Abstract
Helicobacter spp. colonize the gastrointestinal tract of humans and animals and have been associated with gastrointestinal diseases. Antarctic habitats are considered pristine ecosystems, but the increase in human activity could be introducing human bacteria hosted into waters and wildlife. However, Helicobacter spp. occurrence has not been studied in Antarctica. The aim of our study was to detect the Helicobacter DNA in different water sources and penguin feces from Greenwich, Dee and Barrientos Islands during summer of 2012 and 2013. High Helicobacter proportion was observed in water sources amplifying the 16S rRNA (33/40) and 23S rRNA genes (37/40) by semi-nested PCR. Similar results were observed in feces from Gentoo penguins (16S rRNA: 32/39, and 23S rRNA: 28/39) and Chinstrap penguins (16S rRNA: 16/17, and 23S rRNA: 15/17) by PCR. The phylogenetic relationship of 16S rRNA and 23S rRNA sequences from penguin feces was closely related to Helicobacter brantae. Analyses of 16S rRNA sequences showed that the majority of water samples are related to penguin (3/6) and Helicobacter pylori (2/6) sequences, but the 23S rRNA sequences matched with Campylobacter and Arcobacter. These results show for the first time the presence of the genus Helicobacter in different Antarctica water sources and in Gentoo and Chinstrap penguin feces. A few 16S rRNA sequences are very closely related to H. pylori, but specific glmM and ureA H. pylori genes were not detected. More studies are needed to determine the Helicobacter species present in this ecosystem and to establish the human impact in these Antarctic Islands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Helicobacter spp. are gram-negative, microaerophilic, spiral-shaped bacteria that colonize the gastrointestinal tracts of humans and animals and have been associated with gastrointestinal diseases in their hosts. The genus contains 36 formally named species isolated from gastrointestinal tract of birds and mammals. The most studied species is Helicobacter pylori, a human pathogen that causes gastritis and gastric ulcers (Kusters et al. 2006; Parte 2014). Some authors have suggested that H. pylori transmission could occur through contaminated waters. Furthermore, different strategies such as biofilms and viable but non-culturable state have been described for its long-term survival outside (Cellini et al. 2008; García et al. 2014; Santiago et al. 2015). Additionally, waterborne transmission has also been suggested for other Helicobacter species such as H. mustelae, H. muridarum, H. felis, H. canadensis, H. pullorum, H. canis (Azevedo et al. 2008) and H. cetorum (Goldman et al. 2009). Helicobacter spp. culture from environment is difficult. Therefore, molecular techniques have been used to allow the detection of these bacteria in environmental samples (Carbone et al. 2005; Twing et al. 2011).
The Antarctic continent has been geographically isolated from the rest of the world since more than ten million years and some Antarctic habitats are considered to be the most pristine ecosystems on earth. The development of Antarctic research and tourism activities in the past 50 years has resulted in an increase in human presence. Provisions of the 1991 Antarctic treaty have somehow reduced the direct impact of human-derived wastes and thereby their microbial populations (Baker et al. 2003; Bargagli 2008; Grondahl et al. 2009). However, the dramatic increase in human activities in the last decade might be introducing human-associated pathogens into Antarctic wildlife and waters.
Penguins are wild birds that have colonized the Antarctic ecosystem, and they have long lives and a permanent ecological niche and have been considered as environmental bio-indicators (Metcheva et al. 2006; Barbosa 2011). Some studies have reported the presence of microbial human fecal contamination in Antarctic waters, soils and animals (Sjoling and Cowan 2000; Hughes and Thompson 2004; Bonnedahl et al. 2005; Tin et al. 2009; Cowan et al. 2011). Moreover, members of the Helicobacteraceae family were reported in four penguin species from Antarctic, Sub-Antarctic and Australian regions (Dewar et al. 2013). However, the detection of the genus Helicobacter has not been reported in water sources or penguin feces from Antarctica. The main goal of this study was to determine whether Helicobacter genus DNA is present in different water sources and in fecal samples of Gentoo (Pygoscelis papua) and Chinstrap penguins (Pygoscelis antarctica) from Greenwich, Dee and Barrientos Islands, Antarctica.
Materials and methods
Sampling collection and DNA extraction
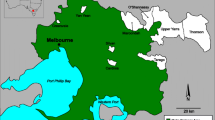
The study was carried out at Greenwich (21E 358256 UTM 3071559), Dee (21E 356244 UTM 3076306) and Barrientos Islands (21E 357986 UTM 3077558), at South Shetland Islands, Antarctica. Water and fecal samples were collected between summer of 2012 and 2013 (Fig. 1). Each island has different human activities; Chilean base Arturo Prat and the Ecuadorian base Pedro Vicente Maldonado are located at Greenwich Island. Dee Island is separated from Greenwich Island to the south by 850 m and has no human settlements. Barrientos Island is a popular tourist site frequented by Antarctic cruise ships visiting penguin reproductive colonies (IAATO 2014).
Forty water samples were collected from coastal, river, lake, glacial meltwater and treated wastewater sources. The physicochemical parameters including temperature, pH, salinity, conductivity and dissolved oxygen were determined with a multiparameter meter (Horiba UX22). All water samples (500 ml) were filtered using Durapore GV membranes (0.22 µm, 47 mm in diameter) in a Millipore filtration system with a vacuum pump (model WP6211560), and each membrane was placed in a sterile 15-ml tube. Freshly dropped fecal samples of Gentoo penguins (P. papua) and Chinstrap penguins (P. antartica) were collected in sterile plastic containers being careful to avoid touching the ground or snow. Waters and fecal samples were kept at −20 °C until arrival to Venezuela; then they were frozen at −80 °C for subsequent molecular analyses.
DNA from filtrated water samples was extracted using QIAamp DNA Mini kit (Qiagen, Valencia, CA). One half of each membrane was cut and resuspended in 180 µl of the first buffer of the QIAamp DNA Mini kit according to the manufacturer recommendations for tissues samples under sterile conditions. Penguin fecal DNA was extracted using Power Soil DNA isolation kit (MOBIO Laboratories), according to the manufacturer recommendations.
PCR assays
Helicobacter DNA was detected in water samples by a semi-nested PCR using Helicobacter genus-specific primers for 16S rRNA gene and 23S rRNA gene. For 16S rRNA gene semi-nested PCR, the first set included the Helicobacter genus-specific forward primer (HeliF) and a reverse primer specific for the Epsilonproteobacteria (EpsilR: 5′-TAT TCA CCG YRR CAT GGC TGA TYY R-3′); the second set included Helicobacter genus-specific primers (HeliF: 5′-AAC GAT GAA GCT TCT AGC TTG CTA G-3′, and HeliR: 5′-GTG CTT ATT CST NAG ATA CCG TCA T-3′) (Germani et al. 1997). Helicobacter 23S rRNA gene was amplified using primers O68 (5′-AGG CGA TGA AGG ACG TA-3′) and V62 (5′-CCC GAC TAA CCC TAC GAT-3′) in the first PCR round and O20 (5′-CAT AAT GAT CCT GCG AGT T-3′) and V62 in the second round (Dewhirst et al. 2005). In Penguin feces, Helicobacter DNA was detected by using the same genus-specific primers for 16S rRNA (HeliF and HeliR) and for 23S rRNA genes (O20 and V62) described above (Germani et al. 1997; Dewhirst et al. 2005). The H. pylori DNA was assessed amplifying glmM (glmMF: 5′-GGA TAA GCT TTT AGG GGT GTT AGG GG-3′, and glmMR: 5′-GCT TAC TTT CTA ACA CTA ACG CGC-3′) (Kansau et al. 1996) and ureA genes (ureAF: 5′-GCC AAT GGT AAA TTA GTT-3′, and ureAR: 5′-CTCCTTAATTGTTTTTAC-3′) (Peek et al. 1995). The reaction mixture contained 3–6 µl of DNA (approximately 50–100 ng) and 0.5 µM of each primer. Amplifications were performed using a Ready-To-Go PureTaq PCR kit (Amersham Biosciences, NJ) in a thermal cycler model GeneAMP 9700 (Applied Biosystems, CA) according to the recommendations for each primer set, except for 23S rRNA gene in penguin fecal samples where the cycling conditions were 95 °C for 3 min, followed by 35 cycles of 95 °C for 1 min, 50 °C for 1.3 min, 72 °C for 2 min and a final extension to 72 °C for 8 min. The positive control was H. pylori DNA, and the negative control was a no-DNA template. The controls for the second round of the semi-nested PCR were prepared by adding PCR amplicons of the first-round negative and positive controls, respectively. An additional negative control was prepared for the second round by adding water to reaction mixture instead of DNA.
16S and 23S rRNA gene sequencing and sequence analysis
Helicobacter genus-specific fragments of the 16S and 23S rRNA genes from water (n = 6) and fecal (n = 12) samples were amplified and purified for sequencing using the QIAquick PCR Purification kit (Qiagen), according to the manufacturer’s recommendations. Purified 16S rRNA amplicons [~300 base pairs (bp)] and 23S rRNA amplicons (~730 bp) were sequenced at Macrogen Inc., Seoul, Korea. The Helicobacter 16S and 23S rRNA gene sequences were deposited in GenBank under the accession numbers KF428985–KF428996 and KP017600–KP017609, respectively. Helicobacter sequences of this study were aligned with the closest GenBank matches using the SINA software (Pruesse et al. 2012). Two phylogenetic trees were constructed using the neighbor-joining method and the Kimura2 model provided in Molecular Evolutionary Genetics Analysis 2.1 software (version 5.0; Tamura et al. 2011).
Statistics
Paired Wilcoxon signed-rank test was used to determine differences in the Helicobacter 16S and 23S rRNA PCR results using the PAST software (Hammer et al. 2001).
Results
The range of the physicochemical parameter values of water samples was: temperature, 2.1–10.27 °C, pH, 4.36–6.9, salinity, 0–4 ‰, conductivity, 0.12–18.06 S/m, and dissolved oxygen 5.50–7.56 mg/l.
Helicobacter spp. 16S rRNA and 23S rRNA genes were detected in more than 70 % of water samples (Table 1), indicating that the Helicobacter genus is present in high proportions for all water sources studied. Not statistical differences were observed between the 16S and 23S rRNA PCR results of glacial meltwater, river, lake and wastewater samples. However, in coastal water samples, the Helicobacter DNA detected with 23S rRNA primers was higher than with 16S rRNA primers in 2013 (p = 0.03) and in both years (p = 0.01).
High proportion of Helicobacter spp. DNA was detected in fecal samples of Gentoo and Chinstrap penguins with no statistical difference between 23S rRNA than 16S rRNA primers including all samples for both years and 2013; however, in 2012, statistical difference in Gentoo penguins was observed (p = 0.01) (Table 2).
The 16S rRNA phylogenetic analysis of water and fecal samples is shown in Fig. 2. Sequences of both penguin species formed a clade with H. brantae. These results confirm the presence of Helicobacter DNA in feces of Gentoo and Chinstrap penguins. This may suggest that penguin sequences are related to Helicobacter species reported in wild birds in other latitudes. Additionally, we observe that sequences from water samples are divided into three groups. Three of water sequences formed a group with penguin sequences and H. brantae; one sequence from coastal seawater (KF428979) forms a group with H. pametensis, and the last group of two sequences (one sequence from coastal seawater and another from wastewater) forms a clade with H. pylori. Interestingly, the sequences associated with H. pylori belonged to water samples associated with human activity such as coastal seawater from Barrientos Island (KF428983) and wastewater effluents from a research base at Greenwich Island (KF428984). However, glmM and ureA H. pylori-specific genes were not found in any of water or penguin fecal samples.
Phylogenetic tree of partial 16S rRNA sequences of the Helicobacter genus obtained from feces of Gentoo and Chinstrap penguins and different water sources. The tree was constructed using the neighbor-joining algorithm. Bootstrap values are based on 10,000 replicates, and no values are given for groups with bootstrap values <50 %. The scale bar represents 0.02 (2 %) nucleotide sequence difference
The 23S rRNA phylogenetic analysis of penguin fecal samples is shown in Fig. 3. In this case, all penguin sequences formed their own cluster and were related to Helicobacter sequences found in avian hosts as H. brantae and H pametensis. Amplicons from three seawater samples were sequenced which showed similarities (between 81 and 95 %) with Arcobacter spp. and in one case (wastewater) 100 % similar to Campylobacter hominis (KP017609). Therefore, these are not included in the phylogenetic analysis presented in Fig. 3.
Phylogenetic tree of partial 23S rRNA sequences of the Helicobacter genus obtained from feces of Gentoo and Chinstrap penguins. The tree was constructed using the neighbor-joining algorithm. Bootstrap values are based on 10,000 replicates, and no values are given for groups with bootstrap values <50 %. The scale bar represents 0.02 (2 %) nucleotide sequence difference
Discussion
Our results show the presence of genus Helicobacter in water samples and both penguin species (Tables 1, 2). The presence of Helicobacter genus detected in the majority of penguin samples may be possibly due to the frequent contact established among penguins, since both species are highly gregarious living in colonies of large numbers of individuals of same and other species (Ancel et al. 2013). Additionally, analyses of 16S rRNA showed that the 50 % of water samples sequenced (3/6) were related to penguin sequences (Fig. 2), suggesting that water sources could be one possible route of transmission, as it has been reported for other Helicobacter species (Azevedo et al. 2008; Goldman et al. 2009).
Phylogenetic relationships of 16S rRNA and 23S rRNA sequences from penguin feces are closely related to Helicobacter species found in wild birds as H. brantae and H. pametensis, which are not closer to human Helicobacter as H. pylori. Helicobacter brantae and H. pamentensis had been reported only in birds and their pathogenic or zoonotic potential had not been demonstrated (Dewhirst et al. 1994; Fox et al. 2006). Helicobacter sequences from penguin feces form their own group in 23S and 16S rRNA trees, suggesting that the Helicobacter species that colonize the digestive tract of Gentoo and Chinstrap penguins are probably new commensal species. Further, Helicobacter culture from these penguin fecal samples is necessary to confirm this assumption.
Comparing 16S and 23S rRNA PCR results, the Helicobacter detection found with 23S rRNA is higher than 16S rRNA primers for coastal water samples (Table 1). Sequences of 23S rRNA amplicons from three coastal seawater samples showed similarities with Arcobacter sequences, while 16S rRNA sequences from these samples had 97–99 % similarity with H. brantae, H. pametensis and H. pylori (Fig. 2). These results suggest that the 23S rRNA primers used in this study amplify other bacterial genera in addition to Helicobacter. This finding was confirmed using the RDP’s ProbeMatch software (Cole et al. 2014). The non-specificity of 23S rRNA primers for Helicobacter might explain the high 23S rRNA detection obtained in coastal seawater samples (Table 1). The Arcobacter genus belongs to the family Campylobacteraceae and has been detected in diverse water sources (drinking water, rivers, lakes, groundwater, seawater, sewage) with many species having a wide range of survival temperatures (Collado and Figueras 2011). Although it is probable that Arcobacter species are present in Antarctica seawater, more studies are necessary to confirm this result.
In the phylogenetic tree of partial 16S rRNA sequences obtained from different water sources, we observed that the sequences are divided into tree groups, the majority of sequences form a clade with penguin feces samples and H. brantae, one sequence (KF428979) is closer to H. pametensis, and the last two sequences (KF428983 and KF428984) form a clade with H. pylori (Fig. 2).
The 16S rRNA amplicon (KF428984) of one sample from water treatment plant effluent had 99 % similar to H. pylori, whereas the 23S rRNA amplicon (KP017609) was identical to Campylobacter homini. Despite the discrepancy in similarity found between both sequences of genes, amplicons showed high similarities with Campylobacter and Helicobacter species associated with humans (Lawson et al. 2001; Kusters et al. 2006), suggesting that human bacterial DNA could be introduced in Antarctica seawater sources. H. pylori detection by PCR and FISH from wastewater effluents had been reported previously and suggests that H. pylori could survive wastewater treatments (Moreno and Ferrús 2012). In our study, the bacterial viability of wastewater samples was not determined. However, the introduction of foreign DNA might have dangerous consequences, since human pathogen genes could be transferred to Antarctic microorganisms.
The other 16S rRNA sequence with 97 % similarity to H. pylori belonged to a seawater sample from Barrientos Island (KF428983) (Fig. 2). There are Gentoo and Chinstrap penguin colonies in this island; therefore, we were expected to obtain a Helicobacter sequence closely related to penguins rather than to humans. However, more than 5000 tourists landed to Barrientos Island in 2012–2013 to watch these penguin colonies (IAATO 2014). Although tourist activities in Antarctica have strong regulations, bacterial contamination has been previously reported in other six penguin colonies (Bonnedahl et al. 2005). However, more studies are necessary to confirm the impact of wastewater discharge as well as the prevalence of waterborne pathogens in this ecosystem. Other possibility could be that our Barrientos Island sequence is related to H. cetorum. Helicobacter cetorum has high 16S rRNA homology to H. pylori and has been cultured from several marine mammals (Goldman et al. 2009). The presence of H. cetorum in Antarctic waters is a possibility because marine mammals like seals and whales are common. Although the seawater sample from Barrientos Island (KF428983) sequence had a 96 % similarity to H. cetorum and forms a group with H. pylori (Fig. 2), we cannot discard the possibility to found more of one Helicobacter sequence in this water sample. Then, we need to use other methods like PCR–DGGE or cloning to determine how many Helicobacter sequences we can find in our samples.
Even though two of our 16S rRNA sequences are closer to H. pylori, the glmM and ureA H. pylori-specific genes were not detected in any of the water samples. This result probably could be due to differences in sensitivity between PCR and semi-nested PCR. Semi-nested PCR is a more sensible method than PCR and allows the amplification of sequences present in extremely low numbers (García-Amado et al. 2011). These results suggest that H. pylori DNA concentration in Antarctica water sources is low; however, water quality monitoring programs including fecal indicators are necessary to confirm the degree of human impact.
In conclusion, the present study is the first report of Helicobacter DNA presence in different water sources and fecal samples of Gentoo (P. papua) and Chinstrap (P. antarctica) penguins from Greenwich, Dee and Barrientos Islands, Antarctica. Our results show that Helicobacter genus is part of the bacterial diversity of the microbiota present in the gastrointestinal tract of these two penguin species; however, more studies are needed to isolate, identify and establish the role of Helicobacter species as commensals. Additionally, two 16S rRNA sequences are closer to human Helicobacter species like H. pylori, and then more studies are needed to determine the Helicobacter species present in this ecosystem and to establish the human impact in these Antarctic Islands.
References
Ancel A, Beaulieu M, Gilbert C (2013) The different breeding strategies of penguins: a review. C R Biol 336:1–12
Azevedo NF, Almeida C, Fernandes I, Cerqueira L, Dias S, Keevil CW, Vieira MJ (2008) Survival of gastric and enterohepatic Helicobacter spp. in water: implications for transmission. Appl Environ Microbiol 74:1805–1811
Baker GC, Tow LA, Cowan DA (2003) PCR-based detection of non-indigenous microorganisms in ‘pristine’ environment. J Microbiol Methods 53:157–164
Barbosa A (2011) Effects of climate change on Antarctic penguins. Ecosystems 20:33–41
Bargagli R (2008) Environmental contamination in Antarctic ecosystems. Sci Total Environ 400:212–226
Bonnedahl J, Broman T, Waldenström J, Palmgren H, Niskanen T, Olsen B (2005) In search of human-associated bacterial pathogens in Antarctic wildlife: report from six penguin colonies regularly visited by tourists. Ambio 34:430–432
Carbone M, Maugeri TL, Gugliandolo C, La Camera E, Biondo C, Fera MT (2005) Occurrence of Helicobacter pylori DNA in the coastal environment of southern Italy (straits of Messina). J Appl Microbiol 98:768–774
Cellini L, Grande R, Di Campli E, Di Bartolomeo S, Di Giulio M, Traini T, Trubiani O (2008) Characterization of an Helicobacter pylori environmental strain. J Appl Microbiol 105:761–769
Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, Brown CT, Porras-Alfaro A, Kuske CR, Tiedje JM (2014) Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res 42(database issue):D633–D642
Collado L, Figueras MJ (2011) Taxonomy, epidemiology, and clinical relevance of the genus Arcobacter. Clin Microbiol Rev 24:174–192
Cowan DA, Chown SL, Convey P, Tuffin M, Hughes K, Pointing S, Vincent WF (2011) Non-indigenous microorganisms in the Antarctic: assessing the risks. Trends Microbiol 19:540–548
Dewar ML, Arnould JPY, Dann P, Trathan P, Groscolas R, Smith S (2013) Interspecific variations in the gastrointestinal microbiota in penguins. MicrobiologyOpen 2:195–204
Dewhirst FE, Seymour C, Fraser GJ, Paster BJ, Fox JG (1994) Phylogeny of Helicobacter isolates from bird and swine feces and description of Helicobacter pametensis sp. nov. Int J Syst Bacteriol 44:553–560
Dewhirst FE, Shen Z, Scimeca MS, Stokes LN, Boumenna T, Chen T, Paster BJ, Fox JG (2005) Discordant 16S and 23S rRNA gene phylogenies for the genus Helicobacter implications for phylogenetic inference and systematics. J Bacteriol 187:6106–6118
Fox JG, Taylor NS, Howe S, Tidd M, Xu S, Paster BJ, Dewhirst FE (2006) Helicobacter anseris sp. nov. and Helicobacter brantae sp. nov., isolated from feces of resident Canada geese in the greater Boston area. Appl Environ Microbiol 72:4633–4637
García A, Salas-Jara MJ, Herrera C, González C (2014) Biofilm and Helicobacter pylori: from environment to human host. World J Gastroenterol 20:5632
García-Amado MA, Bozo-Hurtado L, Astor Y, Suárez P, Chistoserdov A (2011) Denaturing gradient gel electrophoresis analyses of the vertical distribution and diversity of Vibrio spp. populations in the Cariaco Basin. FEMS Microbiol Ecol 77:347–356
Germani Y, Dauga C, Duval P, Huerre M, Levy M, Pialoux G, Sansonetti P, Grimont PAD (1997) Strategy for the detection of Helicobacter species by amplification of 16 s rRNA genes and identification of H. felis in a human gastric biopsy. Res Microbiol 148:315–326
Goldman CG, Matteo MJ, Loureiro JD, Degrossi J, Teves S, Heredia SR, Alvarez K, González AB, Catalano M, Boccio J, Cremaschi G, Solnick JV, Zubillaga MB (2009) Detection of Helicobacter and Campylobacter spp. from the aquatic environment of marine mammals. Vet Microbiol 133:287–291
Grondahl F, Sidenmark J, Thomsen A (2009) Survey of waste water disposal practices at Antarctic research stations. Polar Res 28:298–306
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4:1–9
Hughes KA, Thompson A (2004) Distribution of sewage pollution around a maritime Antarctic research station indicated by faecal coliforms, Clostridium perfringens and faecal sterol markers. Environ Pollut 127:315–321
IAATO (International Association of Antarctica Tour Operators) (2014) 2012–2013 Tourism statistics. http://iaato.org/es/tourism-statistics. Accessed 03 July 2014
Kansau I, Raymond J, Bingen E, Courcoux P, Kalach N, Bergeret M, Braimi N, Dupont C, Labigne A (1996) Genotyping of Helicobacter pylori isolates by sequencing of PCR products and comparison with the RAPD technique. Res Microbiol 147:661–669
Kusters JG, van Vliet AH, Kuipers EJ (2006) Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev 19:449–490
Lawson AJ, On SL, Logan JM, Stanley J (2001) Campylobacter hominis sp. nov., from the human gastrointestinal tract. Int J Syst Evol Microbiol 51:651–660
Metcheva R, Yurukova L, Teodorova S, Nikolova E (2006) The penguin feathers as bioindicator of Antarctica environmental state. Sci Total Environ 362:259–265
Moreno Y, Ferrús MA (2012) Specific detection of cultivable Helicobacter pylori cells from wastewater treatment plants. Helicobacter 17:327–332
Parte AC (2014) LPSN—list of prokaryotic names with standing in nomenclature. Nucleic Acids Res 42:D613–D616. http://www.bacterio.net. Accessed Dec 2015
Peek RM, Miller GG, Tham KT, Perez-Perez GI, Cover TL, Atherton JC, Dunn GD, Blaser MJ (1995) Detection of Helicobacter pylori gene expression in human gastric mucosa. J Clin Microbiol 33:28–32
Pruesse E, Peplies J, Glöckner FO (2012) SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics 28:1823–1829
Santiago P, Moreno Y, Ferrús MA (2015) Identification of viable Helicobacter pylori in drinking water supplies by cultural and molecular techniques. Helicobacter. doi:10.1111/hel.12205
Sjoling S, Cowan DA (2000) Detecting human bacterial contamination in Antarctic soils. Polar Biol 23:644–650
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tin T, Fleming ZL, Hughes KA, Ainley DG, Convey P, Moreno CA, Pfeiffer S, Scott J, Snape I (2009) Impacts of local human activities on the Antarctic environment. Antarctic Sci 21:3–33
Twing KI, Kirchman DL, Campbell BJ (2011) Temporal study of Helicobacter pylori presence in coastal freshwater, estuary and marine waters. Water Res 45:1897–1905
Acknowledgments
This study was accomplished in the Venezuelan Institute for Scientific Research and found by the Venezuelan Antarctic Program, the Ecuadorian Antarctic Institute and Secretariat of Science, Technology and Innovation of the Republic of Ecuador. The authors gratefully acknowledge the Logistics team of Ecuadorian base Pedro Vicente Maldonado, Soraya Silva Ph.D and Nory Gonzales by the help with the sampling collection, Juan Manuel Carrera for the help with map elaboration and M. López Gasca for English improvements of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cunachi, A.M., Fernández-Delgado, M., Suárez, P. et al. Detection of Helicobacter DNA in different water sources and penguin feces from Greenwich, Dee and Barrientos Islands, Antarctica. Polar Biol 39, 1539–1546 (2016). https://doi.org/10.1007/s00300-015-1879-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1879-5