Abstract
The gentoo penguin (Pygoscelis papua) is one of the most widespread penguin species and has been proven to be highly plastic in many aspects of its ecology. However, data from their sub-Antarctic range suggest an unexplained decline of their populations over the last 10–20 years, stressing the need for additional knowledge on their breeding ecology and demography. The present study provides insights into the breeding ecology of the gentoo penguin at a major breeding site, Kerguelen Archipelago, over three breeding seasons (1987, 2002 and 2003). Similarly to other northern populations, gentoo penguins breeding at Kerguelen exhibited winter laying, slow provisioning rate, slow growth rate associated with an extended rearing period and relatively low breeding success compared to southern populations. Our study also revealed interannual differences in the timing of laying and growth parameters as well as unusual sex differences in parental investment. Despite their high plasticity, there are indications that gentoo penguins at the northern edge of their range might work at the upper limit of their capacities. Sub-Antarctic populations would, therefore, be more sensitive to environmental changes than more southerly ones and need to be closely monitored.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the Southern oceans, penguins represent approximately 90% of bird biomass (Williams 1995), and as major predators within the sub-Antarctic and Antarctic ecosystems, consume 1.96 million tons of carbon annually (Woehler 1995). The gentoo penguin (Pygoscelis papua) is one of the most widespread penguin species, breeding on sub-Antarctic and Antarctic Islands and on the Antarctic Peninsula, from 46° to 65°S (Bost and Jouventin 1990). Throughout this large range, the gentoo penguin displays a high degree of plasticity in its life-history strategies, exhibiting contrasting traits between northern and southern populations (Bost and Jouventin 1990). In the sub-Antarctic, the South Georgia, Falkland and Kerguelen Islands are the main breeding sites, holding approximately 70% of the world gentoo population (Woehler 1993). Almost 40,000 pairs of gentoo penguins breed annually on the Kerguelen Archipelago (48°45′–50°00′S, 68°45′–70°58′E), Southern Indian Ocean (Bost and Jouventin 1990), but the population has decreased by approximately 30% over the last 15 years (Lescroël and Bost 2006). A similar decrease is suspected at nearby Heard Island (Woehler 2006). At sub-Antarctic Marion Island, the number of breeding individuals fell by 40% from 1994 to 2002 (Crawford et al. 2003). Thus, greater knowledge about the breeding ecology of gentoo penguins is needed to understand the population trends on sub-Antarctic Islands.
In contrast to the other pygoscelid species, Adélie (P. adeliae) and chinstrap penguins (P. antarctica), there have been few detailed studies of the breeding ecology of gentoo penguins and only three have included data from more than 1 year (King George Island: Trivelpiece et al. 1987; South Georgia Islands: Williams 1991; Crozet Islands: Bost and Jouventin 1991), two of these concerning Antarctic populations. Being one of the biggest populations of gentoo penguins in the world, the Kerguelen population is also unique among sub-Antarctic and Antarctic penguins in beginning to lay in winter—together with the Crozet and Marion Island populations (Bost and Jouventin 1990. 1991). Until now, no detailed study has been published on the breeding ecology of the gentoo penguin at Kerguelen Archipelago.
The objective of this paper is to provide the first description of the breeding ecology of the gentoo penguin at one of its major breeding sites, the Kerguelen Archipelago, from three breeding seasons spread over 15 years (1987–1988, 2002–2003, 2003–2004). Additionally, we will compare our data with that from other localities and investigate the variation of breeding parameters in relation to year and sex.
Methods
Data collection
The study took place during the 1987–1988, 2002–2003 and 2003–2004 austral summers (hereafter called 1987, 2002 and 2003) at Kerguelen Archipelago (Fig. 1). Gentoo breeding ecology was studied on the northeast of the Archipelago in a cluster of five subcolonies (“Estacade”) which included 75–140 nests each. Individuals from this location feed on the rich benthic ichthyofauna of the shelf’s neritic waters (Lescroël and Bost 2005).
The breeding cycle was divided into five stages: (1) Prebreeding, when adults come ashore for courtship activities and pairing; (2) Incubation, when mates take turns to incubate the eggs; (3) Chick guard, when mates take turns to brood and guard chicks at the nest; (4) Crèche, when chicks are left alone and gather with other chicks in crèches and (5) Fledging, when chicks go to sea for the first time, although still being fed by the parents (see also Polito and Trivelpiece 2008).
As gentoo penguins were particularly shy at our study site, care was taken to minimize stress during observations and handling: nests were approached slowly by a crawling observer for checking their content or measuring chicks, and most observations were made from a distance using binoculars. While being handled, birds were blinded with a hood and handling time was restricted to a few minutes. During the three austral summers, 100 study nests were marked with plastic tags in one of the subcolonies. Timing of laying and hatching, as well as clutch size, were assessed by visiting the nests every second day, at approximately the same hour, from mid-August to late October. When laying or hatching occurred between two visits, the event was assumed to have occurred during the previous day. In 1987, we also measured egg length and diameter with a calliper.
To assess relief duration of parents during incubation and chick guard, we marked one mate from each of 30 pairs in a second subcolony using seawater-proof diluted picric acid as a light yellow dye. Birds were marked without catching them, using a long-handled brush. Nest attendance was then monitored from a distance and foraging trip duration of colour-marked birds was gauged from three observations per day during 1 week for both incubation and chick guard stages. Trip durations estimated by such direct observations are in accordance with trip durations estimated by using time-depth recorders (Lescroël and Bost 2005). During the crèche stage, both parents may forage at sea at the same time and bird departures or returns often occurred during periods of darkness. Consequently, direct observations of relief duration were more difficult, and in 2003 we used 10 VHF transmitters (60 mm × 26 mm × 14 mm, i.e. 1.6% of the cross-sectional area of a gentoo penguin, 36 g, Sirtrack Ltd, New Zealand) to assess the feeding frequency. The transmitters were attached to the back feathers using Loctite 401 and were left on birds for three to six consecutive days, providing two to seven trip durations per bird. Presence or absence of the birds in the colony was assessed by scanning the VHF frequencies every 2 h, day and night, using a multidirectional Yagi antenna coupled with a VHF receptor. For statistical analyses, we used individual mean values for each bird.
In a third colony, we followed chick growth from hatching to fledging in 30 nests by weighing known-age chicks every other day in 1987 and 2003. Moult state was also recorded. Up to 15 days old, chicks were marked with a temporary numbered Velcro® band. Then each was marked with a small plastic label with a subcutaneous part and an exterior numbered part (Floy Tag & Manufacturing Inc., USA), which was implanted in the nape of the neck using a tagging gun. Each chick was weighed from age 1–5 days up to 88–140 days old.
In 1987 and 2002, the breeding success of gentoo penguins was assessed in a fourth colony. At the beginning of incubation, the number of occupied nests was counted from a distance using binoculars. This number was taken as the number of breeding pairs. Breeding success was calculated by dividing the number of chicks produced by the colony, i.e. the number of chicks in the crèches immediately prior fledging, by the number of breeding pairs at the beginning of incubation. Using number of chicks raised to crèche stage as a measure of productivity is in accordance with CEMP (Commission for the Conservation of Antarctic Marine Living Resources Ecosystem Monitoring Program) Standard Methods for Monitoring Parameters of Predator Species (CCAMLR 2004) and allows comparisons with other studies (e.g. Cobley and Shears 1999; Crawford et al. 2003).
Data for assessing body weight variation in breeding birds came from measurements made before foraging trips outside the colonies and from demographic, foraging (Lescroël and Bost 2005) and genetic studies undertaken at the same periods. Data were collected during prebreeding, incubation, hatching, crèche and fledging stages in 1987, during incubation, chick guard and crèche stages in 2002 and during prebreeding and crèche stages in 2003. Also, in 1987, stomach contents of 12 females and 8 males were collected during the chick guard stage using a nondestructive method (see details in Lescroël et al. 2004). We report here the drained weight of these contents as well as the reconstituted biomass and the reconstituted mass by prey group (crustaceans, fish, cephalopods and annelids, see Methods in Lescroël et al. 2004).
Breeding birds were sexed by their size (flipper length, bill length and bill depth) and behaviour (particularly their call) or by the presence of a partner of known sex.
Data analysis
Growth data were analysed by fitting each individual chick’s weight curve relative to hatching date to a three-parameter logistic growth curve (Volkman and Trivelpiece 1980; Bost and Jouventin 1991). We fitted only data from chicks measured to at least 80 days of age. Growth parameters were calculated from the logistic equation: \(W=A/(1+{\rm{e}}^{-K(t-t_i)}),\) where W represents weight at time t, A is the asymptotic weight, K is a constant proportional to the overall growth rate and t i is the inflexion point at which 50% of asymptotic weight is achieved, i.e. the age of greatest growth rate.
Data were statistically analysed using Systat 7.0. Values are given as mean ± SD unless otherwise stated. Comparisons of breeding parameters between years, breeding stages and sexes were made using Student’s t tests or ANOVAs. ANOVAs were followed by Tukey post–hoc tests when necessary. Comparisons of shifts duration during incubation and chick guard in 2002 between paired females and males were performed using paired sample t tests. Since the robustness of ANOVA and t test increases when sample sizes are equal or close (Sokal and Rohlf 1995), we randomly selected data whenever possible in order to have similar sample sizes between groups. Normality and homoscedasticity were tested before using parametric tests. Significance was assumed for P < 0.05.
Results
Breeding chronology
Laying
Over the 3 years of study, the mean laying date of the first egg differed by 16 days at Estacade (Table 1). Laying encompassed 26–29 days each year and peaked between late August and mid-September, i.e. at the end of the austral winter. Only 2% of the breeding pairs laid again after a breeding failure (1987, n = 94 pairs). In 2002, laying occurred significantly later than in 1987 or in 2003 (F 2,201 = 244.70, P < 0.001; Tukey post hoc test: 2002–1987, P < 0.001; 2002–2003, P < 0.001; 1987–2003, P = 0.06).
Clutch and egg sizes
In all 3 years, most gentoo pairs laid two eggs (1987: 1.9 ± 0.3 eggs, 2002: 1.8 ± 0.4 eggs, 2003: 1.9 ± 0.2 eggs, n = 77 in each year). The laying interval between eggs was 3.2 ± 0.9 days (range 2–6, n = 127). There was no size dimorphism between the first- and second-laid eggs (egg length: F 1,68 = 0.01, P = 0.92; egg width: F 1,68 = 0.47, P = 0.50; egg volume: F 1,68 = 0.38, P = 0.54; Table 2).
Incubation duration
Incubation of the first-laid egg was significantly longer (36.0 ± 1.1 days, n = 69) than for the second egg (33.8 ± 1.1 days, n = 69) (t 136,138 = 11.81, P < 0.001). No difference occurred among years (F 2,135 = 0.37, P = 0.69).
Hatching
Consistent with the laying schedule, the mean hatch date of the first egg differed by 16 days during the 3 years of the study (Table 3). The hatching interval between the first and second egg was 1.2 ± 0.7 days (range 0–5, n = 65). In 2002, hatching occurred significantly later than in 1987 or in 2003 (F 2,78 = 58.98, P < 0.001; Tukey post hoc test: 2002–1987, P < 0.001; 2002–2003, P < 0.001; 1987–2003, P = 0.07). For all 3 years, hatching of first clutches was spread over nearly 4 weeks. Hatching peaked between the end of September and mid-October, i.e. during the austral spring.
Parental relief duration
Trip duration was significantly longer during incubation (1987: 2.8 ± 1.1 days, n = 35 individuals; 2002: 2.9 ± 1.0 days, n = 46 individuals) than during chick guard (1987: 1.4 ± 0.6 days, n = 34 individuals; 2002: 1.3 ± 0.5 days, n = 46 individuals) or crèche (2003: 1.2 ± 0.5 days, n = 10 individuals, Table 4) (F 2,134 = 50.20, P < 0.001; Tukey post hoc test: incubation–chick guard, P < 0.001; incubation–crèche, P < 0.001; chick guard–crèche, P = 0.86). As a consequence of concomitant foraging by both sexes during crèche, we estimate that gentoo penguin chicks received 0.8 and 1.7 feeding visits per day during chick guard and crèche, respectively. Except in 1987, when males foraged significantly longer than females during chick guard (t 28.7,34 = −2.74, P = 0.01), males and females spent about the same time off the nest throughout the breeding season (t 34,35 = −0.72, P = 0.48, in 1987 during incubation; t 22,23 = −1.51, P = 0.15; t 22,23 = −1.33, P = 0.20, in 2002 during incubation and chick guard, respectively; t 3.9,10 = −1.51, P = 0.65, in 2003 during the crèche stage).
Diet
During the 1987–1988 breeding season, females and males brought back about the same amount of food for their chicks (190 ± 173 and 133 ± 112 g, respectively; t 16.7,19 = 0.88, P = 0.39). In terms of reconstituted biomass, females and males hunted for about the same prey biomass (291 ± 215 and 273 ± 160 g, respectively; t 17,19 = 0.21, P = 0.83), but targeted different prey groups (Table 5). Females exhibited a mixed diet composed about half and half from fish and crustaceans while males preyed mostly on fish (83%) and cephalopods (13%).
Chick growth
Growth parameters
Growth parameters were obtained from 27 individual growth curves in 2003 and from six individual growth curves in 1987 (Table 6). For all fitted curves, mean adjusted R2 = 0.97 and mean standard error = 278.29 g. Chick weight increased linearly from 10 days to about 50 days and then slowed down to attain a plateau at about 80 days (Fig. 2). Maximal growth rates were observed earlier in 2003 (35 days) than in 1987 (41 days; t 31,33 = 2.52, P = 0.02). In 2003, chicks grew faster than in 1987 (t 31,33 = −2.32, P = 0.03) but eventually reached similar asymptotic weights (t 31,33 = 0.85, P = 0.41).
Age at thermal emancipation and moult
Chicks were thermally emancipated (i.e. they do not need an external input of warmth anymore and they can wander in the vicinity of the nest while being still guarded by one parent) at about 25 days after hatching and crèched shortly thereafter. They began moulting at 40–47 days, at a significantly older age in 2003 than in 2002 (t 19.9,28 = −4.99, P < 0.001) and mostly completed their moult before 100 days of age (Table 7).
Breeding success
Breeding success was slightly higher in 1987 (0.75 chick per pair) than in 2002 (0.71 chick per pair). The second-hatched chick always died early (at 7.9 ± 4 days of age, n = 37, 1987).
Body weight variation
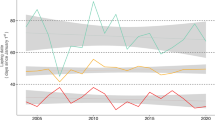
Year and breeding stage significantly influenced body weight (year: F 2,225 = 7.08, P = 0.01, breeding stage nested into year: F 7,225 = 4.83, P < 0.001) (Fig. 3). Breeding birds were lighter in 2002 than in 1987 or 2003 (Tukey post hoc tests: 2002–1987, P = 0.001; 2002–2003, P < 0.01; 2003–1987, P = 0.88). When pooling data from 1987 and 2003 and taking an equal sample size in each breeding stage with a balanced sex-ratio (n = 10), no effect of breeding stage on body weight could be detected (F 3,40 = 0.18, P = 0.91). Since male gentoo penguins are often heavier than females (Bost and Jouventin 1991), we analysed body weight data for females and males separately. In females, breeding stage had a significant effect on body weight variation (F 3,20 = 4.40, P < 0.05) with weights in crèche and fledging being or tending to be lower than prebreeding weight (Tukey post hoc tests: P = 0.02 and 0.06, respectively). In males, there was no significant effect of breeding stage on body weight (F 3,20 = 1.13, P = 0.37). Moreover, body weights of females and males were not statistically different from prebreeding to chick guard stages but females became significantly lighter during the crèche stage (t 7.1,10 = −5.62, P < 0.001) and fledging (t 8.0,10 = −3.02, P < 0.05) (Fig. 4).
Discussion
Comparison with other localities
Gentoo penguins from Kerguelen Archipelago exhibited many general characteristics of the species’ breeding biology. At both northern (e.g. Crozet and Marion Islands) and southern (e.g. South Shetland Islands and Antarctic Peninsula) localities, P. papua populations show a clutch of two eggs, a laying interval of 3 days, an incubation period of 33–37 days, short nest relieves shared between sexes and a relatively slow growth of chicks to reach high asymptotic weights. This latter pattern probably arose as an adaptation to the relatively large body size of the gentoo penguin compared to the other pygoscelid species. Like every other penguin species, except Emperor (Aptenodytes forsteri) and King (A. patagonicus) which lay a single egg, the gentoo penguin lays two eggs, but unlike that of crested penguins (first egg 17–44% smaller) or even that of the closely related Adélie penguin (first egg 8% larger; see Croxall and Davis 1999 for a review), gentoo penguin eggs are of quite similar size (egg size dimorphism <5%).
Interestingly, although gentoo penguins from Kerguelen Archipelago share common characteristics with conspecifics from other localities, they also provide new evidence of the high geographical variability of the ecology of this species (Table 8). The most striking difference among localities lies in the timing of laying. Northern populations of gentoo penguins (within 45–50°S) are unique among sub-Antarctic penguins in laying mainly in winter (from June at Marion Island to early August at Crozet Islands and late August at Kerguelen Archipelago) while middle range (i.e. Falkland Islands: 51–52°S) and southern populations (54–65°S) lay in spring (from October at South Georgia and Falkland Islands to November at South Shetland Islands) as do other pygoscelid species (Bost and Jouventin 1990; Otley et al. 2005). In birds, timing of reproduction is determined by (1) ultimate factors, with favoured genotypes being those which end up in matching chick rearing and peak resource abundance (Lack 1954; Charnov and Krebs 1974), and (2) proximate factors, like photoperiod, climate or state of resources, which allow birds to adjust the timing of laying to an optimal breeding period (Wingfield et al. 1992; Lambrechts et al. 1997). From an ultimate standpoint, winter laying might limit interspecific competition with other summer-breeding marine predators (like the abundant macaroni penguin Eudyptes chrysolophus, Bost and Jouventin 1991). Indeed, the gentoo penguin is a sedentary inshore feeder which has highly flexible feeding habits (Lescroël et al. 2004; Lescroël and Bost 2005) that make possible breeding during the relatively mild winter of the roaring forties. At Crozet Islands, where gentoo penguins strongly depend on euphausiids (also the main prey of the neighbouring five million breeding pairs of macaroni penguins; Ridoux 1994), laying occurs the earliest (about 2 months earlier than at Kerguelen) and the chick rearing period overlaps with the macaroni’s breeding cycle only during a few weeks. On the other hand, in the southernmost localities, the peak of resource availability for gentoo penguins (and for chinstrap penguins as well) will occur after the sea-ice dissipation, i.e. later in the summer season. From a proximate standpoint, while photoperiod and local climate likely play a role in the differences of breeding phenology between northernmost and southernmost localities, these differences have usually been interpreted as consequences of local feeding conditions rather than physical factors (Bost and Jouventin 1990). At Kerguelen Archipelago, the abundance of icefish (Champsocephalus gunnari) between July and September in the eastern coastal waters (for breeding; Duhamel 1987) may facilitate the early acquisition of a good body condition. Icefish indeed account for 35–40% by mass of the gentoo penguin diet in winter at Kerguelen (Lescroël et al. 2004).
Whereas gentoo penguins, in general, perform short parental reliefs compared to other penguin species, gentoo penguins from Kerguelen Archipelago exhibited longest reliefs in comparison to other localities. Trip duration during incubation particularly is the longest mentioned in the literature (see also Lescroël and Bost 2005). Such long trips may be related to abundant but distant resources as Kerguelen Archipelago is surrounded by an extensive and productive shelf (Blain et al. 2001). According to central place foraging theory (Orians and Pearson 1979), birds perform longer trips and return with larger meals when food is abundant (Watanuki et al. 1997), especially when foraging for self-maintenance, as during incubation. However, the low breeding success observed at the northernmost localities (see also below) does not support this hypothesis. At southern localities, parental reliefs are significantly shorter, like at Antarctic Peninsula (1.8 and 0.1–0.3 days during incubation and chick guard, respectively; Cordier et al. 1983; Wilson et al. 1998), probably due to the proximity of more immediately available food resources like Antarctic krill (Euphausia superba). During the crèche stage, both parents foraged at the same time and no longer relieved each other, de facto increasing the feeding frequency of their chicks. At Kerguelen Archipelago, the feeding frequency increased from 0.8 feeding visits per day during the chick guard stage to 1.7 per day during the crèche stage. This is more than at Crozet Islands where the feeding frequency decreased during crèche (from 0.8 to 0.7 per day; Bost and Jouventin 1991) but far less than at South Shetland Islands where chicks in crèches were fed 3.8 times a day (Trivelpiece et al. 1987). Also, the meal size tends to increase from north to south (about 150 g at Crozet Islands and Marion Island, 500 g at Kerguelen Archipelago, 800 g at South Georgia Islands but 400 g at South Shetland Islands; see Bost and Jouventin 1991 for a review; Ridoux 1994).
This provisioning strategy at a relatively slow rate at northern localities is probably responsible for the slow chick growth compared to southern localities. At Kerguelen Archipelago, gentoo penguin chicks took 57–73 days to grow from 10 to 90% of their final weight with a growth constant of 0.064–0.081, which is very similar to the situation at Crozet Islands (65 days, 0.070–0.076) (Bost and Jouventin 1991) but much longer than at South Georgia Islands (46 days, 0.120; Croxall 1984; Croxall and Prince 1987) or South Shetland Islands (39 days, 0.113; Volkman and Trivelpiece 1980). Whatever the locality, the asymptotic weight is always high compared to other pygoscelid species and ranges from 5,061 to 5,336 g at Kerguelen Archipelago to 6,032 g at Crozet Islands.
Our study confirms the findings of Bost and Jouventin (1990) that breeding success of the gentoo penguin generally increases from north to south with Antarctic populations having higher breeding success than sub-Antarctic ones. Hence, on average, a breeding pair is able to rear 0.48 chicks at Crozet Islands (Bost and Jouventin 1990, 1991), between 0.43 and 0.58 at Marion Island (Williams 1980; Crawford et al. 2003), 0.73 at Kerguelen Archipelago (this study), between 0.95 and 1.3 at Falkland Islands (Clausen and Pütz 2002; Otley et al. 2005), 0.98 at Macquarie Island (Robertson 1986) and 0.96 at South Georgia (Williams 1990). At Antarctic Peninsula, a high proportion of pairs (65%) seems able to rear two chicks (Quintana and Cirelli 2000).
Asynchronous hatching, with the resulting potential competitive advantage to the first-hatched chick, is a classic basis for subsequent brood reduction (i.e. a situation where the second-hatched chick survives only in years when it can receive adequate food after its older sibling has been satisfied; Lack 1954, 1966; Clark and Wilson 1981). However, despite a small hatching asynchrony (1.2 days), it was so far commonly assumed that gentoo penguins do not operate a brood reduction strategy (Volkman and Trivelpiece 1980; Williams and Croxall 1991; Croxall and Davis 1999; Polito and Trivelpiece 2008). But this conclusion was drawn from data collected at southernmost breeding localities. At Kerguelen and Crozet Islands, only a very small percentage of breeding pairs (<1%) is able to fledge both chicks and a marked difference was observed in the growth rates of siblings (Bost and Jouventin 1991). Brood reduction, through the starvation to death of the second-hatched chick, does, therefore, occur in northernmost gentoo penguin populations.
To summarize, the breeding ecology of the gentoo penguin at Kerguelen Archipelago fits into a latitudinal gradient from winter breeding with poor synchronous laying, relatively long nest reliefs, slow growth rate of chicks, long rearing period and low breeding success at the northernmost localities—a pattern that we observed in all three study years at Kerguelen Archipelago—, to spring laying, with short nest reliefs, rapid growth, short rearing period and high breeding success at the southernmost localities.
Interannual variation
At Kerguelen Archipelago, gentoo penguins show substantial interannual variation in breeding chronology and chick growth rates. The high year-to-year variability in laying date and growth parameters is typical of a species dependent on resources with fluctuating availability (Montevecchi 1993). Adult body mass also varied significantly among years. In 2002, breeding adults were lighter and laid later than in 1987 and 2003, even though the breeding success was quite similar between 1987 and 2002. In 2003, chicks grew faster than in 1987 but reached about the same asymptotic weights. Due to the higher growth rate, we can hypothesize that 2003 was a “better” food year than 1987, which was itself a better year than 2002. Constancy in chick fledging weight, in spite of large environmental variations, is a well-known characteristic of Adélie penguins, and has been explained by the compensating effects of food delivery, food quality and metabolic processes (Salihoglu et al. 2001). Similar mechanisms might also occur in gentoo penguins. However, despite these indications that the 3 years of study might be years of different food availability, average trip durations did not differ among years. Trip durations at the study colony (on the eastern part of Kerguelen Archipelago) are among the longest ever reported and they might represent a maximum effort ceiling which cannot be further increased.
Intersexual variation
Provisioning is a large part of parental care and biparental care is the norm among bird species (Clutton-Brock 1991) and especially seabirds (Croxall 1984). Until now, it has been assumed that breeding duties are equally shared between sexes in gentoo penguins (Williams 1995) and that parental expenditure was, therefore, the same between males and females. Parental expenditure is the amount of resources allocated to care for offspring and may include time and energy (Clutton-Brock 1991).
In our study, females exhibited higher body reserve depletion during the chick rearing period than did males. Thus, as reported for Crozet Islands (Bost and Jouventin 1991), female gentoo penguins from Kerguelen Archipelago seem to produce a greater parental effort than males. This is an unexpected result in species which show little sexual dimorphism (Lewis et al. 2002). At the beginning of the breeding cycle, body mass did not differ between sexes and both sexes lost mass between prebreeding and incubation. After that, females lost weight between hatching and chick guard and then remained at low body mass levels until chick fledging. On the other hand, males tended to gain weight continuously from incubation to crèche and then remained at high body mass levels. Females seem, therefore, to lose energy during chick rearing whereas males seem able to forage both for themselves and for the chicks. This hypothesis is further supported by the comparison of the reconstituted prey biomass in stomach contents and the drained weight of meals brought back to the colony by females and males: males brought back 49% of what they captured versus 65% for females.
This pattern was also reported in the Adélie penguin, where females expend more energy during the post-hatch period than males, show longer trips and range over greater distances, but also bring back more food than their male mates do (Chappell et al. 1993; Clarke et al. 1998). As female gentoo penguins from Kerguelen Archipelago did not bring back significantly heavier meals than males, they appear to bear higher foraging costs (based on body weight variations) for a similar foraging success, though the results from 1987 and 2002 on foraging trip durations are inconsistent.
These sexual differences in foraging behaviour might be mediated mainly (1) by morphology-based mechanisms that would lead to differences in ability to capture prey and/or to exploit some areas or depths (although gentoo penguins are only slightly dimorphic: e.g. females have flippers about 4% shorter than males and are about 8% lighter than males during the prebreeding period), or (2) by physiology-based mechanisms such as differences in energy or nutrient (i.e. calcium) requirements. The important fraction of crustaceans, whose exoskeleton is rich in calcium carbonate, in the female diet could support this second hypothesis. Clearly, however, more investigations are needed to fully understand the pattern of parental investment in gentoo penguins.
In short, the breeding biology and parental investment of gentoo penguins vary extensively as a function of locality, year and sex, as has been demonstrated previously for their diet (Lescroël et al. 2004) and foraging ecology (Lescroël and Bost 2005). Inshore feeding, flexible behaviour and sedentary nature appear to be key features determining the biology of the species (Bost and Jouventin 1991). Such variations in their overall ecology are indicators of the ability of these birds to modify their strategies to take advantage of the spatial and temporal availability of prey near their breeding sites. However, low provisioning rates associated with slow chick growth and reduced breeding success clearly reflects lower food availability at the northern edge of the gentoo penguin range. From this viewpoint, sub-Antarctic populations might be more sensitive than Antarctic populations to perturbations of the food chain they belong to by industrial fisheries and climate modifications. This stresses the need for more systematic monitoring of these northern populations.
References
Adams NJ, Klages NT (1989) Temporal variation in the diet of the gentoo penguin Pygoscelis papua at sub-Antarctic Marion Island. Colon Waterbirds 12:30–36. doi:10.2307/1521309
Blain S, Tréguer P, Belviso S, Bucciarelli E, Denis M, Desabre S, Fiala M, Martin Jézéquel V, Le Fèvre J, Mayzaud P, Marty J-C, Razouls S (2001) Amphipod-based food web: Themisto gaudichaudii caught in nets and by seabirds in Kerguelen waters, southern Indian Ocean. Mar Ecol Prog Ser 223:261–276. doi:10.3354/meps223261
Bost C-A, Jouventin P (1990) Evolutionary ecology of the gentoo penguin Pygoscelis papua. In: Davis L, Darby J (eds) Penguin biology. Academic, San Diego, pp 85–112
Bost C-A, Jouventin P (1991) The breeding performance of the gentoo penguin Pygoscelis papua at the northern edge of its range. Ibis 133:14–25. doi:10.1111/j.1474-919X.1991.tb04804.x
CCAMLR (2004) Standard methods for monitoring parameters of predators species. CCAMLR Ecosystem Monitoring Program. Hobart, Australia
Chappell MA, Janes DN, Shoemaker VH, Bucher TL, Maloney SK (1993) Reproductive effort in Adélie penguins. Behav Ecol Sociobiol 33:173–182. doi:10.1007/BF00216598
Charnov EL, Krebs JR (1974) On clutch-size and fitness. Ibis 116:217–219. doi:10.1111/j.1474-919X.1974.tb00241.x
Clark AB, Wilson DS (1981) Avian breeding adaptations: hatching asynchrony, brood reduction, and nest failure. Q Rev Biol 56(3):253–277. doi:10.1086/412316
Clarke J, Manly B, Kerry K, Gardner H, Franchi E, Corsolini S, Focardi S (1998) Sex differences in Adélie penguin foraging strategies. Polar Biol 20:248–258. doi:10.1007/s003000050301
Clausen A, Pütz K (2002) Recent trends in diet composition and productivity of gentoo, magellanic and rockhopper penguins in the Falkland Islands. Aquat Conserv 12:51–61. doi:10.1002/aqc.476
Clausen AP, Arkhipkin AI, Laptikhovsky VV, Huin N (2005) What is out there: diversity in feeding of gentoo penguins (Pygoscelis papua) around the Falkland Islands (Southwest Atlantic). Polar Biol 28:653–662. doi:10.1007/s00300-005-0738-1
Clutton-Brock TH (1991) The evolution of parental care. Princeton University Press, Princeton
Cobley ND, Shears JR (1999) Breeding performance of gentoo penguins (Pygoscelis papua) at a colony exposed to high levels of human disturbance. Polar Biol 21:355–360
Cordier JR, Mendez A, Mougin JL, Visbeek G (1983) Les oiseaux de la baie de l’Espérance, Péninsule Antarctique (63°24′S, 56°59′W). L’Oiseau Rev Fr Ornithol 51:147–160
Crawford RJM, Cooper J, Du Toit M, Greyling MD, Hanise B, Holness CL, Keith DG, Nel JL, Petersen SL, Spencer K, Tshingana D, Wolfaardt AC (2003) Population and breeding of the gentoo penguin Pygoscelis papua at Marion Island, 1994/95–2002/03. Afr J Mar Sci 25:463–474
Croxall JP (1984) Seabirds. In: Laws RM (ed) Antarctic ecology. Academic, New York, pp 533–620
Croxall JP, Davis LS (1999) Penguins: paradoxes and patterns. Mar Ornithol 27:1–12
Croxall JP, Prince PA (1980) Food, feeding ecology and ecological segregation of seabirds at South Georgia. Biol J Linn Soc Lond 14:103–131. doi:10.1111/j.1095-8312.1980.tb00101.x
Croxall JP, Prince PA (1987) Seabirds as predators on marine resources, especially krill, at South Georgia. In: Croxall JP (ed) Seabirds: feeding ecology and role in marine ecosystems. Cambridge University Press, Cambridge, pp 347–368
Croxall JP, Reid K, Prince PA (1999) Diet, provisioning and productivity responses of marine predators to differences in availability of Antarctic krill. Mar Ecol Prog Ser 177:115–131. doi:10.3354/meps177115
Duhamel G (1987) Ichtyofaune des Secteurs Indien Occidental et Atlantique Oriental de l’océan austral: Biogéographie, cycles biologiques et dynamique des populations. Thèse de doctorat, Université Pierre et Marie Curie, Paris
Hindell MA (1989) The diet of gentoo penguins Pygoscelis papua at Macquarie Island: winter and early breeding season. Emu 89:71–78
Holmes ND, Giese M, Achurch H, Robinson S, Kriwoken LK (2006) Behaviour and breeding success of gentoo penguins Pygoscelis papua in areas of low and high human activity. Polar Biol 29:399–412
Kato A, Williams TD, Barton TR, Rodwell S (1991) Short-term variation in the winter diet of gentoo penguins Pygoscelis papua at South Georgia during July 1989. Mar Ornithol 19:31–38
Klages NTW, Pemberton D, Gales RP (1990) The diets of king and gentoo penguins at Heard Island. Aust Wildl Res 17:53–60. doi:10.1071/WR9900053
Lack D (1954) The natural regulation of animal numbers. Oxford University Press, Oxford
Lack D (1966) Population studies of birds. Clarendon Press, Oxford
Lambrechts MM, Blondel J, Maistre M, Perret P (1997) A single response mechanism is responsible for evolutionary adaptive variation in a bird’s laying date. Proc Natl Acad Sci USA 94:5153–5155. doi:10.1073/pnas.94.10.5153
Lescroël A, Bost C-A (2005) Foraging under contrasting oceanographic conditions: the gentoo penguin at Kerguelen Archipelago. Mar Ecol Prog Ser 302:245–261. doi:10.3354/meps302245
Lescroël A, Bost C-A (2006) Recent decrease in gentoo penguin populations at Iles Kerguelen. Antarct Sci 18(2):171–174. doi:10.1017/S0954102006000198
Lescroël A, Ridoux V, Bost C-A (2004) Spatial and temporal variation in the diet of the gentoo penguin (Pygoscelis papua) at Kerguelen Islands. Polar Biol 27:206–216. doi:10.1007/s00300-003-0571-3
Lewis S, Benvenuti S, Dall’Antonia L, Griffiths R, Money L, Sherratt TN, Wanless S, Hamer KC (2002) Sex-specific foraging behaviour in a monomorphic seabird. Proc R Soc Lond B Biol Sci 269:1687–1693
Montevecchi WA (1993) Birds as indicators of change in marine prey stocks. In: Furness RW, Greenwood JJD (eds) Birds as monitors of environmental change. Chapman & Hall, London, pp 217–266
Orians GH, Pearson NE (1979) On the theory of central place foraging. In: Horm DJ, Mitchell RD, Stairs GR (eds) Analysis of ecological systems. Ohio University Press, Columbus, pp 154–177
Otley HM, Clausen AP, Christie DJ, Pütz K (2005) Aspects of the breeding biology of the gentoo penguin Pygoscelis papua at Volunteer Beach, Falkland Islands, 2001/02. Mar Ornithol 33:167–171
Polito MJ, Trivelpiece WZ (2008) Transition to independence and evidence of extended parental care in the gentoo penguin (Pygoscelis papua). Mar Biol 154:231–240. doi:10.1007/s00227-008-0919-x
Pütz K, Ingham RJ, Smith JG, Croxall JP (2001) Population trends, breeding success and diet composition of gentoo Pygoscelis papua, magellanic Spheniscus magellanicus and rockhopper Eudyptes chrysocome penguins in the Falkland Islands. Polar Biol 24:793–816. doi:10.1007/s003000100293
Quintana RD, Cirelli V (2000) Breeding dynamics of a gentoo penguin Pygoscelis papua population at Cierva Point, Antarctic Peninsula. Mar Ornithol 28:29–35
Ridoux V (1994) The diets and dietary segregation of seabirds at the subantarctic Crozet Islands. Mar Ornithol 22:1–192
Robertson G (1986) Population size and breeding success of the gentoo penguin Pygoscelis papua at Macquarie Island. Aust Wildl Res 13:583–587. doi:10.1071/WR9860583
Robinson SA, Hindell MA (1996) Foraging ecology of gentoo penguins Pygoscelis papua at Macquarie Island during the period of chick care. Ibis 138:722–731
Salihoglu B, Fraser WR, Hofmann EE (2001) Factors affecting fledging weight of Adélie penguin (Pygoscelis adeliae) chicks: a modeling study. Polar Biol 24:328–337. doi:10.1007/s003000000215
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn edn. W.H. Freeman and Co, New York
Trathan PN, Forcada J, Atkinson R, Downie RH, Shears JR (2008) Population assessments of gentoo penguins (Pygoscelis papua) breeding at an important Antarctic tourist site, Goudier Island, Port Lockroy, Palmer Archipelago, Antarctica. Biol Conserv 141:3019–3028. doi:10.1016/j.biocon.2008.09.006
Trivelpiece WZ, Trivelpiece SG, Volkman NJ (1987) Ecological segregation of Adélie, gentoo and chinstrap penguins at King George Island, Antarctica. Ecology 68:351–361. doi:10.2307/1939266
Volkman NJ, Trivelpiece W (1980) Growth in pygoscelid penguin chicks. J Zool 91:521–530
Volkman NJ, Jazdzewski K, Kittel W, Trivelpiece WZ (1980) Diets of Pygoscelis penguins at King George Island, Antarctica. Condor 82:373–378. doi:10.2307/1367558
Watanuki Y, Kato A, Robertson G (1997) Diving and foraging behaviour of Adélie penguins in areas with and without fast sea-ice. Polar Biol 17:296–304. doi:10.1007/PL00013371
Williams AJ (1980) Aspects of the breeding biology of the gentoo penguin Pygoscelis papua. Gerfaut 70:283–295
Williams TD (1990) Annual variation in breeding biology of gentoo penguins, Pygoscelis papua, at Bird Island, South Georgia. J Zool (Lond) 222:247–258. doi:10.1111/j.1469-7998.1990.tb05675.x
Williams TD (1991) Foraging ecology and diet of Gentoo Penguins Pygoscelis papua at South Georgia during the winter and an assessment of their winter prey consumption. Ibis 133:3–13. doi:10.1111/j.1474-919X.1991.tb04803.x
Williams TD (1995) The penguins. Oxford University Press, Oxford
Williams TD, Croxall JP (1991) Chick growth and survival in gentoo penguins (Pygoscelis papua): effects of hatching asynchrony and variation in food supply. Polar Biol 11:197–202. doi:10.1007/BF00240208
Wilson RP, Alvarrez B, Latorre L, Adelung D, Culik B, Bannasch R (1998) The movements of gentoo penguins Pygoscelis papua from Ardley Island Antarctica. Polar Biol 19:407–413. doi:10.1007/s003000050266
Wingfield JC, Hahn TP, Levin R, Honey P (1992) Environmental predictability and control of gonadal cycles in birds. J Exp Zool 261:214–231. doi:10.1002/jez.1402610212
Woehler EJ (1993) The distribution and abundance of Antarctic and sub-Antarctic penguins. Scientific Committee on Antarctic Research, Cambridge
Woehler EJ (1995) Consumption of Southern Ocean marine resources by penguins. In: Dann P, Norman I, Reilly P (eds) The penguins. Surrey Beatty & Sons, Chipping Norton, pp 266–295
Woehler EJ (2006) Status and conservation of the seabirds of Heard Island and the McDonald Islands. In: Green K, Woehler EJ (eds) Heard Island: Southern Ocean Sentinel. Surrey Beatty & Sons, Chipping Norton, pp 128–165
Acknowledgments
This work was supported by the Institut Polaire Paul Emile Victor (IPEV, Programme No. 394, resp. C.A. Bost), the Terres Australes et Antarctiques Françaises and the Centre National de la Recherche Scientifique. We would like to thank Alain Lamalle and the members of the 37th, 39th, 52nd, 53rd and 54th missions at Kerguelen, and especially F. Genevois, V. Chartendrault, E. Pettex, J.-L. Chil, C. Marteau and F. Le Bouard for their help in the field. Finally, we are especially indebted to Pierre Jouventin for his thoughtful comments and inspiration over the whole study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lescroël, A., Bajzak, C. & Bost, CA. Breeding ecology of the gentoo penguin Pygoscelis papua at Kerguelen Archipelago. Polar Biol 32, 1495–1505 (2009). https://doi.org/10.1007/s00300-009-0647-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-009-0647-9