Abstract
Key message
Banana MaBZR1/2 interact with MaMPK14 to enhance the transcriptional inhibition of cell wall modifying genes including MaEXP2, MaPL2 and MaXET5.
Abstract
Fruit ripening and softening, the major attributes to perishability in fleshy fruits, are modulated by various plant hormones and gene expression. Banana MaBZR1/2, the central transcription factors of brassinosteroid (BR) signaling, mediate fruit ripening through regulation of ethylene biosynthesis, but their possible roles in fruit softening as well as the underlying mechanisms remain to be determined. In this work, we found that MaBZR1/2 directly bound to and repressed the promoters of several cell wall modifying genes such as MaEXP2, MaPL2 and MaXET5, whose transcripts were elevated concomitant with fruit ripening. Moreover, yeast two-hybrid (Y2H) and bimolecular fluorescence complementation (BiFC) assays indicated that MaBZR1/2 physically interacted with a mitogen-activated protein kinase MaMPK14, and this interaction strengthened the MaBZR1/2-mediated transcriptional inhibitory abilities. Collectively, our study provides insight into the mechanism of MaBZR1/2 contributing to fruit ripening and softening, which may have potential for banana molecular improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brassinosteroids (BRs), a kind of plant unique steroid hormones known by their polyhydroxylated sterol structure, modulate a wide range of physiological events consisting of cell expansion, vasculature differentiation, photomorphogenic development, senescence and environmental stimuli (Wang et al. 2012). BRs are perceived at the plasma membrane by the BRI1 receptor, a leucine-rich receptor-like kinase (LRR-RLK) that can phosphorylate and dimerize with BAK1 kinase. Activated BRI1 or BAK1 subsequently modulates, presumably indirectly, the BIN2 kinase and/or BSU1 phosphatase activities, which in turn, control directly the phosphorylation level and nuclear localization of two critical transcription factors (TFs), namely BZR1 and BES1. Dephosphorylated BZR1 and BES1 bind to the promoters of numerous BR-responsive genes to modulate their transcription, thereby leading to BR response (Kim and Wang 2010).
BZR1 and BES1 are two plant-specific TFs and show high similarity at the protein level (Wang et al. 2002). Proteins in this family contain a well-conserved N-terminal domain called the BZR domain (He et al. 2005), which preferentially binds to E-box (CANNTG) and/or BRRE (CGTGT/CG) motifs present in the promoters of target genes. Extensive research has implicated that BZR TF family is involved in plant diverse physiological and biochemical processes. For example, Arabidopsis BZR1 and BES1 act as the primary transcriptional regulators through modulating a great deal of genes related to BR response (Sun et al. 2010; Yu et al. 2011). In wheat, TaBZR2 functions positively in manipulating drought response by facilitating the expression of TaGST1 (Cui et al. 2019). More recently, tomato BZR1 bound to the promoters of ATG2 and ATG6, thus resulting in up-regulated expression of ATGs and autophagosome formation (Wang et al. 2019). In addition, BZR TFs also have a significant role in fruit ripening. Overexpression of the Arabidopsis BZR1-1D in tomato fruit resulted in elevated carotenoid accumulation, soluble solid, and vitamin C contents, highlighting the importance of BZR in regulating secondary metabolism and fruit ripening (Liu et al. 2014). Our prior study indicated that MaBZR1/2 were involved in banana fruit ripening by binding to BRRE motifs in the promoters of ethylene biosynthetic genes such as MaACS1, MaACO13 and MaACO14 (Guo et al. 2019). However, BZR TFs and their regulatory network controlling fruit ripening remain poorly understood and, thus, need further investigation.
Mitogen-activated protein kinase (MAPK) cascades are critical signal transduction pathways to translate extracellular stresses into intercellular responses in most organisms (Rodriguez et al. 2010; Moustafa et al. 2014). A MAPK cascade mainly comprises three sequentially phosphorylated protein kinases including MAPKKKs, MAPKKs and MAPKs, which are linked in various ways to upstream receptors and downstream targets (Jonak et al. 2002). A growing body of evidence indicates that MAPKs have a role in plant growth and development, as well as biotic and abiotic stresses. For example, cold-activated MPK3 and MPK6 were able to phosphorylate ICE1 and cause its degradation, thus negatively controlling the cold response in Arabidopsis (Zhao et al. 2017). In rice, OsMKK10-2–OsMPK6 cascade plays an important role in salicylic-acid (SA)-signaling-dependent pathogen defence via phosphorylation of WRKY45 (Ueno et al. 2015). More recently, MaMPK2 interacts with and phosphorylates MabZIP93 to strengthen MabZIP93-mediated activation of cell wall modifying genes during banana ripening, suggesting the involvement of MAPKs in fruit ripening (Wu et al. 2019). Albeit large amount of information on the various roles that MAPKs implicate, few substrate proteins have been identified so far (Dóczi and Bögre 2018). Therefore, identifying potential MAPK interacting proteins and characterizing the biological functions of this interaction are of great significance for understanding the MAPK signaling cascades.
Banana ranks as the fourth most important global food commodity, after rice, wheat and maize. The fruit not only serves as a primary source of carbohydrates for many millions people in the world, but also provides a staple food and economic resource in many developing countries. As a typical climacteric fruit, banana is commonly harvested at the mature-green stage when the angularity on fruit surface disappears, and fruit ripening is artificially initiated with exogenous ethylene prior to commercialization. Postharvest ripening is essential for the quality formation of the fruit. However, excessive ripening causes undesirable postharvest deterioration, which limits storability, transportability, and shelf life (Costa et al. 2010). Generally, about 35–40% of the banana is lost due to improper ripening (Mohapatra et al. 2010). Textural change is a constrained factor affecting the shelf life of banana fruit, which is at least in part, due to the degradation of cell wall polysaccharides (Li et al. 2010). Genes encoding cell wall modifying enzymes or proteins, such as pectin lyases (PL), xyloglucan endotransglycosylase/hydrolases (XTH), polygalacturonases (PG), pectin methylesterases (PME), celluloses, and expansins (EXP) are involved in this process (Tucker et al. 2017). How these cell wall modifying genes are regulated during fruit ripening is poorly understood and a better understanding of the molecular basis of banana ripening and softening is indispensable for extending the shelf life and improving the postharvest quality of the fruit. Although MaBZR1/2 were found to mediate banana ripening via the direct control of ethylene biosynthetic genes (Guo et al. 2019), whether MaBZR1/2 control other ripening-associated genes such as cell wall modifying genes remains largely unknown.
In this work, the transcript levels of several cell wall modifying genes during banana ripening were studied. Moreover, the interaction of MaBZR1/2 with the promoters of these cell wall modifying genes was determined. Importantly, MaMPK14 was identified to physically interact with MaBZR1/2. It was found that the interaction between MaBZR1/2 and MaMPK14 influenced MaBZR1/2-mediated transcriptional inhibitory capacities. Our results reveal the transcriptional controlling module mediated by MaBZR1/2 in banana ripening, which would provide valuable genetic resource for banana quality improvement.
Materials and methods
Plant materials and treatments
Commercially mature banana (Musa acuminata AAA group, cv. Cavendish) fruits were picked from a local farm in Guangzhou, China. The same fruit samples from our previous study (Guo et al. 2019), which consist of control (natural ripening), ethylene-stimulated ripening (100 μl l−1 ethylene for 18 h) and 1-methylcyclopropene (1-MCP)-retarded ripening (0.5 μl l−1 1-MCP for 18 h), as well as BR treatment (2 μM brassinolide for 30 min at about 0.1 MPa) were used in this work.
RNA isolation and gene expression quantification
Frozen banana pulp was powdered in the presence of liquid N2 using a mortar and pestle. Total RNA was isolated utilizing the hot borate method (Wan and Wilkins 1994) and converted to cDNA using PrimerScript RT reagent kit (TaKaRa, Dalian division) following the manufacturer’s guidelines. Quantitative real-time PCR (RT-qPCR) reactions were performed as reported by Chen et al. (2011). Expression levels were calculated relative to MaRPS2 (ribosomal protein 2) using the 2−ΔΔCt method. All the primers used in this study are illustrated in Supplementary Table S1.
Electrophoretic mobility shift assay (EMSA)
The N terminus of MaBZR1 (N1-100 aa) and MaBZR2 (N1-100 aa) were inserted into pGEX-4T-1 (Amersham Biosciences) to fuse in-frame with the glutathione S-transferase (GST) tag and transformed into Escherichia coli strain Rosetta (DE3), respectively. The recombinant proteins were purified using the Protein Purification System (Clontech) according to the manufacturer’s protocols. The fragments of ~ 59 bp containing BRRE (CGTGT/CG) in the promoters of cell wall modifying genes were generated (Sangon Biotech) and biotin-labeled using Pierce™ Biotin 3′ End DNA Labeling Kit (Thermo Scientific). The EMSA was conducted using LightShift Chemiluminescent EMSA Kit (Pierce) according to the manufacturer’s directions.
Yeast two-hybrid screening and validation
For yeast two-hybrid screening, the Matchmaker Gold Yeast Two-Hybrid System (Clontech) was used based on the manufacturer’s recommendations. The coding region of MaBZR1 was cloned into pGBKT7 as bait, and the banana cDNA library was inserted into pGADT7-Rec vector as prey. For yeast two-hybrid validation, the coding regions of MaBZR1 and MaBZR2 were cloned into pGBKT7 as bait, while MaMPK14 was cloned into pGADT7 as prey. Bait and prey constructs were co-transformed into the yeast strain Y2HGold using a lithium acetate method, and possible interaction was quantified according to the method described in the handbook (Clontech). Interaction assays were repeated two times with equivalent results.
Bimolecular fluorescence complementation analysis
Plasmids containing N- and C-terminal YFP fusions were transiently co-expressed in tobacco (Nicotiana benthamiana) leaf epidermal cells as reported by Song et al. (2018). After infiltration, tobacco plants were kept in a greenhouse at 22 °C under a 16-h light period for about 2–3 days. YFP fluorescence was captured with a Zeiss fluorescence microscope.
Subcellular localization
The full-length MaMPK14 was inserted into the pEAQ-GFP vector (Sainsbury et al. 2009) with a C-terminal green fluorescent protein (GFP) tag. The constructed plasmid and the control GFP vector were transiently introduced in tobacco (Nicotiana benthamiana) through Agrobacterium tumefaciens infiltration. Plants were incubated under a 16-h light period at room temperature for at least 2–3 days, and the GFP fluorescence was observed using a Zeiss fluorescence microscope.
Dual-luciferase reporter assay
To investigate the transcriptional activities of MaBZR1, MaBZR2 and MaMPK14 on MaEXP2, MaPL2 and MaXET5 promoters, the full-lengths of MaBZR1, MaBZR2 and MaMPK14 were cloned into the pEAQ vector as effectors; while MaEXP2, MaPL2 and MaXET5 promoters were individually inserted into pGreenII 0800-LUC double-reporter vector as reporters. Single or combination of effector(s) as well as reporter plasmid was transiently transformed into tobacco leaves by Agrobacterium infiltration. LUC and REN luciferase activities were monitored using the Dual-Luciferase Assay reagents (Promega) in the Luminoskan Ascent Microplate Luminometer equipment (Thermo). The value of LUC/REN was analyzed for assessing the transcriptional abilities of MaBZR1/2 or interaction between MaBZR1/2 and MaMPK14 on MaEXP2, MaPL2 and MaXET5 promoters. Experiments included at least six replicates.
Results
Association of MaBZR1/2 with the promoters of several cell wall modifying genes
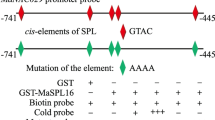
Our recent study indicated that MaBZR1/2 directly bound to and repressed the promoters of several ethylene biosynthetic genes through direct BRRE-motif interactions (Guo et al. 2019). Therefore, we assumed that MaBZR1/2 might control other ripening-associated genes such as cell wall modifying genes. To confirm this assumption, we analyzed the promoters of cell wall modifying genes from banana. As anticipated, several cell wall modifying genes such as MaEXP2 (Sane et al. 2007), MaPL2 (Pua et al. 2001) and MaXET5 (Mbéguié-A-Mbéguié et al. 2009) contain at least one BRRE motif in their promoters (Supplementary Text S1). We then conducted an electrophoretic mobility shift assay (EMSA) with the GST-MaBZR1/2 recombinant proteins to determine the in vitro binding of MaBZR1/2 to these regions. As shown in Fig. 1, shift-band was obviously observed when the probes containing the BRRE motifs derived from MaEXP2, MaPL2 and MaXET5 promoters were incubated with the GST-MaBZR1/2 recombinant proteins, respectively. Furthermore, the binding signals were reduced following the addition of increasing amount of unlabeled competitors with the same sequence, but not of the mutant probes carrying the mutated BRRE motifs (Fig. 1), supporting the direct binding of MaBZR1/2 with the MaEXP2, MaPL2 and MaXET5 promoters.
MaBZR1/2 bind directly to the promoters of MaEXP2, MaPL2 and MaXET5 in vitro. The probe sequences corresponding to each of the MaEXP2, MaPL2 and MaXET5 promoters are illustrated with underline representing the BRRE element and the mutant BRRE. The purified GST or recombinant GST-MaBZR1/2 fused proteins were incubated with probes, and the protein–DNA complexes were separated on native polyacrylamide gels. − or + represents absence or presence, respectively. ++ indicates increasing amounts of unlabeled wild-type or mutated probes for competition
To further decipher the regulatory function of MaBZR1/2 on these cell wall modifying genes, we utilized a dual-luciferase reporter assay system in Nicotiana benthamiana leaves. The constructive plasmids (MaEXP2pro::Luc, MaPL2pro::Luc, and MaXET5pro::Luc) containing the MaEXP2, MaPL2 and MaXET5 promoters fused to the reporter gene Luciferase, respectively, were used as reporters; while the coding regions of MaBZR1/2 driven by 35S promoter were used as effectors (Fig. 2a). As illustrated in Fig. 2b, transient expression of either MaBZR1 or MaBZR2 apparently inhibited the expression of LUC reporter gene driven by MaEXP2, MaPL2 and MaXET5 promoters, respectively, which is in good agreement with the previous findings showing MaBZR1/2 to be transcriptional repressors (Guo et al. 2019). Altogether, these data indicate that MaBZR1/2 directly bind to the BRRE motifs present in the promoters of MaEXP2, MaPL2 and MaXET5 to inhibit their transcription.
MaBZR1/2 repress the transcription of MaEXP2, MaPL2 and MaXET5. a Schematic diagram of the reporter and effector constructs used in the dual-luciferase reporter assay. b MaBZR1/2 inhibit transcription of MaEXP2, MaPL2 and MaXET5. The suppression of MaEXP2, MaPL2 and MaXET5 by MaBZR1/2 was shown by LUC/REN. The value of LUC/REN of the empty vector plus promoter reporter was set as 1. Value presented as mean ± SE of six biological repeats. The ** and * represent significant differences at 0.01 and 0.05 levels according to Student’s t test, respectively
Expression of cell wall modifying genes during banana ripening
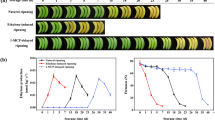
We detected the expression pattern of MaEXP2, MaPL2 and MaXET5 during banana fruit ripening. Banana fruits were subjected to natural ripening, ethylene-promoted ripening and 1-MCP-delayed ripening. RT-qPCR analysis demonstrated that all these genes exhibited a similar trend, with maximal expression levels in the ripening stage (Fig. 3a) when the fruit firmness decreases significantly. Moreover, these genes were also enhanced by BR treatment (Fig. 3b), which was shown to accelerate the ripening of banana fruit (Guo et al. 2019). The higher accumulation of MaEXP2, MaPL2 and MaXET5 transcripts particularly in the ripening phase suggests that they might play a role in fruit softening during banana ripening, as previous studies reported (Pua et al. 2001; Sane et al. 2007; Mbéguié-A-Mbéguié et al. 2009).
Expression profiles of MaEXP2, MaPL2 and MaXET5. a Expression of MaEXP2, MaPL2 and MaXET5 in bananas with natural, ethylene-induced and 1-MCP-retarded ripening. b Expression of MaEXP2, MaPL2 and MaXET5 in bananas treated by BR. Gene expression is shown as a ratio relative to 0 days of natural ripening, which was set at 1. Value presented as mean ± SE of three replicates
Identification of MaBZR1/2 interaction partner
To study the potential components involved in controlling the activity of MaBZR1/2 transcription factors, a yeast two-hybrid (Y2H) screen of a banana cDNA library using MaBZR1 as a bait was carried out to identify its potential interaction partners. Yeast cells harboring the bait were transformed with a library of cDNAs containing inserts of prey proteins fused to GAL4-AD, and positive clones were selected on histidine (His)- and adenine (Ade)-depleted selection plates. After screening, a mitogen-activated protein kinase MaMPK14 (Ma04_g03880.1) was identified as one of the MaBZR1-interacting proteins based on prototrophy for His and Ade. To verify this interaction, the coding region of MaMPK14 was cloned into pGADT7 for Y2H assays. Further experiments indicated that MaBZR1 indeed interacted with MaMPK14 in yeast cells (Fig. 4a). In addition to MaBZR1, we observed that MaBZR2 was able to interact with MaMPK14 as well in yeast two-hybrid system (Fig. 4a).
Physical protein interaction between MaBZR1/2 and MaMPK14. a Yeast two-hybrid assay analysis. Interaction was indicated by the ability of yeast cells to grow on a synthetic medium lacking tryptophan, leucine, histidine and adenine (SD/-Leu-Trp-His-Ade), and to turn blue in the presence of X-α-Gal. Yeast cells transformed with pGBKT7-53 + pGADT7-T were used as positive control, while those transformed with pGBKT7-MaBZR1/2 + pGADT7-T, pGADT7-MaMPK14 + pGBKT7 and pGBKT7-Lam + pGADT7-T served as negative controls. b Bimolecular fluorescence complementation analysis. Fluorescence signal of YFP indicates protein–protein interaction. Combinations of YN or YC with the corresponding MaBZR1/2 and MaMPK14 constructs were used as negative controls. Scale bar = 25 μm
Bimolecular fluorescence complementation (BiFC) assays were applied to validate these protein–protein interactions in tobacco epidermal cells. When transiently co-expressed MaMPK14 together with MaBZR1 or MaBZR2 in N. benthamiana leaves, a strong fluorescent signal was captured in the nucleus (Fig. 4b). In contrast, no fluorescence was observed in cells expressing with combination of the single construct and the opposite empty vector. Despite the fact that expression of MaMPK14-GFP resulted in accumulation in both the nucleus and the cytoplasm in N. benthamiana leaves (Fig. 5), MaMPK14 might be translocated to the nucleus through the interaction with MaBZR1/2 for transcriptional regulation. Collectively, these data suggest that MaBZR1/2 could physically interact with MaMPK14.
Subcellular localization of MaMPK14 in tobacco (N. benthamiana) leaf epidermal cells. A plasmid harboring GFP or MaMPK14-GFP was transformed into N. benthamiana leaves by Agrobacterium tumefaciens strain EHA105. GFP signals were captured with a fluorescence microscope after 2 days of infiltration. Bar = 25 μm
MaMPK14 increases MaBZR1/2 repression of cell wall modifying genes
To determine whether MaMPK14 could affect MaBZR1/2-mediated regulation of cell wall modifying genes, we carried out the transient expression assays in N. benthamiana leaves in which the cell wall modifying gene promoters were separately fused with Luciferase (LUC) gene to generate reporters. The effector constructs included MaBZR1, MaBZR2 or MaMPK14 gene driven by the 35S promoter (Fig. 6a). Figure 6b shows that expression of MaBZR1 or MaBZR2 alone significantly suppressed the transcription of MaEXP2, MaPL2 and MaXET5, consistent with the notion of MaBZR1/2 functioning as transcriptional repressors. Notably, expression of MaMPK14 alone could not facilitate the transcription of MaEXP2, MaPL2 and MaXET5, implying that MaMPK14 being a kinase could not bind directly to the DNA. When MaBZR1 or MaBZR2 was co-expressed with MaMPK14, further repression of the reporter activity was detected, supporting a potential interplay among MaBZR1/2 and MaMPK14 in controlling target gene expression. Overall, these findings indicate that MaBZR1/2 and MaMPK14 may function additively to control the transcription of cell wall modifying genes.
MaBZR1/2 corporate with MaMPK14 to regulate the downstream target genes. a Schematic representation of the double reporters and effectors plasmids used in the transient expression assays. b The reporter and effectors vectors were co-introduced into tobacco leaves by Agrobacterium EHA105. The reporter plasmid together with different combinations of effectors plasmids were infiltrated into tobacco leaves, and the tobacco leaves were incubated for 2–3 days, the activities of LUC and REN were measured sequentially, and the LUC/REN ratio was calculated as the final transcriptional activity. The symbols – and + represent absence or presence, respectively. Each value represents the means of six biological replicates, and vertical bars represent the SE. The ** and * represent significant differences at 0.01 and 0.05 levels according to Student’s t test, respectively
Discussion
BZR TFs are not only important positive regulators of BR signaling pathway, but also key connecting points that integrate diverse signaling cascades (Li et al. 2018). Recently, identification of BZR TFs in non-model plants has been reported. For example, 15 BZR TFs were found in Brassica rapa, which exhibited differential expression responding to low temperature stress (Saha et al. 2015). Moreover, 6 BZR TFs were found in Eucalyptus grandis, among which 4 EgrBZRs accumulated in vascular tissue, pointing out their possible roles in wood formation (Fan et al. 2018). In maize, 11 ZmBZR were characterized, and most of them manifested up-regulation in different organ development or against nitrogen starvation, hypoxia and salt stress (Manoli et al. 2018; Yu et al. 2018). Our prior investigation have identified 3 BZR TFs from banana fruit namely MaBZR1 to MaBZR3, in which MaBZR1/2 are likely involved in banana ripening through transcriptional repression of ethylene biosynthetic genes (Guo et al. 2019). Considering that BZRs serve as major integrators among various signaling pathways, it is interesting to study whether MaBZR1/2 control other ripening-associated genes such as cell wall modifying genes, which would be useful for better understanding the contribution of MaBZR1/2 to banana fruit ripening.
Fruit softening is an essential trait constraining the shelf life and postharvest quality of harvested fruits, which involves physiological modification of the cell wall matrix polysaccharide structure caused by various cell wall modifying enzymes and/or proteins like pectin methylesterase (PME), polygalacturonase (PG), pectate lyase (PL), xyloglucan endotransglycosylase (XET), and expansin (EXP) (Tucker et al. 2017). Many studied have revealed that the cell wall modifying genes are regulated at the transcriptional level, which includes the action of TFs. Participation of several TFs in regulation of cell wall modifying genes during fruit ripening has been found. For example, banana MaLBD1/2/3 and MaBSD1 act as transcriptional activators of MaEXP1/2 that may function in fruit softening (Ba et al. 2014a, b). On the contrary, MaDEAR1 is likely involved in banana ripening through transcriptional inhibition of a subset of cell wall modifying genes like MaEXP1/3, MaPG1, MaXTH10, MaPL3, and MaPME3 (Fan et al. 2016). Similarly, papaya CpERF9 represses the transcription of CpPME1/2 and CpPG5 by directly binding to their promoters (Fu et al. 2016). Recently, MaTCP5 and MaTCP20 activate the transcription of cell wall modifying genes MaXTH10/11, while MaTCP19 represses their transcription, and interaction of MaTCP20 with MaTCP5 or MaTCP19 may dynamically manipulate MaXTH10/11 transcription (Song et al. 2018). In this context, we found that MaBZR1/2 were capable of binding to and repressing the promoters of several cell wall modifying genes including MaEXP2, MaPL2 and MaXET5 (Figs. 1, 2), which were significantly elevated in the banana ripening (Fig. 3). Similarly, using ChIP-chip analysis, BZR1 regulates numerous target genes, which consist of cell wall-related genes such as cellulose synthase, pectinesterases, xyloglucosyl transferases, and expanins (Sun et al. 2010). In soybean, EXPA1 and EXPA8 that are likely involved in BR-mediated cell elongation and expansion were also enriched by GmBZL3 (Song et al. 2019). Based on these findings, it seems likely that BZR-mediated transcriptional regulation of cell wall modifying genes is one of the major regulatory nodes conferring BR response.
Interaction of BZR1 with other partners plays a pivotal role in the functional activity of BZR1 participating in various signaling. For example, BZR1 interacts with RGA to block its transcriptional activity on target genes associated with cell growth (Li et al. 2012). BZR1 interacts with RD26 and WRKY54 to regulate the transcription of CBF, WRKY6, PYL6 and RD26 genes that relate to abiotic stress tolerance (Chen et al. 2017; Li et al. 2017b; Ye et al. 2017). In this study, we identified MaMPK14 as an interaction partner of MaBZR1/2 using yeast two-hybrid assays (Fig. 4a). Further experiment revealed that MaBZR1/2 formed a complex with MaMPK14 in the nucleus (Fig. 4b), despite the fact that the localization of MaMPK14 was distributed throughout the entire cells (Fig. 5). Given that MaBZR1/2 interact with MaMPK14, we speculated that MaBZR1/2 might be substrates of MaMPK14 and likely be phosphorylated by MaMPK14. It should be pointed out that phosphorylated BZR1/BES1 is unstable and likely to be degraded by the proteasome, and that phosphorylated BZR1/BES1 indicates an inactive form of BZR1/BES1 (Tang et al. 2011). However, we found that interaction of MaBZR1/2 with MaMPK14 led to enhanced MaBZR1/2-mediated transcriptional inhibitory capacities in tobacco epidermal cells tested (Fig. 6). Similarly, BES1 interacts with MPK6 and is phosphorylated by MPK6 in vitro; moreover, MAPK-mediated BES1 phosphorylation positively modulates plant immunity, underlying a contrary regulatory mechanism of BES1 phosphorylation by BIN2 in BR signaling (Kang et al. 2015). It should be worthy mentioning that phosphorylation of a given protein at different sites may have diverse functions. A well-known example is ICE1, which is phosphorylated by different protein kinases at different sites. For example, OST1, a Ser/Thr protein kinase in ABA signaling, phosphorylates ICE1 at Ser278 to prohibit its 26S proteasome-mediated degradation by HOS1 (Ding et al. 2015). By contrast, MPK3/MPK6 interacts with and phosphorylate ICE1 at conserved Ser/Thr residues (Ser94/203/403, Thr366/382/384), which decreases the stability and transcriptional activity of ICE1, thereby negatively affecting cold tolerance (Li et al. 2017a; Zhao et al. 2017). Therefore, whether MaBZR1/2 are phosphorylated by MaMPK14 or the phosphorylation pattern of MaBZR1/2 is different with that of Arabidopsis BZR1 needs to be investigated in future study.
In conclusion, based on these findings, together with our previous report (Guo et al. 2019), a working model of MaBZR1/2 in controlling banana fruit ripening was proposed (Fig. 7). Ethylene inhibits the expression of MaBZR1/2. The banana ripening negative regulators MaBZR1/2 targeted and repressed the promoters of ripening-associated genes including ethylene biosynthetic genes such as MaACS1, MaACO13 and MaACO14, as well as cell wall modifying genes such as MaEXP2, MaPL2 and MaXET5, the accumulation of which was up-regulated in the ripening stage. Moreover, protein–protein interaction reveals direct interaction between MaBZR1/2 and MaMPK14, and the interaction strengthens MaBZR1/2-mediated transcriptional repressive capacities (Fig. 7). Our findings that MaBZR1/2 physically interact with MaMPK14 provide another level for controlling the transcription of cell wall modifying genes during banana ripening. Further investigation is necessary to study how MaBZR1/2 work together with MaMPK14 to regulate the cell wall modifying gene expression and ultimately the banana ripening.
A postulated model of MaBZR1/2 in controlling banana fruit ripening. Ethylene inhibits the activity of MaBZR1/2. The banana ripening negative regulators MaBZR1/2 bound to and repressed the promoters of ripening-associated genes including ethylene biosynthetic genes such as MaACS1, MaACO13 and MaACO14, as well as cell wall modifying genes such as MaEXP2, MaPL2 and MaXET5. Moreover, MaBZR1/2 interact with MaMPK14, which enhances MaBZR1/2-mediated transcriptional repression on the cell wall modifying genes. Arrow indicates activation, whereas T bar indicates inhibition
Abbreviations
- 1-MCP:
-
1-Methylcyclopropene
- BAK1:
-
BRI1-associated receptor kinase 1
- BES1:
-
Brassinosteroid Insensitive 1-EMS-Suppressor 1
- BiFC:
-
Bimolecular fluorescence complementation
- BIN2:
-
Brassinosteroid insensitive 2
- BR:
-
Brassinosteroid
- BRI1:
-
Brassinosteroid insensitive 1
- BZR1:
-
Brassinazole resistant 1
- EMSA:
-
Electrophoretic mobility shift assay
- EXP:
-
Expansin
- MAPK:
-
Mitogen-activated protein kinase
- MAPKK:
-
Mitogen-activated protein kinase kinase
- MAPKKK:
-
Mitogen-activated protein kinase kinase kinase
- PCR:
-
Polymerase chain reaction
- PL:
-
Pectate lyase
- TF:
-
Transcription factor
- XET:
-
Xyloglucan endotransglycosylase
- Y2H:
-
Yeast two-hybrid
References
Ba LJ, Shan W, Xiao YY et al (2014a) A ripening-induced transcription factor MaBSD1 interacts with promoters of MaEXP1/2 from banana fruit. Plant Cell Rep 33:1913–1920
Ba LJ, Shan W, Kuang JF et al (2014b) The banana MaLBD (LATERAL ORGAN BOUNDARIES DOMAIN) transcription factors regulate EXPANSIN expression and are involved in fruit ripening. Plant Mol Biol Rep 32:1103–1113
Chen L, Zhong HY, Kuang JF et al (2011) Validation of reference genes for RT-qPCR studies of gene expression in banana fruit under different experimental conditions. Planta 234:377–390
Chen J, Nolan TM, Ye H et al (2017) Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought responses. Plant Cell 29:1425–1439
Costa F, Peace CP, Stella S et al (2010) QTL dynamics for fruit firmness and softening around an ethylene-dependent polygalacturonase gene in apple (Malus × domestica Borkh.). J Exp Bot 61:3029–3039
Cui XY, Gao Y, Guo J et al (2019) BES/BZR transcription factor TaBZR2 positively regulates drought responses by activation of TaGST1. Plant Physiol 180:605–620
Ding Y, Li H, Zhang X et al (2015) OST1 kinase modulates freezing tolerance by enhancing ICE1 stability in Arabidopsis. Dev Cell 32:278–289
Dóczi R, Bögre L (2018) The quest for MAP kinase substrates: gaining momentum. Trends Plant Sci 23:918–932
Fan ZQ, Kuang JF, Fu CC et al (2016) The banana transcriptional repressor MaDEAR1 negatively regulates cell wall-modifying genes involved in fruit ripening. Front Plant Sci 7:1021
Fan C, Guo G, Yan H et al (2018) Characterization of Brassinazole resistant (BZR) gene family and stress induced expression in Eucalyptus grandis. Physiol Mol Biol Plants 24:821–831
Fu CC, Han YC, Qi XY et al (2016) Papaya CpERF9 acts as a transcriptional repressor of cell-wall-modifying genes CpPME1/2 and CpPG5 involved in fruit ripening. Plant Cell Rep 35:2341–2352
Guo YF, Shan W, Liang SM et al (2019) MaBZR1/2 act as transcriptional repressors of ethylene biosynthetic genes in banana fruit. Physiol Plant 165:555–568
He JX, Gendron JM, Sun Y et al (2005) BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science 307:1634–1638
Jonak C, Okresz L, Bogre L, Hirt H (2002) Complexity, cross talk and integration of plant MAP kinase signalling. Curr Opin Plant Biol 5:415–424
Kang S, Yang F, Li L et al (2015) The Arabidopsis transcription factor BRASSINOSTEROID INSENSITIVE1-ETHYL METHANESULFONATE-SUPPRESSOR1 is a direct substrate of MITOGEN-ACTIVATED PROTEIN KINASE6 and regulates immunity. Plant Physiol 167:1076–1086
Kim TW, Wang ZY (2010) Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu Rev Plant Biol 61:681–704
Li X, Xu C, Korban SS, Chen K (2010) Regulatory mechanisms of textural changes in ripening fruits. Crit Rev Plant Sci 29:222–243
Li QF, Wang C, Jiang L et al (2012) An interaction between BZR1 and DELLAs mediates direct signaling crosstalk between brassinosteroids and gibberellins in Arabidopsis. Sci Signal 5:ra72
Li H, Ding Y, Shi Y et al (2017a) MPK3- and MPK6-mediated ICE1 phosphorylation negatively regulates ICE1 stability and freezing tolerance in Arabidopsis. Dev Cell 43:630–642
Li H, Ye K, Shi Y et al (2017b) BZR1 positively regulates freezing tolerance via CBF-dependent and CBF-independent pathways in Arabidopsis. Mol Plant 10:545–559
Li QF, Lu J, Yu JW et al (2018) The brassinosteroid-regulated transcription factors BZR1/BES1 function as a coordinator in multisignal-regulated plant growth. BBA Gene Regul Mech 1861:561–571
Liu L, Jia C, Zhang M et al (2014) Ectopic expression of a BZR1-1D transcription factor in brassinosteroid signaling enhances carotenoid accumulation and fruit quality attributes in tomato. Plant Biotechnol J 12:105–115
Manoli A, Trevisan S, Quaggiotti S, Varotto S (2018) Identification and characterization of the BZR transcription factor family and its expression in response to abiotic stresses in Zea mays L. Plant Growth Regul 84:423–436
Mbéguié-A-Mbéguié D, Hubert O, Baurens FC et al (2009) Expression patterns of cell wall modifying genes from banana during ripening in relationship with finger drop. J Exp Bot 60:2021–2034
Mohapatra D, Mishra S, Sutar N (2010) Banana post harvest practices: current status and future prospects. Agric Rev 31:56–62
Moustafa K, AbuQamar S, Jarrar M et al (2014) MAPK cascades and major abiotic stresses. Plant Cell Rep 33:1217–1225
Pua EC, Ong CK, Liu P, Liu JZ (2001) Isolation and expression of two pectate lyase genes during fruit ripening of banana (Musa acuminata). Physiol Plant 113:92–99
Rodriguez MC, Petersen M, Mundy J (2010) Mitogen-activated protein kinase signaling in plants. Annu Rev Plant Biol 61:621–649
Saha G, Park JI, Jung HJ et al (2015) Molecular characterization of BZR transcription factor family and abiotic stress induced expression profiling in Brassica rapa. Plant Physiol Biochem 92:92–104
Sainsbury F, Thuenemann EC, Lomonossoff GP (2009) pEAQ: versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotechnol J 7:682–693
Sane AVA, Sane AP, Nath P (2007) Multple forms of α-expansin genes are expressed during banana fruit ripening and development. Postharvest Biol Technol 45:184–192
Song CB, Shan W, Yang YY et al (2018) Heterodimerization of MaTCP proteins modulates the transcription of MaXTH10/11 genes during banana fruit ripening. BBA Gene Regul Mech 1861:613–622
Song L, Chen W, Wang B et al (2019) GmBZL3 acts as a major BR signaling regulator through crosstalk with multiple pathways in Glycine max. BMC Plant Biol 19:86
Sun Y, Fan XY, Cao DM et al (2010) Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev Cell 19:765–777
Tang W, Yuan M, Wang R et al (2011) PP2A activates brassinosteroid-responsive gene expression and plant growth by dephosphorylating BZR1. Nat Cell Biol 13:124–131
Tucker G, Yin X, Zhang A et al (2017) Ethylene and fruit softening. Food Qual Saf 1:253–267
Ueno Y, Yoshida R, Kishi-Kaboshi M et al (2015) Abiotic stresses antagonize the rice defence pathway through the tyrosine-dephosphorylation of OsMPK6. PLoS Pathog 11:e1005231
Wan CY, Wilkins TA (1994) A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.). Anal Biochem 223:7–12
Wang ZY, Nakano T, Gendron J et al (2002) Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev Cell 2:505–513
Wang ZY, Bai MY, Oh E, Zhu JY (2012) Brassinosteroid signaling network and regulation of photomorphogenesis. Annu Rev Genet 46:701–724
Wang Y, Cao JJ, Wang KX et al (2019) BZR1 mediates brassinosteroid-induced autophagy and Nitrogen starvation in tomato. Plant Physiol 179:671–685
Wu C, Shan W, Liang S et al (2019) MaMPK2 enhances MabZIP93-mediated transcriptional activation of cell wall modifying genes during banana fruit ripening. Plant Mol Biol 101:113–127
Ye H, Liu S, Tang B et al (2017) RD26 mediates crosstalk between drought and brassinosteroid signaling pathways. Nat Commun 8:14573
Yu X, Li L, Zola J et al (2011) A brassinosteroid transcriptional network revealed by genome-wide identification of BESI target genes in Arabidopsis thaliana. Plant J 65:634–646
Yu H, Feng W, Sun F et al (2018) Cloning and characterization of BES1/BZR1 transcription factor genes in maize. Plant Growth Regul 86:235–249
Zhao C, Wang P, Si T et al (2017) MAP kinase cascades regulate the cold response by modulating ICE1 protein stability. Dev Cell 43:618–629
Acknowledgements
This work was funded by the National Natural Science Foundation of China (Grant no. 31401922, 31772021), Guangdong Special Support Program (Grant no. 2017TQ04N512), China Agriculture Research System (Grant no. CARS-31-11), and Guangdong Provincial Special Fund For Modern Agriculture Industry Technology Innovation Teams.
Author information
Authors and Affiliations
Contributions
JK and XS conceived and designed the research. WS and YG carried out most of the experiments. WW performed some of the experiments. JC, WL, and DY analyzed the data. JK, XS, JC and WL wrote the manuscript. All the authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Communicated by Sukhpreet Sandhu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shan, W., Guo, YF., Wei, W. et al. Banana MaBZR1/2 associate with MaMPK14 to modulate cell wall modifying genes during fruit ripening. Plant Cell Rep 39, 35–46 (2020). https://doi.org/10.1007/s00299-019-02471-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-019-02471-5