic matter quality in the Bay of Marseilles (NW Mediterranean) assessed by biochemical and isotopic analyses. Mar Pollut Bull 64:1112–1121
D’Angelo C, Wiedenmann J (2014) Impacts of nutrient enrichment on coral reefs: new perspectives and implications for coastal management and reef survival. Curr Opin Environ Sustainy 7:82–93
Dalsgaard J, St John M, Kattner G, Müller-Navarra D, Hagen W (2003) Fatty acid trophic markers in the pelagic marine environment. Adv Mar Biol 46:225–340
Danovaro R, Dell’Anno A, Fabiano M, Pusceddu A, Tselepides A (2001) Deep-sea ecosystem response to climate change: the eastern Mediterranean case study. TREE 16:505–510
Dolan JR, Ritchie ME, Ras J (2007) The ‘neutral’ community structure of planktonic herbivores, tintinnid ciliates of the microzooplankton, across the SE Tropical Pacific Ocean. Biogeosciences Discus 4:561–593
Frisch AJ, Ireland M, Baker R (2014) Trophic ecology of large predatory reef fishes: energy pathways, trophic level, and implications for fisheries in a changing climate. Mar Biol 161:61–73
Gazeau F, Smith SV, Gentili B, Frankignoulle M, Gattuso JP (2004) The European coastal zone: characterization and first assessment of ecosystem metabolism. Est Coast Shelf Sci 60:673–694
Haas AF, Nelson CE, Wegley Kelly L, Carlson CA, Rohwer F, Leichter JJ, Wyatt A, Smith JE (2011) Effects of coral reef benthic primary producers on dissolved organic carbon and microbial activity. PLoS ONE 6:e27973
Harmelin-Vivien ML, Loizeau V, Mellon C, Beker B, Arlhac D, Bodiguel X, Ferraton F, Hermand R, Philippon X, Salen-Picard C (2008) Comparison of C and N stable isotope ratios between surface particulate organic matter and microphytoplankton in the Gulf of Lions (NW Mediterranean). Cont Shelf Res 28:1911–1919
Hedges JI, Clark WA, Quay PD, Richey JE, Devol AH, Santos UD (1986) Compositions and fluxes of particulate organic material in the Amazon river. Limnol Oceanogr 31:717–738
Hubert N, Paradis E, Bruggemann H, Planes S (2011) Community assembly and diversification in Indo-Pacific coral reef fishes. Ecol Evol 1:229–277
Jacquin AG, Donval A, Guillou J, Leyzour S, Deslandes E, Guillou M (2006) The reproductive response of the sea urchins Paracentrotus lividus (G.) and Psammechinus miliaris (L.) to a hyperproteinated macrophytic diet. J exp Mar Biol Ecol 339:43–54
Jaschinski S, Brepohl DC, Sommer U (2011) Seasonal variation in carbon sources of mesograzers and small predators in an eelgrass community: stable isotope and fatty acid analyses. Mar Ecol Progr Ser 431:69–82
Koussoroplis A-M, Bec A, Perga ME, Koutrakis E, Desvilettes C, Bourdier G (2010) Nutritional importance of minor dietary sources for leaping grey mullet Liza saliens (Mugilidae) during settlement: insights from fatty acid δ13C analysis. Mar Ecol Progr Ser 404:202–217
Laurent V (2016) Le climat aux îles Marquises. In: Galzin R, Duron SD, Meyer JY (eds) Biodiversité terrestre et marine des îles Marquises, Polynésie française. Société Française d’Ichtyologie, Paris, p 526
Le Bourg B, Letourneur Y, Bănaru D, Blanchot J, Chevalier C, Mou-Tham G, Lebreton B, Pagano M (2017) The same but different: stable isotopes reveal two distinguishable, yet similar, neighbouring food chains in a coral reef. J Mar Biol Ass UK 98:1589–1597
Letourneur Y, Lison de Loma T, Richard P, Harmelin-Vivien ML, Cresson P, Bănaru D, Fontaine M-F, Gref T, Planes S (2013) Identifying carbon sources and trophic position of coral reef fishes using diet and stable isotope (δ15N and δ13C) analyses in two contrasted bays in Moorea, French Polynesia. Coral Reefs 32:1091–1102
Liénart C, Susperregui N, Rouaud V, Cavalheiro J, David V, Del Amo Y, Duran R, Lauga B, Monperrus M, Pigot T, Bichon S, Charlier K, Savoye N (2016) Dynamics of particulate organic matter in a coastal system characterized by the occurrence of marine mucilage–a stable isotope study. J Sea Res 116:12–22
Liénart C, Savoye N, David V, Ramond P, Rodriguez Tressa P, Hanquiez V, Marieu V, Aubert F, Aubin S, Bichon S, Boinet C, Bourasseau L, Bozec Y, Bréret M, Breton E, Caparros J, Cariou J, Claquin P, Conan P, Corre A-M, Costes L, Crouvoisier M, Del Amo Y, Derriennic H, Dindinaud F, Duran R, Durozier M, Devesa J, Ferreira S, Feunteun E, Garcia N, Geslin S, Grossteffan E, Gueux A, Guillaudeau J, Guillou G, Jolly O, Lachaussée N, Lafont M, Lagadec V, Lamoureux J, Lauga B, Lebreton B, Lecuyer E, Lehodey J-P, Leroux C, L'Helguenm S, Macé E, Maria E, Mousseau L, Nowaczyk A, Pineau P, Petit F, Pujo-Pay M, Raimbault P, Rimmelin-Maury P, Rouaud V, Sauriaud P-G, Sultan E, Susperregui E (2018) Dynamics of particulate organic matter composition in coastal systems: forcing of spatio-temporal variability at multi-systems scale. Progr Oceanogr 162:271–289
Liu KK, Kaplan IR (1989) The eastern tropical Pacific as a source of 15N-enriched nitrate in seawater off southern California. Limnol Oceanogr 34:820–830
Mann KH (1988) Production and use of detritus in various freshwater, estuarine and coastal marine ecosystems. Limnol Oceanogr 33:910–930
Martinez E, Rodier M, Maamaatuaiahutapu K (2016) Environnement océanique des Marquises. In: Galzin R, Duron SD, Meyer JY (eds) Biodiversité terrestre et marine des îles Marquises, Polynésie française. Société Française d’Ichtyologie, Paris, p 526
Meziane T, d’Agata F, Lee SY (2006) Fate of mangrove organic matter along a subtropical estuary: small-scale exportation and contribution to the food of crab communities. Mar Ecol Progr Ser 312:15–27
Meziane T, Lee SY, Mfilinge PL, Shin PKS, Lam MHW, Tsuchiya M (2007) Inter-specific and geographical variations in the fatty acid composition of mangrove leaves: implications for using fatty acids as a taxonomic tool and tracers of organic matter. Mar Biol 150:1103–1113
Moynihan MA, Barbier P, Olivier F, Toupoint N, Meziane T (2016) Spatial and temporal dynamics of nano- and pico-size particulate organic matter (POM) in a coastal megatidal marine system: dynamics of nano- and pico-POM. Limnol Oceanogr 61:1087–1100
Parnell AC, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5:e9672
Payri C, De Ramon N’Yeurt A, Fiat S, Andrefouët S (2016) La macroflore marine des îles Marquises. In: Galzin R, Duron SD, Meyer JY (eds) Biodiversité terrestre et marine des îles Marquises, Polynésie française. Société Française d’Ichtyologie, Paris, p 526
Peterson BJ (1999) Stable isotopes as tracers of organic matter input and transfer in benthic food webs: a review. Acta Oecologica 20:479–487
Phillips D (2001) Mixing models in analyses of diet using multiple stable isotopes: a critique. Oecologia 127:166–170
Phillips D, Gregg JW (2003) Source partitioning using stable isotopes: coping with too many sources. Oecologia 136:261–269
Pinnegar JK, Polunin NVC (1999) Differential fractionation of δ13C and δ15N among fish tissues: implications for the study of trophic interactions. Funct Ecol 13:225–231
Post D (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecol Soc America 83:703–718
Raapoto H, Martinez E, Petrenko A, Doglioli AM, Maes C (2018) Modeling the wake of the Marquesas Archipelago. J Geophys Res Oceans 123:1213–1228
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for statistical computing, Vienna. https://www.R-project.org. Accessed Jan–Apr 2018
Richoux NB, Froneman PW (2008) Trophic ecology of dominant zooplankton and macrofauna in a temperate, oligotrophic South African estuary: fatty acid approach. Mar Ecol Progr Ser 357:121–137
Riera P, Richard P (1996) Isotopic determination of food sources of Crassostrea gigas along a trophic gradient in the Estuarine Bay of Marennes-Oléron. Est Coast Shelf Sci 42:347–360
Savoye N, Aminot A, Tréguer P, Fontugne M, Naulet N, Kérouel R (2003) Dynamics of particulate organic matter δ15N and δ13C during spring phytoplankton blooms in a macrotidal ecosystem (Bay of Seine, France). Mar Ecol Progr Ser 255:27–41
Schaal G, Riera P, Leroux C (2009) Trophic significance of the kelp Laminaria digitate (Lamour.) for the associated food web: a between-sites comparison. Est Coast Shelf Sci 85:565–572
Sorokin YI, Sorokin PY (2010) Plankton of the central Great Barrier Reef: abundance, production and trophodynamic roles. J Mar Biol Ass UK 90:1173–1187
Vermeij MJ, van Moorselaar I, Engelhard S, Hörnlein C, Vonk SM, Visser PM (2010) The effects of nutrient enrichment and herbivore abundance on the ability of turf algae to overgrow coral in the Caribbean. PLoS ONE 5:e14312
Volkman JK, Tanoue E (2002) Chemical and biological studies of particulate organic matter in the ocean. J Oceanogr 58:265–279
Wakeham SG, Hedges JI, Lee C, Peterson ML, Hernes PJ (1997) Compositions and transport of lipid biomarkers through the water column and surficial sediments of the equatorial Pacific Ocean. Deep-Sea Res part II 44:2131–2162
Wyatt ASJ, Lowe RJ, Humphries S, Waite AM (2010) Particulate nutrient fluxes over a fringing coral reef: relevant scales of phytoplankton production and mechanisms of supply. Mar Ecol Progr Ser 405:113–130
Wyatt ASJ, Lowe RJ, Humphries S, Waite AM (2013) Particulate nutrient fluxes over a fringing coral reef: source–sink dynamics inferred from carbon to nitrogen ratios and stable isotopes. Limnol Oceanogr 58:409–427
Acknowledgements
This work is a part of the first author’s PhD work, funded by the LabEx “Corail” (project RETROMAR). We would like to thank the Centre Plongée Marquises (X. Curvat “Pipapo”, J. Augereau, M. Edragas and D. Taupotini), the Délégation à la Recherche de la Polynésie française, the mayor and people of Nuku Hiva for their support. We are also grateful to C. Berthe, F. Boulileret, R. Sauze, D. Lecchini and M. Taquet for their field assistance. We thank the staff of the CRIOBE for logistical support and laboratory assistance, especially C. Sidobre, and B. Espiau, and N. Thiney for her invaluable help for FAs analyses in Paris, Muséum National d’Histoire Naturelle. Thanks to M. Paul, a native English-speaker, for improving the English of the article. Gaël Guillou is acknowledged for running stable isotope analyses. We also thank the anonymous referees for their useful and constructive comments that allowed us to improve the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This research received no specific grant from any funding agency, commercial or not-profit sectors. The authors declare that they have no conflicts of interest. No benthic/coral habitat was disturbed during this research. Because this work did not use animals, no international, national, local and/or institutional guidelines for sampling was applicable.
Additional information
Responsible Editor: N. Aberle-Malzahn.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fey, P., Parravicini, V., Lebreton, B. et al. Sources of organic matter in an atypical phytoplankton rich coral ecosystem, Marquesas Islands: composition and properties. Mar Biol 167, 92 (2020). https://doi.org/10.1007/s00227-020-03703-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-020-03703-z