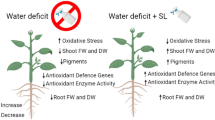
Abstract
Key message
Exogenously supplied BR and JA help KS101 and KBS3 genotypes of Brassica rapa to alleviate drought stress by modifying osmolyte concentration, levels of antioxidant enzymes and photosynthetic system.
Abstract
Oilseed plants are susceptible to drought stress and a significant loss in yield has been reported during recent decades. Thus, it is imperative to understand the various underlying drought response mechanisms in Brassica oilseed plants to formulate the sustainable strategies to protect the crop yield under water-limiting conditions. Phytohormones play a key role in fine-tuning various regulatory mechanisms for drought stress adaptation in plants, and the present study explores the response of several physiological stress markers by exogenous supplementation of 24-epibrassinolide (EBL) and jasmonic acid (JA) on two genotypes of Brassica rapa, KS101 and KBS3 under drought stress conditions. The exogenous application of BR and JA, separately or in combination, significantly alleviated the drought stress by improving photosynthetic rate, photosynthetic pigments, stomatal conductance, transpiration rate and antioxidant defence. We observed that concentration of different osmolytes increased and membrane damage significantly reduced by the application of BR and JA. The overall activity of antioxidant enzymes POD, CAT, GR, APX and CAT elevated during all the treatments, be it stress alone or in combination with BR and JA, compared to the control. However, we observed that the BR was much better in mitigating the drought stress compared to JA. Thus, the present study suggests that BR and JA supplementation improves the performance of B. rapa on exposure to drought stress, which hints at the critical role of BR and JA in improving crop productivity in drought-prone areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant growth and development is hampered by several abiotic stresses which are caused by a number of ecological influences (Ma et al. 2020). Drought stress is described as a combination of insufficient rainfall and low soil moisture. It is a major environmental obstacle that plants in arid and semi-arid ecosystems are often exposed to (Fang et al. 2015). Drought overweighs all other “crop production affecting factors” by hampering the plant growth at its all-developmental stages, thereby decreasing crop productivity and incurring substantial economic loss worldwide (Fang et al. 2015; Farooq et al. 2009). A recent report by Climate Change Science Program mentions that the drought is inevitable in many parts of the world since the average temperature is expected to increase by 1.8–4.0 °C in 2100 (IPCC 2018).
Phytohormones, besides being important in various physiological mechanisms, are also key components for stress tolerance in plants (Shahzad et al. 2018). In fact, exogenous application of hormones leads to the rapid, although transient alteration of genome-wide transcriptome in plants. In Arabidopsis, exogenous hormonal supply for less than 1 h led to the changed expression of about 10–300 genes by upregulation and downregulation of an equal number of genes (Goda et al. 2008). Therefore, it becomes important to investigate the relationship between plant cell physiology and phytohormones, as well as the role of plant hormones in modulation of osmoprotectants under drought stress. Among phytohormones, brassinosteroid (BR) isolated from Brassica pollens have been known to regulate the temperature, salt, water, pesticide and other environmental cues in many plants (Shahzad et al. 2018) by regulating several osmolytes (Parvez et al. 2020). Although under stress conditions many biological processes in plants are controlled by BR signalling, BRL3 (BRI1-LIKE receptor) is believed to control the biogenesis of important osmolytes (Fàbregas et al. 2018). Jasmonates (JAs) represent another class of important phytohormones having important role under abiotic stress conditions. Under a number of stressful conditions, JA controls gene regulation, secondary metabolism and protein biosynthesis (Ahmad et al. 2018). Its increased endogenous levels indicate its significance in abiotic stress tolerance, while exogenous supplementation significantly improves plant growth in response to various environmental cues. JA has been shown in various studies to increase the production of non-enzymatic antioxidants including proline (Kaya et al. 2019; Shan et al. 2015).
Osmoprotectants are low molecular weight molecules that protect the membranes/proteins from osmotic shock and maintain the homeostatic osmotic balance in plant cells under drought stress (McNeil et al. 1999). Various abiotic stresses including drought stress induce the formation and buildup of several osmolytes such as sugars, glycine betaine (GB), proline (Pro) and proteins/free amino acids, which increase the cellular osmotic potential (Ramazan et al. 2021; Ozturk et al. 2020). Since Pro acts as a buffer to resolve lower water capacity, its accumulation serves as a significant indicator of diverse stress response mechanisms, particularly in plants grown under drought-stressed conditions (Jaleel et al. 2007). Drought causes plants to produce reactive oxygen species (ROS) namely, O2–, 1O2 and H2O2, injuring living tissues/macromolecules (DNA, lipids, proteins and carbohydrates), thereby leading to trapid induced programmed cell death (PCD) (McNeil et al. 1999). Plants, on the other hand, have evolved complex antioxidant defence system such as superoxide dismutase (SOD) and enzymes of the Halliwell–Asada pathway to quench deleterious ROS (Alam et al. 2019). Importantly, regulating enzymatic activity apparently seems to be the key process in plant resistance to environmental perturbations (Noctor and Foyer 1998).
Growth, development, yield and crop quality of Brassica crops in arid, semi-arid and Mediterranean regions get severely affected by drought stress (Zhang et al. 2014). In terms of abiotic stress responses in Brassica crops, Chinese cabbage (B. rapa) is the most studied species, with genome-wide analyses indicating that different plant hormones play role in different drought stress responses. However, systemic profiling of different phytohormones has yet to be investigated (Paul et al. 2016; Saha et al. 2016). We believe that in Brassica crops, certain plant hormones surely play their part in the drought tolerance. On these lines, our research offers an analysis of BR and jasmonic acid in two varieties of Brassica rapa. The outcome of hormonal profiles was explained in relation to morphological, physiological and biomolecular parameters.
Materials and methods
Plant material, growth conditions and hormonal treatments
Seeds for two genotypes of Brassica rapa used in this investigation, viz. KS-101 and Shalimar Sarson-3 (KBS3) were obtained from Sher-i-Kashmir University of Agricultural Science and Technology, Srinagar. Earthen pots with autoclaved soil, sand and vermiculite (2:1:1 w/w/w) were used for seed germination and kept under controlled conditions of the plant growth chamber (Blue Star-NKL-750) set at 25 °C ± 1 °C, light intensity of 200 mol photons m−2 s−1and 60–80% of relative humidity. Plants from each variety were distributed into the following five sets—(1) set of control plants with all optimum environmental conditions, (2) set of drought-stressed plants, (3) set of drought-stressed plants with exogenously applied BR, (4) set of drought-stressed plants with exogenously applied JA and (5) set of drought-stressed plants with exogenously applied BR (0.01 µM) as well as JA (10 µM). The 21-day-old seedlings of B. rapa were exposed to water deficit stress. Plants were exogenously supplied with a foliar spray of BR and JA during the drought period. Plant tissue was harvested after 10 days of drought stress for several physio-biochemical as well as antioxidant enzyme assays. Plants collected at the end of drought stress were either used afresh or stored for further analysis at − 80 °C. For biochemicals and antioxidant enzymes, the homogenate was extracted and stored at − 80 °C.
Estimation of morphological parameters
At the end of the drought period, morphological parameters such as plant height, shoot length, root length, petiole length, leaf length and leaf breadth were measured. These values were calculated using a scale and expressed in centimetres.
Estimation of pigments
Chlorophyll and carotenoid content estimation was done by Holden’s (1961) method using UV–Vis spectrophotometer (Shimadzu-1800). Anthocyanin estimation was done by using the method of Nakata and Ohme-Takagi (2014).
Estimation of proline and glycine betaine
Estimation of Pro and GB content was done by following the protocol of Bates et al. (1973) and Grieve et al. (1983), respectively.
Estimation of total phenolic content
The protocol of Apak et al. (2008) was used to estimate the phenolic content in the plant tissue. Leaf tissue (50 mg) was homogenised in 80% acetone and centrifuged for 10 min at 10,000g. 1 mL Folin–Ciocalteau’s reagent and 2 mL distilled water were applied to 100 µL of the supernatant and vigorously shaken. 20% sodium carbonate was added to this mixture, and the volume was increased with distilled water to 10 mL. At 750 nm, the absorbance was measured using UV–Vis spectrophotometer (Shimadzu, Japan).
Estimation of total soluble sugar
The total soluble sugar was calculated as in Dey (1990). A UV–Vis spectrophotometer was used to read the absorbance at 485 nm (Shimadzu-1800). Sugar concentration was measured using a standard curve.
Estimation of malondialdehyde
MDA was estimated by following the method of Hodges et al. (1999). OD was measured at 532 nm and 600 nm using UV–Vis spectrophotometer (Shimadzu-1800). Histochemical approach was also followed to analyse lipid peroxidation qualitatively through Schiff’s reagent as in Awasthi et al. (2018).
Estimation of relative water content
FW (fresh weight), TW (turgid weight) and DW (dry weight) of leaves were calculated to estimate relative water content (RWC). The RWC was then calculated by the equation:
RWC = [FW − DW]/[TW − DW] × 100.
Estimation of photosynthetic parameters
To calculate the different photosynthetic parameters, an infra-red gas analyser (IRGA) (LICOR-6400XT) was used. At the end of the drought period, from 9 am to 11 am, various photosynthetic parameters were measured, including photosynthetic rate, water usage efficiency, stomatal conductance and transpiration rate.
DAB assay
Histochemical detection of hydrogen peroxide (H2O2) was done by following the protocol of Kumaret al. (2014). 3, 3’-Diaminobenzidine (DAB) was used as the chromogenic substrate which gets oxidised by H2O2 and producs a reddish brown precipitate.
Estimation of total soluble protein and antioxidant enzyme activity
Leaf tissue was crushed in 100 mM and 7.8 pH sodium phosphate buffer. After centrifugation for 20 min at 10,000 rpm at 4 °C, OD was measured from the supernatant at 260 nm and 280 nm for protein estimation by using UV–Vis spectrophotometer (Shimadzu-1800) (Chen et al. 2015). The remaining supernatant was utilised for estimating the enzyme activities of SOD, CAT, POD, GR and APX.
Estimation of superoxide dismutase (SOD) activity
The activity of SOD was measured using the Zhang et al. (2005) protocol. The reaction mixture contained 0.05 M phosphate buffer (pH 7.8), 750 µmol/L NBT, 20 µmol/L riboflavin, 130 mM methionine, 100 mol/L EDTA-Na2 and 0.05 mL of enzyme extract. The reaction mixture tubes were exposed to twin 15 W fluorescent lamps for 50 s, while the control set was kept in the dark for the same time. 560 nm wavelength was used to check the optical density using a UV–Vis spectrophotometer (Shimadzu-1800) (Simonian et al. 2006).
Estimation of peroxidase (POD) activity
POD activity was accessed by following the protocol of Chen and Zhang (2016). The reaction mixture comprised 100 mM phosphate buffer, 0.2% guaiacol, 30% H2O2 and 0.05 ml of enzyme extract. To calculate POD activity, the absorbance was read at 470 nm after every 15 s for 1 min by using a UV–Vis spectrophotometer (Shimadzu-1800).
Estimation of catalase (CAT) activity
The protocol of Zhang et al. (2005) was used to calculate the CAT activity. 2.25 mL sodium phosphate buffer (pH 7.8), 1.5 mL deionised water, 0.45 mL 0.1 M H2O2 and 0.3 mL enzyme extract made up the reaction mixture. The decline in optical density at 240 nm was used to estimate the CAT activity.
Estimation of glutathione reductase (GR) activity
To assess the GR activity, Halliwell and Foyer (1978) was followed. 0.5 mM glutathione (oxidised), 0.15 mM NADPH, 3 mM MgCl2 in 50 mM Tris (pH 7.5) and 0.06 mL enzyme extract were included in the reaction mixture. The oxidation of NADPH by glutathione at 340 nm was then monitored using a UV–Vis spectrophotometer to evaluate GR activity (Shimadzu-1800).
Estimation of ascorbate peroxidase (APX) activity
The activity of APX was estimated by following the method of Harb et al. (2015). The reaction mixture with pH 7.0 potassium phosphate buffer (25 mM), 0.1 mM EDTA, 0.25 mM ascorbate and H2O2 of 1 mM was mixed with 0.04 mL of enzyme extract. Enzyme activity was measured by observing the decline in absorbance at 290 nm.
Statistical analysis
With three replicates, the experiments were carried out in a fully randomised design. SPSS software (Version 21) was used to analyse the data. Data shown are the means (sum of three replicates) and standard deviations. Vertical bars signify standard deviation and, on the top, different letters represent significant difference among the means according to Tukey’s test (P < 0.05). Correlation between variables, hierarchical cluster analysis (HCA) and principal component analysis (PCA) were conducted in R version 4.0.3 (R Core Team 2020).
Results
Effect of drought on morphological characteristics
Drought stress affected both elongation and expansion growth in both the cultivars of B. rapa. KJD and BJDB displayed up to 63% and 59% increase in overall plant height compared to the control plants (KC and BC), while in KD and BD it increased by 4% and 1.5% only when compared to the control. Root growth increased in the JA-supplemented drought-stressed plants (360% in KJD and 463% in BJDB) than the BR-supplied drought-stressed plants (54% in BDB and 1% in KDB). During drought stress, root length in both KD and BD increased up to 20% and 24% compared to the control KC and BC. Stem length was significantly reduced in JA- and BR-treated plants, but KJD and KJDB displayed reduction in stem length, while in case of KD and KDB stem length was increased by 18 and 5%. Petiole length was decreased during all the treatments in the KS101 and KBS3 genotypes, except in KD in which it was increased up to 8%. The leaf length and leaf breadth in both the cultivars were decreased during all the treatments when compared to control (see Fig. 1, Table 1).
The effect of drought stress with and without the combination of phytohormones. (a) Control plants (KC), (b) drought plants without the exogenous application of phytohormones (KD), (c) drought plants with brassinosteroid treatment (KDB), (d) drought plants with JA treatment (KJD), (e) drought plants with exogenously supplied Br and JA treatment (KJDB), (f) control plants (BC), (g) plants during drought without phytohormone treatment (BD), (h) plants with drought and exogenously supplied brassinosteroid (BDB), (i) plant with drought and JA treatment (BJD), (j) plants during drought with exogenously supplied JA and Br (BJDB) (colour figure online)
Photosynthetic pigments
The total chlorophyll content increased during all the treatments except KD and BJD, where it declined by 15 and 24% compared to control plants (KC and BC). We observed an increase in the total chlorophyll content in both the BR- and JA-treated plants during drought as illustrated in Fig. 2a. The maximum chlorophyll content was observed in KDB and BDB which increased by 17 and 13%. Analysis of KJD, BJD, KJDB and BJDB revealed an increase of 5, 3, 8 and 5%, respectively, compared to the control plants.
Change in photosynthetic pigments in the KS101 and KBS3 genotypes: (a) total chlorophyll, (b) anthocyanin content, (c) carotenoid content. Data shows the mean ± SD of three or more replicates and vertical bars signify standard deviation and, on the top, different letters represent significant difference among the means according to Tukey’s test (P < 0.05). In legends, C represents KC and BC, D represents KDB and BDB, DB represents KDB and BDB, JD represents KJDB and BJDB, JDB represents KJDB and BJDB; this will be followed throughout the manuscript (colour figure online)
The carotenoid content increased significantly in all the treatments; however, the increase was more in KD and BD than the other treatments in comparison to the control (Fig. 2c).
Anthocyanin shows a significant increase of 28 and 19% in case of KDB and BDB. However, it increased only by 12 and 5% in KD and BD. Anthocyanin content was also increased to various extents in other treatments as depicted in Fig. 2b.
Photosynthetic parameters
Drought stress resulted in reduced photosynthesis in both cultivars. KD and BD displayed reduction in photosynthesis by 86 and 89%, while in KJDB and BJDB the minimal reduction of 21% was observed (Fig. 3a). Drought stress reduced the transpiration rate in both the genotypes. We observed significant decline in transpiration rate in KD and BD by 90 and 89%, respectively, when compared to KC and BC. However, transpiration rate increased by 31% in the BJDB genotype when treated with both BR and JA as compared to it control (Fig. 3b). Stomatal conductance was reduced in both KD and BD by 84 and 54%, respectively, but increased by 6 and 52% in BJD and BJDB (Fig. 3c). Water use efficiency was highest in KJDB and BDB (Fig. 3d).
Change in photosynthetic parameters in KS101 and KBS3 genotypes. (a) Photosynthetic rate. (b) Transpiration rate. (c) Stomatal conductance. (d) Water use efficiency. Data show the mean ± SD of three or more replicates and vertical bars signify standard deviation and, on the top, different letters represent significant difference among the means according to Tukey’s test (P < 0.05) (colour figure online)
Effect of drought stress on osmolytes
Drought stress led to the elevation of glycine betaine by 11 and 22% in KD and BD, respectively. Maximum levels of GB were observed in KDB (34%) and BDB (36%), indicating that BR induced glycine betaine formation in drought-stressed genotypes of B. rapa. Similarly, JA too led to the increase in glycine betaine concentration in both the genotypes (Fig. 4a).
Change in osmolytes and protein in KS101 and KBS3 genotypes. (a) Glycine betaine, (b) proline content, (c) sugar content. (d) Protein concentration. Data show the mean ± SD of three or more replicates and vertical bars signify standard deviation and, on the top, different letters represent significant difference among the means according to Tukey’s test (P < 0.05) (colour figure online)
The proline content increased significantly in all the treatments in comparison to control; however, the percentage increase was more in BR- and JA-treated plants than the simple drought-stressed plants (Fig. 4b).
Drought stress led to the increase in total soluble sugars; however, drought stress along with BR supplement led to maximum elevation, as we observed in KDB (32%), BDB (42%). The slight increase in the sugar content was found in KD and BD which increased by 23 and 22% in KC and BC. Total soluble sugars also increased in drought-stressed plants supplemented with JA, KJD, BJD, KJDB and BJDB (Fig. 4c).
Change in protein content in response to drought stress and exogenous supply of BR and JA
Protein content decreased significantly by 79 and 77% in KD and BD in response to drought in both the genotypes. However, in case of KDB and BDB only 12 and 7% decrease in protein content was observed. At the same time, decrease in the protein content in KJD, BJD, KJDB and BJDB was less than that of KD and BD (Fig. 4d).
Lipid peroxidation
All the treatments displayed a slight increase in the MDA content as compared to the control during the drought stress. We observed a highest increase in MDA content by 2% and 7% in KD and BD, while only slight increase of the MDA content by 0.01 and 0.3% was observed in KDB and KJD. The increase in other cases was also much less than that of KD and BD as can be clearly seen in Fig. 5a–J and Fig. 6a.
Change in different biochemical parameters in KS101 and KBS3 genotypes, (a) MDA content (b) relative water content, (c) phenolic content. Data show the mean ± SD of three or more replicates and vertical bars signify standard deviation and, on the top, different letters represent significant difference among the means according to Tukey’s test (P < 0.05) (colour figure online)
Relative water content
Relative water content changed in all the treatments as illustrated in Fig. 6b. In both genotypes of B. rapa, RWC reduced during the drought treatment compared to their control. RWC significantly decreased in both KD and BD by 58% and 52%; however, it increased by 23, 27, 66, 55, 40 and 86% in KJD, BJD, BJDB, KDB, BDB and KJDB respectively compared to KD and BD as depicted in Fig. 6b.
Change in phenolic content
Phenolic content showed a significant increase during all the treatments except in BJD where it decreased significantly by 65% compared to BC. The highest phenolic content was found in KJD (237%) and KDB (179%), followed by BJDB (112%). The phenolic content in both KD and BD elevated significantly by 75% (Fig. 6c).
Change in antioxidant enzyme activities
The overall activity of antioxidant enzymes POD, CAT and GR increased during all the treatments, be it stress alone or in combination with BR and JA, compared to the control. APX activity reduced in case of BJD by 4% and its highest activity was observed in KDB (42%) and BDB (43%). In the case of KD and BD, APX activity increased by 1 and 0.1%. The APX activity in the case of KJDB, KJD and BJDB also increased during drought stress as shown in Fig. 7a.
Change in the antioxidant enzyme activity in KS101 and KBS3 genotypes. (a) APX activity, (b) catalase activity, (c) SOD activity, (d) GR activity, (e) POD activity. Data show the mean ± SD of three or more replicates and vertical bars signify standard deviation and, on the top, different letters represent significant difference among the means according to Tukey’s test (P < 0.05) (colour figure online)
SOD activity increased significantly by 239- and 244-fold in KDB and BDB, while in the case of KD and BD its activity increased only by 102 and 70% when compared to the control. Its activity was also increased by 202 and 187% in KJDB and BJDB (Fig. 7c). Maximum CAT activity was found in KDB (210%) and BDB (264%), while its minimum activity was observed in KD (23%), BD (11%) and KJD (23%) (Fig. 7b). In case of KDB and BDB, GR activity was increased significantly by 271 and 312% and that of KJD and BJD was increased by 25 and 45% as compared to KC and BC (Fig. 7d). Increased POD activity by 384 and 323% during the drought was found in KDB and BDB and its enzymatic activity was only 0.7, 0.9 and 6% in KD, KJD and BJD (Fig. 7e).
ROS accumulation in leaves
We assessed the accumulation of ROS by allowing DAB–H2O2 reaction in leaves which resulted in the formation of localised brown pigment after the leaves were decolourised. Drought-treated plants had noticeably more brown pigmentation than control as well as BR- and JA-treated plants (Fig. 8a–j).
Statistical analysis
Hierarchical cluster analysis (HCA) and principal component analysis (PCA) have shown that the exogenous application of BR and JA results in strong correlations among the vectors such as SOD, POD, CAT, GR, APX, carotenoids, phenolics, GB, proline, sugar and total chlorophyll in KS101 genotype (Fig. 9). Further, in the KBS3 genenotype, a strong correlation was found among the vectors such as SOD, POD, CAT, GR, APX, GB, phenolics and sugar (Fig. 10). Further, cluster analysis has shown that the BR- and JA-applied plants form clusters which are close to control plants. However, the drought-stressed plants form a separate cluster distant from the other treatments (Figs. 9, 10).
Discussion
Drought stress was induced in two genotypes of B. rapa in 21-day-old seedling stage by withholding water supply which created water deficit in these seedlings. We observed decreased fresh and dry weights along with reduced RWC in leaves of drought-stressed plants. According to several research reports, the most common impacts of drought on plants is the decrease in plant biomass and RWC (Mahmood et al. 2012). Drought stress greatly influences plant–water relationship, resulting in osmotic stress in plants which finally results in reduction in growth and affects plant development (Xu et al. 2014). However, the research studies have revealed that this reduction in growth and osmotic stress under drought can be improved by the exogenous supplementation of hormones such as BR and JA. Plant height, leaf length, FW and DW declined considerably in both the KS101 and KBS3 genotypes under drought stress conditions during the present study. Further, it was observed that the exogenous application of JA and BR counters the drought stress significantly. In addition, it was found that the exogenous supplementation of drought-stressed plants with the two hormones was effective in improving the RWC of plants (Fig. 6b). Several other studies have also revealed that BR and JA are effective agents in restoring the water content in plants during water shortage (Alam et al. 2014). The drought stress has also resulted in increased growth of roots than the control plants. This root length was further increased with the exogenous application of JA and BR. However, the increased root length was more significantly increased by JA than the BR treatment. Crops grown in drought stress require a large root system to absorb more soil water and alleviate drought stress (Ehdaie et al. 2012), because JA transitory buildup is required for abscisic acid-regulated increase in root length during drought stress (Ollas et al. 2013). Higher root dispersion at depth in the subsurface layers is thought to be a potential characteristic for plant adaptation to water stress, enhancing the subsoil profile’s water extraction capability for grain filling and enhanced grain production (Ehdaie et al. 2012).
The abiotic stress environments lead to overproduction of ROS resulting in oxidative stress. The DAB assay showed that the drought stress leads to production of H2O2 which was reduced by the application of BR and JA. MDA, an indicator of oxidative stress and membrane impairment in plants, is formed when unsaturated fatty acids in the phospholipid layer of membranes experience peroxidation owing to increased production of ROS inside cells (Martinez et al. 2018). The increased MDA content under drought stress compared to control genotypes (KC and BC) indicates membrane lipid peroxidation and, thus, membrane integrity is lost. Various studies have shown that the exogenous supplementation of BR and JA decreases the levels of MDA due to production of various osmolytes which scavenge the ROS and prevent peroxidation of lipids (Mir et al. 2018). The histochemical analysis of roots also revealed that the MDA levels were decreased in the BR- and JA-applied plants (Fig. 5a–j).
Drought stress leads to decrease in photosynthetic pigments such as chlorophyll, carotenoid and anthocyanins in different crop plants. Furthermore, it negatively impacts the photosynthesis due to ROS-induced oxidation of photosynthetic pigments, impaired biosynthesis of pigments, destruction of chlorophyll structure and stomatal closure (Banks and botany 2018). Our study revealed that under drought stress conditions, the chlorophyll content reduces to a large degree than in the control plants in both KS101 and KBS3 genotypes of B. rapa (Fig. 2a). Reduced activity of the enzymes—proto-chlorophyllide reductase and aminolevulinic acid dehydratase (ALA-dehydratase)—both important components in chlorophyll biogenesis, could explain the decrease in chlorophyll material (Padmaja et al. 1990). A similar trend was observed for carotenoids which decreased under drought stress compared to the control set of both the genotypes of B. rapa. Supplementation of both BR and JA reduced the reduction in the pigment concentration due to drought stress irrespective of the genotype. However, the positive impact of BR was more as compared to JA, showing that BR has a more dominant role in drought stress compared to JA. Both the hormones have indeed significant positive impact on photosynthetic pigments (Mahmood et al. 2012). Carotenoids are known to prevent chlorophyll molecules from photo-oxidative damage (Prasad et al. 2005). Janeczko et al. (2007) revealed that BR application results in augmented carotenoid content when applied to drought-stressed plants. The anthocyanin contents showed a significant increase under water deficit conditions than the control in both KS101 and KBS3 genotypes of B. rapa. Further, it was observed that the exogenous application of BR and JA increased the anthocyanin content in both the genotypes. However, it was found the BR treatment was more effective in improving the anthocyanin content in both the genotypes (Fig. 2b). Previous studies also show that exogenous supply of BRs upregulates the anthocyanin content by increasing the expression of BR genes (Luan et al. 2013). The photosynthetic investigation analysed by IRGA revealed that the rate of photosynthesis, water use efficiency, conductance of stomata and the rate of transpiration reduced considerably due to drought stress. It has been found that drought conditions affect photosynthesis and growth by disturbing the water exchange properties and leaf gas exchange attributes (Farooq et al. 2010). Nevertheless, the exogenous supply of BR and JA improves these attributes significantly (Fig. 3) and this corroborates with the research study by (Behnamnia et al. 2009) which showed that BR and JA had positive impact under drought stress in normalising the water use efficiency, photosynthetic rate, stomatal conductance and transpiration rate. By stabilising membrane structures under stressful conditions, BRs improve photosynthetic rate and carbon absorption in PSII antennae (Farooq et al. 2010), as well as increase efficiency in light utilisation and dissipation of excessive excitation energy (Hu et al. 2013).
Plants amass diverse non-toxic compounds such as sugars, proline, GB, phenolics and other compounds that help them withstand osmotic stress (Jan et al. 2018). Many studies reveal that proline content upsurges by several folds in response to drought stress, not only for osmotic equilibrium but also for free radical scavenging, cellular structure stability and redox potential (Alam et al. 2013). Further it was found that the exogenous application of JA and BR augmented the proline levels in all treatments in both the genotypes (4A). In our study, the concentration of GB increased significantly in reaction to water deficit. Further, in response to exogenous supplementation of BR and JA, the GB increased to a larger extent even more than the plants facing drought. However, the increase in GB was higher in BR-applied plants than the JA-applied ones. GB has been found to provide plants with abiotic stress tolerance by activating LOX (lipid peroxidase) and FAD (fatty acid desaturase), which preserve the stability of membrane or mediate the regulation of various genes (Karabudak et al. 2014). Sugars are main molecules present in the cells under drought stress and uphold osmotic balance in plants (Fariduddin et al. 2013). The present study observed that, although the water deficit elevated the levels of soluble sugars in both the genotypes, exogenous application of BR and JA further increased the soluble sugar content to larger extent (Fig. 4c). However, the increase in sugars was found more in BR-applied plants than the JA-applied ones, similar to proline and GB. The PCA and HCA analysis has shown that strong correlation exists among the different osmolytes under DS, and the application of BR and JA further strengthens this correlation. In this study, BR and JA stimulated the synthesis of phenolics, which are important for DS tolerance in several plants (Anuradha et al. 2007; Wahid and Ghazanfar 2006; Wahid 2007). However, the increase was more in JA-applied plants. Showing the role of JA in upregulating phenolics under DS owing to increased PAL (phenylalanine ammonia lyase) activity (Fig. 6c) (Yoshida et al. 2020). The phenolics have phenol ring which plays significant role in the stabilisation of membrane during drought stress and creates a network of ROS scavenging in plants (Farooq et al. 2009).
Exogenous supplementation of BR and JA has been shown in several studies to upregulate the levels of antioxidant enzymes in drought-stressed plants. SOD is one of the first antioxidant enzymes against oxidative stress, converting O2− to O2 and H2O2 (Cruz de Carvalho 2008), which is then decomposed in the cytosol and peroxisomes by APX and CAT, respectively (Zhao et al. 2018). Under DS, the exogenous supplementation of BR and JA results in strong correlations among antioxidant enzymes such as SOD, POD, CAT, GR, and APX (Fig. 7). Several studies have revealed that BRs and JA can activate the transcription of various genes coding for antioxidant enzymes and increase the activity of key enzymes of the antioxidant pathway, i.e. SOD, APX, POD, GR and CAT (Alam et al. 2014; Vardhini et al. 2010).
Conclusion
Plants are vulnerable to drought stress, and significant yield reductions have been documented in recent decades; therefore, understanding the many underlying drought response mechanisms in plants is critical. Exogenous treatment of BR and JA, either separately or together, considerably reduced the effect of drought stress by increasing the photosynthetic rate, photosynthetic pigments, stomatal conductance, transpiration rate and antioxidant defence. The application of BR and JA resulted in a rise in the concentration of various osmolytes and antioxidants such as sugars, glycine betaine (GB), proline (Pro), proteins/free amino acids and SOD, APX, POD, GR and CAT, which increase the cellular osmotic potential, ROS scavenging and a considerable reduction in membrane damage. We conclude that the exogenous supply of BR and JA has significant role in alleviating drought stress in KS101 and KBS3 genotypes of B. rapa. However, BR has more effective role in imparting drought tolerance to both the genotypes of B. rapa compared to JA.
Abbreviations
- KC/KS101C:
-
KS-101control
- KD/KS101D:
-
Drought-treated KS-101 genotype
- KDB/KS101DB:
-
Drought-treated KS-101genotype with exogenously supplied BR
- KJD/KS101JD:
-
Drought-treated KS-101genotype with exogenously supplied JA
- KJDB/KS101JDB:
-
Drought-treated KS-101 genotype with exogenously supplied JA and BR
- BC/KBS3C:
-
KBS-3 control
- BD/KBS3D:
-
Drought-treated KBS-3 genotype
- BDB/KBS3DB:
-
Drought-treated KBS-3 genotype with exogenously supplied BR
- BJD/KBS3JD:
-
Drought-treated KBS-3 genotype with exogenously supplied JA
- BJDB/KBS3JDB:
-
Drought-treated KBS-3 genotype with exogenously supplied JA and BR
References
Ahmad P, Abass Ahanger M, Nasser Alyemeni M, Wijaya L, Alam P, Ashraf M (2018) Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. J Plant Interact 13:64–72
Alam MM, Hasanuzzaman M, Nahar K, Fujita M (2013) Exogenous salicylic acid ameliorates short-term drought stress in mustard (Brassica juncea L.) seedlings by up-regulating the antioxidant defense and glyoxalase system. Aust J Crop Sci 7:1053
Alam MM, Nahar K, Hasanuzzaman M, Fujita M (2014) Exogenous jasmonic acid modulates the physiology, antioxidant defense and glyoxalase systems in imparting drought stress tolerance in different Brassica species. Plant Biotechnol Rep 8:279–293
Alam P, Albalawi TH, Altalayan FH, Bakht MA, Ahanger MA, Raja V, Ashraf M, Ahmad P (2019) 24-Epibrassinolide (EBR) confers tolerance against NaCl stress in soybean plants by up-regulating antioxidant system, ascorbate-glutathione cycle, and glyoxalase system. Biomolecules 9:640
Anuradha S, Rao S (2007) The effect of brassinosteroids on radish (Raphanus sativus L.) seedlings growing under cadmium stress. Plant Soil Environ 53:465
Apak R, Güclü K, Özyürek M, Celik SE (2008) Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Mikrochim Acta 160:413–419
Awasthi JP, Saha B, Chowardhara B, Devi SS, Borgohain P, Panda SK (2018) Qualitative analysis of lipid peroxidation in plants under multiple stress through Schiff’s reagent: a histochemical approach. Bio-Protocol 8:e2807
Banks JM (2018) Chlorophyll fluorescence as a tool to identify drought stress in Acer genotypes. Environ Exp Bot 155:118–127
Bates LS, Waldren RP, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Behnamnia M, Kalantari KM, Ziaie J (2009) The effects of brassinosteroid on the induction of biochemical changes in Lycopersicon esculentum under drought stress. Turk J Bot 33:417–428
Chen T, Zhang B (2016) Measurements of proline and malondialdehyde contents and antioxidant enzyme activities in leaves of drought stressed cotton. Bio-Protoc 6:e1913–e1913
Chen T, Li W, Hu X, Guo J, Liu A, Zhang B (2015) A cotton MYB transcription factor, GbMYB5, is positively involved in plant adaptive response to drought stress. Plant Cell Physio 56:917–929
Cruz de Carvalho MH (2008) Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signal 3:156–165
De Ollas C, Hernando B, Arbona V, Gómez-Cadenas A (2013) Jasmonic acid transient accumulation is needed for abscisic acid increase in citrus roots under drought stress conditions. Physiol Plan 147:296–306
Dey P (1990) Oligosaccharides. In: Dey PM (Ed) Methods in plant biochemistry. Carbohydrates, vol 2. New York, Academic Press, pp 189–218
Ehdaie B, Layne AP, Waines JG (2012) Root system plasticity to drought influences grain yield in bread wheat. Euphytica 186:219–232
Fàbregas N, Lozano-Elena F, Blasco-Escámez D, Tohge T, Martínez-Andújar C, Albacete A, Osorio S, Bustamante M, Riechmann JL, Nomura T (2018) Overexpression of the vascular brassinosteroid receptor BRL3 confers drought resistance without penalizing plant growth. Nat Commun 9:1–13
Fang Y, Xiong L (2015) General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol Life Sci 72:673–689
Fariduddin Q, Khalil RR, Mir BA, Yusuf M, Ahmad A (2013) 24-Epibrassinolide regulates photosynthesis, antioxidant enzyme activities and proline content of Cucumis sativus under salt and/or copper stress. Environ Monit Assess 185:7845–7856
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra S (2009) Plant drought stress: effects, mechanisms and management. Int J Sustain 29:185–212
Farooq M, Wahid A, Lee DJ, Cheema S, Aziz T (2010) Drought stress: comparative time course action of the foliar applied glycinebetaine, salicylic acid, nitrous oxide, brassinosteroids and spermine in improving drought resistance of rice. J Agron Crop Sci 196:336–345
Goda H, Sasaki E, Akiyama K, Maruyama-Nakashita A, Nakabayashi K, Li W, Ogawa M, Yamauchi Y, Preston J, Aoki K (2008) The AtGenExpress hormone and chemical treatment data set: experimental design, data evaluation, model data analysis and data access. Plant J 55:526–542
Grieve C, Grattan S (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307
Halliwell B, Foyer C (1978) Properties and physiological function of a glutathione reductase purified from spinach leaves by affinity chromatography. Planta 139:9–17
Harb A, Awad D, Samarah N (2015) Gene expression and activity of antioxidant enzymes in barley (Hordeum vulgare L.) under controlled severe drought. J Plant Interact 10:109–116
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Holden M (1961) The breakdown of chlorophyll by chlorophyllase. Biochem J 2(78):359
Hu W-h, Yan X-h, Xiao Y-a, Zeng J-j, Qi H-j, Ogweno JO (2013) 24-Epibrassinosteroid alleviate drought-induced inhibition of photosynthesis in Capsicum annuum. Sci Hortic 150:232–237
IPCC (2018) Summary for Policymakers. In: Masson-Delmotte V, Zhai P, Pörtner H-O, Roberts D, Skea J, Shukla PR, Pirani A, Moufouma-Okia W, Péan C, Pidcock R, Connors S, Matthews JBR, Chen Y, Zhou X, Gomis MI, Lonnoy E, Maycock T, Tignor M, Waterfield T (eds) Global Warming of 1.5 °C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty
Jaleel CA, Gopi R, Sankar B, Manivannan P, Kishorekumar A, Sridharan R, Panneerselvam R (2007) Studies on germination, seedling vigour, lipid peroxidation and proline metabolism in Catharanthus roseus seedlings under salt stress. Colloids Surf B 73:190–195
Jan N, Majeed U, Andrabi KI, John R (2018) Cold stress modulates osmolytes and antioxidant system in Calendula officinalis. Acta Physiol Plant 40:1–16
Janeczko A, Gullner G, Skoczowski A, Dubert F, Barna B (2007) Effects of brassinosteroid infiltration prior to cold treatment on ion leakage and pigment contents in rape leaves. Biol Plant 51:355–358
Karabudak T, Bor M, Özdemir F, Türkan İ (2014) Glycine betaine protects tomato (Solanum lycopersicum) plants at low temperature by inducing fatty acid desaturase7 and lipoxygenase gene expression. Mol Biol Rep 41:1401–1410
Kaya C, Ashraf M, Wijaya L, Ahmad P (2019) The putative role of endogenous nitric oxide in brassinosteroid-induced antioxidant defence system in pepper (Capsicum annuum L.) plants under water stress. Physiol Biochem 143:119–128
Kumar D, Yusuf MA, Singh P, Sardar M, Sarin NB (2014) Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio-protocol 4:e1108–e1108
Luan L-Y, Zhang Z-W, Xi Z-M, Huo S-S, Ma LN (2013) Brassinosteroids regulate anthocyanin biosynthesis in the ripening of grape berries. South African J Enol Vitic 34:196–203
Ma X, Su Z, Ma HJ (2020) Molecular genetic analyses of abiotic stress responses during plant reproductive development. J Exp Bot 71:2870–2885
Mahmood M, Bidabadi SS, Ghobadi C, Gray D (2012) Effect of methyl jasmonate treatments on alleviation of polyethylene glycol-mediated water stress in banana (Musa acuminata cv. ‘Berangan’, AAA) shoot tip cultures. Plant Growth Regul 68:161–169
Martinez V, Nieves-Cordones M, Lopez-Delacalle M, Rodenas R, Mestre TC, Garcia-Sanchez F, Rubio F, Nortes PA, Mittler R, Rivero RM (2018) Tolerance to stress combination in tomato plants: new insights in the protective role of melatonin. Molecules 23:535
McNeil SD, Nuccio ML, Hanson AD (1999) Betaines and related osmoprotectants. Targets for metabolic engineering of stress resistance. Plant Physiol 120:945–949
Mir MA, John R, Alyemeni MN, Alam P, Ahmad P (2018) Jasmonic acid ameliorates alkaline stress by improving growth performance, ascorbate glutathione cycle and glyoxylase system in maize seedlings. Sci Rep 8:1–13
Nakata M, Ohme-Takagi M (2014) Quantification of anthocyanin content. Bioprotocol 4:1098
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Ozturk M, Turkyilmaz Unal B, García-Caparrós P, Khursheed A, Gul A, Hasanuzzaman M (2020) Osmoregulation and its actions during the drought stress in plants. Physiol Plant 172:1321–1335
Padmaja K, Prasad D, Prasad A (1990) Inhibition of chlorophyll synthesis in Phaseolus vulgaris L. seedlings by cadmium acetate. Photosynthetica 24:399–405
Parvez S, Abbas G, Shahid M, Amjad M, Hussain M, Asad SA, Imran M, Naeem MA (2020) Effect of salinity on physiological, biochemical and photostabilizing attributes of two genotypes of quinoa (Chenopodium quinoa Willd.) exposed to arsenic stress. Ecotoxicol Environ Saf 187:109814
Paul A, Rao S, Mathur S (2016) The α-crystallin domain containing genes: identification, phylogeny and expression profiling in abiotic stress, phytohormone response and development in tomato (Solanum lycopersicum). Front 7:426
Prasad SM, Kumar D, Zeeshan M (2005) Growth, photosynthesis, active oxygen species and antioxidants responses of paddy field cyanobacterium Plectonema boryanum to endosulfan stress. J Gen Appl Microbiol 51:115–123
Ramazan S, Qazi HA, Dar ZA and John R (2021) Low temperature elicits differential biochemical and antioxidant responses in maize (Zea mays) genotypes with different susceptibility to low temperature stress. Physio Mol Biol Plants 27:1395–1412
Saha G, Park J-I, Kayum M, Nou I (2016) A genome-wide analysis reveals stress and hormone responsive patterns of TIFY family genes in Brassica rapa. Front 7:936
Shahzad B, Tanveer M, Che Z, Rehman A, Cheema SA, Sharma A, Song H, ur Rehman S, Zhaorong D (2018) Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: a review. Ecotoxicol Environ Saf 147:935–944
Shan C, Zhou Y, Liu M (2015) Nitric oxide participates in the regulation of the ascorbate-glutathione cycle by exogenous jasmonic acid in the leaves of wheat seedlings under drought stress. Protoplasma 252:1397–1405
Simonian MH, Smith JA (2006) Spectrophotometric and colorimetric determination of protein concentration. Curr Protoc Mol Biol 76:10–11
Team RC (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Vardhini BV, Anuradha S, Sujatha E, Rao S (2010) Role of brassinosteroids in alleviating various abiotic and biotic stresses—a review. Plant Stress 4:55–61
Wahid A, Ghazanfar A (2006) Possible involvement of some secondary metabolites in salt tolerance of sugarcane. J Plant Physiol 163:723–730
Wahid A (2007) Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. J Plant Res 120:219–228
Xu Y, Hu W, Liu J, Zhang J, Jia C, Miao H, Xu B, Jin Z (2014) A banana aquaporin gene, MaPIP1; 1, is involved in tolerance to drought and salt stresses. BMC Plant Biol 14:1–14
Yoshida CHP, Pacheco AC, Lapaz AdM, Gorni PH, Vítolo HF, Bertoli SC (2020) Methyl jasmonate modulation reduces photosynthesis and induces synthesis of phenolic compounds in sweet potatoes subjected to drought. Bragantia 79:319–334
Zhang H, Jiang Y, He Z, Ma M (2005) Cadmium accumulation and oxidative burst in garlic (Allium sativum). J Plant Physiol 162:977–984
Zhang XK, Lu GY, Long WH, Zou XL, Li F, Nishio T (2014) Recent progress in drought and salt tolerance studies in Brassica crops. Breed Sci 64:60–73
Zhao G, Zhao Y, Yu X, Kiprotich F, Han H, Guan R, Wang R, Shen W (2018) Nitric oxide is required for melatonin-enhanced tolerance against salinity stress in rapeseed (Brassica napus L.) seedlings. Int J Mol Sci 19:1912
Acknowledgements
Financial support in the form of Junior Research Fellowship to WA, NM and UM is acknowledged. RJ also received a research grant from CSIR (Council for Scientific and Industrial Research), India [Sanction No 38(1459)/18/EMR-II, dated 04/04/2018].
Author information
Authors and Affiliations
Contributions
WA, NM and UY did all the experiments, analysis of data and manuscript writing. RJ conceived the idea, supervised the work and finalised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Manzer H. Siddiqui.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmad Lone, W., Majeed, N., Yaqoob, U. et al. Exogenous brassinosteroid and jasmonic acid improve drought tolerance in Brassica rapa L. genotypes by modulating osmolytes, antioxidants and photosynthetic system. Plant Cell Rep 41, 603–617 (2022). https://doi.org/10.1007/s00299-021-02763-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-021-02763-9