Abstract
Key message
Silencing SlAGL6 in tomato leads to fused sepal and green petal by influencing the expression of A-, B-class genes.
Abstract
AGAMOUS-LIKE6 (AGL6) lineage is an important clade MADS-box transcription factor and plays essential roles in various developmental programs especially in flower meristem and floral organ development. Here, we isolated a tomato AGL6 lineage gene SlAGL6 and successfully obtained several RNA interference (RNAi) lines. Silencing SlAGL6 led to abnormal fused sepals and light green petals with smaller size. The total chlorophyll content in transgenic petals increased and the morphology of epidermis cells altered. Further analysis showed that A-class gene MACROCALYX (MC) participating in sepal development and a NAC-domain gene GOBLET involving in boundary establishment were down-regulated in transgenic lines. In transgenic petals, two chlorophyll synthesis genes, Golden2-like1 (SlGLK1) and Golden2-like2 (SlGLK2), two photosystem-related genes, ribulose bisphosphate carboxylase small chain 3B (SlrbcS3B) and chlorophyll a/b-binding protein 7 (SlCab-7) were induced and three B-class genes TM6, TAP3 and SlGLO1 were repressed. These results suggest that SlAGL6 involves in tomato sepal and petal development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Decades ago, ABC model was proposed to illustrate the relationship between genes and the formation of floral organs according to the research on various flower mutations of model plants. In ABC model, genes in three classes control the initiation of four floral organs: sepals, petals, stamens and carpels and the development of four whorls are separately determined by A class alone, A and B together, B and C together and C alone (Coen and Meyerowitz 1991; Schwarzsommer et al. 1990). In Arabidopsis, the A-class contains two genes APETALA1 (AP1) and APETALA2 (AP2), B-class contains APETALA3 (AP3) and PISTILLATA (PI) and C-class contains AGAMOUS (AG). A few years later, the model was expanded by finding out D- and E-class genes in the process of flower development. D-class is involved in ovule development (Angenent et al. 1995; Colombo et al. 1995), while E-class genes are considered participating in the identity of flower meristem and all flower organs (Angenent et al. 1994; Ditta et al. 2004; Pelaz et al. 2000; Vandenbussche et al. 2003). E function is controlled by SEPALLATA (SEP) clade genes, previously known as AGAMOUS LIKE 2 (AGL2) clade (Pelaz et al. 2001; Theissen 2001). All these genes belong to MADS-box family except for AP2 and its homologs (Theissen et al. 2000). Further study revealed that besides SEP clade, MADS-box gene AGL6 and its homologs also function like SEP genes (Hsu et al. 2014; Rijpkema et al. 2009; Smaczniak et al. 2012).
AGL6 clade genes have been proved functioning in manifold developmental events, especially in controlling flower development. For example, AGL6 lineage members have been confirmed to play role in lateral organ initiation (Koo et al. 2010), flowering time regulation (Koo et al. 2010; Yoo et al. 2011b), circadian-clock control (Yoo et al. 2011a) and male and female gametophyte morphogenesis construction (Hsu et al. 2014) in Arabidopsis. Ectopic expression of wintersweet and orchid AGL6 gene in Arabidopsis also alter flowering time and morphology of floral organs (Hsu et al. 2003; Wang et al. 2011). Rice AGL6 lineage gene MADS6 has been proved interacting with multiple floral homeotic genes to specifying floral organ identities and meristem fate (Duan et al. 2012, Hsu et al. 2014; Li et al. 2011, 2010). Maize AGL6 mutation bearded-ear (bed) exhibited extra mosaic or fused floral organs in upper floral meristem and additional floral meristems in lower floral meristem (Thompson et al. 2009). In Petunia, research on mutants clearly demonstrated that PhAGL6 (pMADS4) functions redundantly in petal and anther development with E-function genes FBP2 and FBP5, in addition, PhAGL6 plays role in ovule development (Rijpkema et al. 2009). In tomato, recent research revealed that Slagl6 mutations confer facultative parthenocarpic, leading to high quality seedless fruits under heat stress conditions (Klap et al. 2016), which indicates that SlAGL6 plays an essential role in the regulation of fruit set.
According to phylogenetic analysis, AGL6 is sister to SEP and SQUAMOSA (SQUA, also known as AP1) (Hileman et al. 2006; Kim et al. 2013). AGL6 lineage is considered as an ancient clade. No distinctive AGL6 members have been found in ferns so far and AGL6 exists in both gymnosperms and angiosperms indicating that the emergence of AGL6 lineage dates back to the initiation of seed plants about 400 MYA (million years ago) (Theissen et al. 2000). Along the evolutionary process of AGL6 lineage, several duplications were proposed and four duplications were proved: one at the base of core eudicots, one during basal angiosperm diversification and two in monocot evolution which suggest that the function of AGL6 become diversified (Viaene et al. 2010). Duplication usually causes subfunctionalization and new function. It is considered as a strong positive selection pressure on AGL6 by functioning redundantly with SEP but exiting stably in different angiosperm lineages which also indicating that function of AGL6 is not exactly identical to SEP genes (Dreni and Zhang 2016).
Though the protein sequence of AGL6 is conserved among angiosperms, the functions are not the same in different species. To know more comprehensively about the function of AGL6 lineage genes in floral organ development, the study on diverse species is necessary. Previous study showed tomato Slagl6 mutations have effect on parthenocarpic fruits in tomato cv. M82 (Klap et al. 2016), but precise functional study of SlAGL6 on floral organ and inflorescence has not been reported. To explore the function of SlAGL6 in flower development, we used tomato (Solanum lycopersicon) Mill. cv. Ailsa Craig++, a common material for tomato research, as material to detect the expression of SlAGL6 in different tissues, floral organs and flowers at different developmental stages. Transgenic tomato plants with SlAGL6 silenced were also obtained by RNA interference using S. lycopersicon Mill. cv. Ailsa Craig++ as wild-type tomato. Down-regulation of SlAGL6 produced fused sepals and pale green petals indicating that SlAGL6 plays an important role on the development of floral organs.
Materials and methods
Plant material and growth condition
Tomato plants (S. lycopersicon Mill.cv. Ailsa Craig++) were grown in controlled greenhouse conditions of 16-h-day (25 °C)/8-h-night (18 °C). Materials of roots, stems, young leaves, mature leaves, senescent leaves, flowers and fruits of different stages were collected from tomato plants. Flower parts (sepals, petals, stamens and carpels) were collected from flowers at anthesis. Different stages of flowers were determined by size (2, 5, 7, and 10 mm) before anthesis and by time after anthesis. Samples were frozen in liquid nitrogen immediately and stored at −80 °C for expression analysis.
Total RNA extraction and quantitative real-time PCR analysis
Total RNA was isolated using Trizol (Takara, Dalian, China) according to the manufacture’s instructions. M-MLV reverse transcriptase (Promega, Beijing, China) was used for the synthesis of first strand cDNA with oligo (dT)18 as primer. Quantitative RT-PCR was performed using CFX96™ Real-Time System (Bio-Rad, USA). The 10 μL reaction system contained 5 μL 2× SYBR Premix Ex Taq (Takara), 0.5 μL primers, 1 μL cDNA and 3.5 μL of distilled deionized water. Tomato CAC gene was selected as internal standard for tissue-specific expression analysis (Exposito-Rodriguez et al. 2008). All primers used in this study are exhibited in Supplementary Table S1. The analysis of relative gene expression levels were performed using the 2−ΔΔCT method (Livak and Schmittgen 2001). Samples of three biological repeats were employed in this study.
Structure and phylogenetic analysis of SlAGL6
Multiple sequence alignment of SlAGL6 with other MADS-box proteins was conducted by DNAMAN programs. Phylogenetic tree of SlAGL6 and other reported proteins was constructed using MEGA5.05 software. The accession numbers of proteins contained in multiple sequence alignment and phylogenetic analysis are listed in Supplementary Table S2.
Construction of SlAGL6 RNAi vector and plant transformation
To repress the expression of SlAGL6 gene, we constructed an RNAi vector. Primers of SlAGL6-i-F and SlAGL6-i-R (sequences are listed in Supplementary Table S1) were used for the amplification of specific DNA fragment of SlAGL6 gene. The purified products of 393 bp were digested with Xho I/ Kpn I and inserted into pHANNIBAL plasmid at Xho I/Kpn I restriction site in the sense orientation while products tailed with Xba I/Cla I restriction enzyme in the antisense orientation. At last, the double-stranded RNA expression unit, containing the cauliflower mosaic virus (CaMV) 35 S promoter, SlAGL6 fragment in the sense orientation, PDK intron, SlAGL6 fragment in the antisense orientation and OCS terminator was digested by Sac I/Xba I and linked into plant binary vector pBIN19 in Sac I/Xba I restriction sites.
The generated binary plasmids were translated into Agrobacterium tumefaciens LBA4404 strain, and A. tumefaciens-mediated transformation procedure according to the methods of Chen et al. (2004) was applied to WT tomato cotyledon explants. The transgenic plants were screened by kanamycin (50 mg L−1) resistance and detected with primers NPTII-F and NPTII-R (sequences are listed in Supplementary Table S1). The positive transgenic lines were screened out and prepared for the subsequent experiments.
Extraction and quantitation of chlorophyll in petals
Petals of WT and transgenic lines were milled into powder with liquid nitrogen after weighting. Samples were transferred into tubes and 5 mL 80% of acetone was added for extracting. The extraction was centrifuged at 5000 rpm for 15 min at 4 °C after 24 h in dark. The supernatant was carefully moved out and the absorbance at 645 and 663 nm was recorded. Total chlorophyll was calculated using formulas: Chl (mg g−1) = (20.29 × A645 + 8.02 × A663) (Wellburn 1994).
Statistics of petal length and fused sepals
Petal length of WT and transgenic flowers were measured at 2 dpa (day post anthesis) and the flowers employed in statistics were all the first flowers of inflorescences. The fused sepals were observed at 2 dpa. At least ten flowers per lines were contained in statistics.
Anatomical analyses of flowers
Flowers at 2 dpa were fixed by 70% ethanol/acetic acid/formaldehyde (18:1:1, by volume; FAA). The samples were conducted with dehydration, paraffin embedding, sectioning and dewaxing in proper order. The cross section of one third from bottom of flowers was observed by microscope (OLYMPUS IX71) and photographed.
Scanning electron microscopy
Flowers of wild type and transgenic plants at anthesis were fixed in FAA and then dehydrated in gradient ethanol–water series. After vacuum drying, petals were separated and sputtered gold for scanning electron microscopy observation and photography.
Statistical analyses
Data were presented as mean ± standard deviation. Significant difference between transgenic lines and WT was analyzed using Student’s t test (*P < 0.05).
Results
Sequence and expression analysis of SlAGL6 gene
To investigate the function of tomato AGL6 lineage in floral organ development, we concentrated our work on SlAGL6 (GenBank accession number: XM_004229820.1). Sequence analysis manifested that SlAGL6 contains an open reading frame (ORF) of 759 bp encoding 253 amino acids. Multiple sequence alignment with other typical MADS-box proteins indicated that SlAGL6 is a conservative MADS-box protein (Fig. 1a) sharing 85.2% identity with Petunia PhAGL6. Phylogenic analysis implied that SlAGL6 belongs to conserved AGL6 lineage which is a sister clade of SEP class (Fig. 1b).
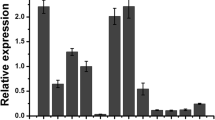
Sequence, phylogenic and expression analysis of SlAGL6 gene. a Multiple sequence alignment of SlAGL6 with other MADS-proteins. SlAGL6 is marked with asterisk. b Phylogenic analysis of SlAGL6 and other MADS-box proteins. The phylogenic tree was constructed by the neighbor-joining method, bootstrap analysis of 1000 replicates. SlAGL6 is marked with asterisk. Accession numbers of other proteins contained in phylogenic analysis are listed in Supplement Table S2. c Expression profile of SlAGL6 in WT. RT root, ST stem, YL young leaf, ML mature leaf, SL senescent leaf, FL flower, IMG immature green fruit, MG mature green fruit, B breaker fruit, B + 4 4 days after breaker, B + 7 7 days after breaker. d Expression profile of SlAGL6 in four-whorl floral organs of WT. Se sepal, Pe petal, St stamen, Ca carpel. Four floral organs were sampled at 2 dpa. e Expression of SlAGL6 in different stages of flower development. F1 2 mm flower, F2 5 mm flower, F3 7 mm flower, F4 10 mm flower, AD anthesis day, 2 dpa 2 days post anthesis, 4 dpa 4 days post anthesis, 7 dpa 7 days post anthesis. Each value represents the mean ± SE of three replicates
In addition, the expression profile of SlAGL6 was investigated in various tomato tissues (including roots, stems, young leaves, mature leaves, senescent leaves, flowers and fruits in different stages) and four floral organs (sepals, petals, stamens and carpels). Interestingly, we found that the expression levels of SlAGL6 in flowers were significantly higher than in other tissues (Fig. 1c). Furthermore, the profile of SlAGL6 in four floral organs revealed the highest transcript level appeared in petals and carpels, followed by sepals, and the lowest in stamens (Fig. 1d). These results indicated the possibility of SlAGL6 participating in the development of petals, carpels and probably in sepals. The expression of SlAGL6 existed in the whole development process of flowers and the expression at and post anthesis was higher than pre-anthesis. The transcript accumulation of SlAGL6 reached peak at anthesis (Fig. 1e).
Repression of SlAGL6 produces fused sepals
Tomato transgenic plants expressing the sense and antisense RNA of SlAGL6 were generated to examine the function of SlAGL6 and seven transgenic lines were obtained. To further determine the repression efficiency of SlAGL6 in transgenic lines, total RNA of flower was extracted and qRT-PCR was conducted. The result displayed that SlAGL6 in three transgenic lines (RNAi2, RNAi6 and RNAi11) was down-regulated by more than 80% (Supplementary Fig. 1) compared with the control and the three transgenic lines were selected for subsequent experiments. In each floral organ, SlAGL6 was down-regulated by more than 80% while in sepals, the efficiency of silencing reached 90% (Supplementary Fig. 1).
In SlAGL6 silenced lines, the sepals of almost all flowers fused in varying degrees (Fig. 2c, d) while the normal sepals split completely each other (Fig. 2b). Some sepals fused together all along, even when the flower completely opened. Petals could not unfold normally due to the constraint of fused sepals. The fused sepals in SlAGL6-RNAi lines were easily separated by hand. By paraffin section, the cross section showed that sepals of transgenic lines at 2 dpa jointed each other by few layers of cells (Fig. 2f) while WT sepals was separated (Fig. 2e). A quantitative statistics of fused sepals was conducted. We defined two sepals adhering less than one third of sepal length as normal dehiscence while more than one third as fusion. The data illustrated that the numbers of sepals in WT and transgenic line had no difference, but the numbers of normal dehisced sepals in transgenic lines were obviously inferior to WT (Supplementary Table S3).
Phenotype of sepal in SlAGL6 RNAi lines. a Sepals of transgenic lines are fused (separated from flower) Bars 1 cm. b–d Normal sepals of WT (b) and fused sepals (c, d) in transgenic lines. e, f Cross sections of WT (e) and transgenic (f) flowers at 2 dpa by paraffin section. Arrows mark the connectives between sepals. Se sepal, Pe petal, St stamen, Ca carpel. Bars 9 μm
Transcription of genes concerned with sepal development are repressed
To figure out the possible genes affected in sepals of transgenic lines, we examined the transcription of a known A-class gene MC (Vrebalov et al. 2002) as well as two reported genes GOBLET (Berger et al. 2009) and SlNAM2 (Hendelman et al. 2013) that can also lead to sepal fusion in sepals of 2 dpa. For MC, the expression reduced by half in transgenic lines compared with WT (Fig. 3a). The transcription of GOBLET was slightly down-regulated in transgenic lines (Fig. 3b) while SlNAM2 had no apparent difference in transcriptional level (Supplementary Fig. S2a). Together, repression of SlAGL6 had effect on the expression of some genes central to sepal development.
Expression analysis of genes involving in sepal development in WT and transgenic lines. Relative expression of MC (a) and GOBLET (b) in sepal, sepals were obtained at 2 dpa. Each value represents the mean ± SE of three replicates. Asterisks indicate a significant difference (P < 0.05) between WT and transgenic lines
Silencing of SlAGL6 generates shorter, light green petals with altered epidermis cells
Usually, the normal petals of WT flowers at anthesis are yellow, but in transgenic flowers, the color of petals was more green than wild type at the same time (Fig. 4a, b). Scanning electron microscopy analysis revealed the adaxial epidermis cells which were protruding and clear-cut in WT petals (Fig. 4c) converted into cave-shape and fuzzy-outline cells in transgenic lines (Fig. 4d) at 2 dpa. At the same time, we measured the petal length and found that transgenic petals were shorter than WT (Fig. 4e).
Phenotype of petal in SlAGL6 RNAi lines. a Flowers of WT and transgenic lines at 2 dpa. Bars 1 cm. b Separated petals of WT and transgenic lines. Bars 1 cm. Surface of adaxial petal of WT (c) and transgenic (d) flower. Bars 60 μm. e Petal length of WT and transgenic lines. Error bars represent the standard error of the mean (n = 10). Asterisks indicate a significant difference (P < 0.05) between WT and transgenic lines
Total chlorophyll content increases and chlorophyll synthesis genes of transgenic plant are up-regulated
To further confirm the phenotype of green petal in transgenic flowers, we measured the content of total chlorophyll in petals of WT and transgenic lines. The content of total chlorophyll in transgenic petals rose to 1.3–1.8-fold compared with WT at 2 dpa (Fig. 5a). At the same time, chlorophyll synthesis related genes were also detected by quantitative real-time PCR. The chlorophyll biosynthesis genes, SlGLK1 and SlGLK2 (Powell et al. 2012) were significantly up-regulated (SlGLK1 to 2.8-–fourfolds and SlGLK2 to 1.8–3.6-folds) in petals of transgenic lines (Fig. 5b, c). Similarly, the expression of two photosystem-related genes also increased (SlCab-7 to 1.2-–twofolds and SlrbcS3B to 1.9–3.2-folds) (Fig. 5d, e).
Total chlorophyll content and detection of chlorophyll synthesis genes in petal of WT and transgenic lines. a Total chlorophyll content of petals from WT and transgenic flowers. b–d Expression analysis of SlGLK1, SlGLK2, SlCab-7 and SlrbcS3B in petals of WT and transgenic lines at 2 dpa. Each value represents the mean ± SE of three replicates. Asterisks indicate a significant difference (P < 0.05) between WT and transgenic lines
Transcripts of tomato B-class floral organ identity genes in petals are reduced
Since the SlAGL6 was highly expressed in petals and repressing of SlAGL6 caused the conversion of color and cellular morphology, we detected the expression of four reported tomato B-class genes TAP3 (Kramer et al. 1998; Quinet et al. 2014), TPI (Geuten and Irish 2010; Kim et al. 2005), TM6 (Busi et al. 2003; Sung et al. 2000) and SlGLO1 (Geuten and Irish 2010; Guo et al. 2016) in petals of WT and transgenic lines. For TPI, the transcript accumulation in transgenic lines remained nearly at the same level as WT except in line RNAi-2 (Supplementary Fig. 2c). However, the expression of TM6, TAP3 and SlGLO1 decreased a little in transgenic lines (Fig. 6). The transcription of MC in transgenic petals was also examined and except for RNAi-2, other lines showed no significant difference with WT (Supplementary Fig. 2b). The results illustrated that SlAGL6 moderately affected partial B-class genes and had no influence on A-class genes in petals.
Expression of C, D and E-class genes were affected in transgenic lines
Although no obviously difference was observed in stamens and carpels in SlAGL6 transgenic lines, we still detected the transcription of C-class gene TAG1 and D-class gene AGL11 in stamens and carpels. As supplementary, two E-class genes, TAGL2 and TM5 were detected in each floral organ. Results showed that in stamen and carpel, TAG1 had no significant difference in stamen between WT and transgenic lines while in carpel, the expression of TAG1 reduced a little in two transgenic lines (Supplementary Fig. 2d). AGL11 was repressed in both stamen and carpel (Supplementary Fig. 2e). The expression of TAGL2 had no difference between WT and transgenic lines in petals and stamens, while in sepals, the transcription of TAGL2 was repressed in only one transgenic line. It is worth noting that the expression of TAGL2 was down-regulated remarkably in carpels (Supplementary Fig. 2f). The transcription of TM5 raised in two transgenic lines in petals and elevated in carpels obviously. The expression of TM5 in stamens was barely affected while in sepals, it was only affected in RNAi-2 (Supplementary Fig. 2g).
Discussion
In previous research, it was extensively accepted that AGL6 linage was active in specifying the identity of the floral meristem, determining the flowering time and development of floral organs. But the function of AGL6 is still not completely clarified. In some basal angiosperms, AGL6 lineage genes have been considered to be A-function genes for their high expression in sepals (Chanderbali et al. 2006; Kim et al. 2005; Wang et al. 2016) and floral induction function by affecting flowering time (Hsu et al. 2003; Wang et al. 2011). However, in Petunia, PhAGL6 was proved to be more similar to SEP (E-function) than to AP1 (A-function) genes in both sequence and expression pattern (Rijpkema et al. 2009). Arabidopsis AGL6 lineage member, AGL13 was claimed to be a putative ancestor for the E-functional genes (Hsu et al. 2014). In this study, expression pattern showed that tomato AGL6 lineage member SlAGL6 expressed highly in flowers and four whorls organ expression profile revealed that the highest expression of SlAGL6 located in petals and carpels, while the expression in sepals was relatively lower. The expression profile was similar to Petunia AGL6 lineage member PhAGL6 whose expression resembled E-class gene FBP2 (Rijpkema et al. 2009) suggesting SlAGL6 is a potential E-function gene. Silencing SlAGL6 caused abnormality in sepals and petals. While along with the development of flower, the transcript accumulation of SlAGL6 maintained at a stable level indicated that SlAGL6 is likely to play role in the whole development process of flowers.
In SlAGL6 RNAi lines, we found that the number of flowers with fused sepals and fused sepals in a single flower were remarkably higher than in WT (Supplementary Table S3). The few layers of cells between sepals in cross section of transgenic flowers (Fig. 2f) suggested that the formation of partial boundaries in the first whorl was probably influenced. A tomato MADS-box gene MC and two NAC-domain transcription factors GOBLET and SlNAM2 were detected in sepals of transgenic lines and WT. MC is a well-known tomato A-function gene involving in sepal development (Vrebalov et al. 2002). Previous research showed that SlNAM2 participates in the establishment of tomato flower whorl and sepal boundaries (Hendelman et al. 2013), while GOBLET is required to inhibit congenital fusion with primordia in the same whorl (Berger et al. 2009). Based on our results, we draw a conclusion that SlAGL6 affects the development of sepals via A-function genes and leads to sepals fusion by influencing genes participating in boundary establishment.
Down-regulation of SlAGL6 led to light green petals with increased total chlorophyll content and up-regulated chlorophyll biosynthesis genes SlGLK1 and SlGLK2. In previous research, repression of tomato E-function genes TM5 (Pnueli et al. 1994b) and TAGL2 (TM29) (Ampomah-Dwamena et al. 2002), B-function genes TPI (Mazzucato et al. 2008) and SlGLO1 (Guo et al. 2016) also produced green petals. What’s more, the expression of SlGLK1 and SlGLK2 also increased in SlGLO1 RNAi lines (Guo et al. 2016). Taken together, we proposed that SlAGL6 affected the synthesis of chlorophyll in petals likely by influencing SlGLO1. In our study, expression of the two E-function genes TAGL2 and TM5 was also detected, results showed that TAGL2 had no difference between WT and transgenic lines in petals, while TM5 raised in two transgenic lines (Supplementary Fig. 2f, g). SlCab-7 encodes the type LHCI Type II chlorophyll a/b-binding polypeptide of Photosystem I (Pichersky et al. 1988) and Slrbcs3B encodes the small subunit of ribulose-l, 5-bisphosphate carboxylase (Sugita et al. 1987). The repression of SlAGL6 in petals of transgenic lines might maintain more active photosynthetic function than wild type for the increasing expression of SlCab-7 and Slrbcs3B. Petals of SlAGL6 RNAi lines also exhibited shorter size than WT and alteration in epidermis cells morphology. The same phenotype was also appeared in Petunia. Lack of function of PhAGL6 produced petals with smaller size and conversed petal epidermis cells into cells similar to those found in sepal epidermis redundantly with PhFBP2 and PhFBP5 (Rijpkema et al. 2009). According to the former reports in tomato, repressing B-class genes SlGLO1 and TM6 generated shorter petals probably caused by a decrease in cell proliferation (de Martino et al. 2006; Guo et al. 2016). The alteration in morphology of epidermis cells in petal was observed in tap3 homozygous mutant while in SlGLO1 RNAi lines, the adaxial cells were more sparse than WT (de Martino et al. 2006; Guo et al. 2016). In SlAGL6 transgenic lines, the expression of these genes reduced a little indicating that the same alteration may also appeared in SlAGL6 transgenic lines. In this study, we indeed observed that the morphology of cells in transgenic lines altered. These results revealed that SlAGL6 may function directly or indirectly by affecting B-class genes in petal development.
In this study, we did not observed any differences between WT and transgenic plants in stamen and carpel as well as flowering time, inflorescence structure and flower number under normal growth condition. But we also examined the expression of C-class gene TAG1 and D-class gene AGL11. Repression of TAG1 displays homeotic conversion of stamens into petaloid organs and the replacement of carpels with pseudocarpels bearing indeterminate floral meristems with nested perianth flowers (Pnueli et al. 1994a). As a D-class member, tomato AGL11 is responsible for seed development and silencing tomato AGL11 produces seedless fruits (Ocarez and Mejia 2016). However, in our study, no obvious difference was observed in seed development although the expression of AGL11 was down-regulated (Supplementary Fig. 2e), but recent study showed that Slagl6 mutation set seedless fruits under heat stress condition (Klap et al. 2016) which indicating that repression of SlAGL6 affects the expression of D-class gene and has impact on seed development in abnormal environment.
The regulatory network between MADS-box floral homeotic proteins is huge and complex based on DNA structure and combinatorial interactions (Yan et al. 2016). As an important floral homeotic gene, AGL6 works by affecting the expression of other genes especially MADS-box genes. In Petunia, PhAGL6 was proved by yeast-two-hybrid assay to interact with SOC1-clade proteins, C- and D-function proteins and other SEP-clade proteins, however, it does not interact with B-function proteins (Rijpkema et al. 2009). In Arabidopsis, AGL6 was confirmed to inhibit the transcription of FLOWER LOCUS C/MADS AFFECTING FLOWERING (FLC/MAF) genes and up-regulate FLOWERING LOCUS T (FT) to promote flowering (Yoo et al. 2011b). The other Arabidopsis AGL6 lineage member AGL13 was proved to regulate the expression of AG/AP3/PI by positive regulatory feedback loops and suppress its own expression through negative regulatory feedback loops by acting as a repressor, AGL6 (Hsu et al. 2014). Ectopic expressing Orchid AGL6-like gene OMADS1 in Arabidopsis changed the expression of FT, SUPPRESSOR OF OVEREXPRESSION OF CO1 (SOC1) and flower meristem identity genes LEAFY (LFY) and AP1 (Hsu et al. 2003). In rice, the transcript levels of A-, B-, C- and E-class genes were affected in mutant of AGL6 lineage gene MADS6 (Li et al. 2011; Ohmori et al. 2009). In this study, A-class gene MC was only reduced in sepals of transgenic lines (Fig. 3a) and three B-function genes TM6, TAP3 and SlGLO1were down-regulated in petals of transgenic lines (Fig. 6). The detection of MADS-box genes in RNAi flower suggested that SlAG6 participates in the development of tomato sepals by regulating A-class genes and petals by regulating B-class genes. In Petunia and rice, AGL6 genes were all testified to function redundantly with other AGL6 members or E-function genes and lead to a minor phenotype in single mutant or RNAi plants (Ohmori et al. 2009; Rijpkema et al. 2009). In tomato, by sequence alignment, only one member of the AGL6 lineage was identified. The phenotype appeared in SlAGL6 transgenic lines was quite feeble, so whether redundancy with genes in other classes exists in the functioning process of SlAGL6 is still a question to be solved.
In summary, SlAGL6 participates in the development of tomato sepals and petals. So far, the functions of AGL6 lineage members in Arabidopsis, rice, Petunia and tomato have been studied and there are both similarities and differences among different species. AGL6 lineage is an ancient clade in MADS transcription factor and focusing on the functional study of AGL6 lineage members in different species will help us get better acquaintance about evolution and functional divergence of MADS-box genes.
Author contribution statement
GC and ZH designed and managed the research work and improved the manuscript. XY, XG, YL, JZ, JH and ST performed the experiments. XY wrote the manuscript.
References
Ampomah-Dwamena C, Morris BA, Sutherland P, Veit B, Yao JL (2002) Down-regulation of TM29, a tomato SEPALLATA homolog, causes parthenocarpic fruit development and floral reversion. Plant Physiol 130:605–617. doi:10.1104/pp.005223
Angenent GC, Franken J, Busscher M, Weiss D, Vantunen AJ (1994) Co-suppression of the petunia homeotic gene Fbp2 affects the identity of the generative meristem. Plant J 5:33–44. doi:10.1046/j.1365-313X.1994.5010033.x
Angenent GC, Franken J, Busscher M, van Dijken A, van Went JL, Dons HJ, van Tunen AJ (1995) A novel class of MADS box genes is involved in ovule development in petunia. Plant Cell 7:1569–1582
Berger Y et al (2009) The NAC-domain transcription factor GOBLET specifies leaflet boundaries in compound tomato leaves. Development 136:823–832. doi:10.1242/dev.031625
Busi MV, Bustamante C, D’Angelo C, Hidalgo-Cuevas M, Boggio SB, Valle EM, Zabaleta E (2003) MADS-box genes expressed during tomato seed and fruit development. Plant Mol Biol 52:801–815
Chanderbali AS, Kim S, Buzgo M, Zheng Z, Oppenheimer DG, Soltis DE, Soltist PS (2006) Genetic footprints of stamen ancestors guide perianth evolution in Persea (Lauraceae). Int J Plant Sci 167:1075–1089. doi:10.1086/507586
Chen GP, Hackett R, Walker D, Taylor A, Lin ZF, Grierson D (2004) Identification of a specific isoform of tomato lipoxygenase (TomloxC) involved in the generation of fatty acid-derived flavor compounds. Plant Physiol 136:2641–2651. doi:10.1104/pp.104.041608
Coen ES, Meyerowitz EM (1991) The war of the whorls—genetic interactions controlling flower development. Nature 353:31–37. doi:10.1038/353031a0
Colombo L, Franken J, Koetje E, van Went J, Dons HJ, Angenent GC, van Tunen AJ (1995) The petunia MADS box gene FBP11 determines ovule identity. Plant Cell 7:1859–1868
de Martino G, Pan I, Emmanuel E, Levy A, Irish VF (2006) Functional analyses of two tomato APETALA3 genes demonstrate diversification in their roles in regulating floral development. Plant Cell 18:1833–1845. doi:10.1105/tpc.106.042978
Ditta G, Pinyopich A, Robles P, Pelaz S, Yanofsky MF (2004) The SEP4 gene of Arabidopsis thaliana functions in floral organ and meristem identity. Curr Biol 14:1935–1940. doi:10.1016/j.cub.2004.10.028
Dreni L, Zhang DB (2016) Flower development: the evolutionary history and functions of the AGL6 subfamily MADS-box genes. J Exp Bot 67:1625–1638. doi:10.1093/jxb/erw046
Duan YL et al (2012) Characterization of Osmads6-5, a null allele, reveals that OsMADS6 is a critical regulator for early flower development in rice (Oryza sativa L.). Plant Mol Biol 80:429–442. doi:10.1007/s11103-012-9958-2
Exposito-Rodriguez M, Borges AA, Borges-Perez A, Perez JA (2008) Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. Bmc Plant Biol. doi:10.1186/1471-2229-8-131
Geuten K, Irish V (2010) Hidden variability of floral homeotic B genes in solanaceae provides a molecular basis for the evolution of novel functions. Plant Cell 22:2562–2578. doi:10.1105/tpc.110.076026
Guo XH, Hu ZL, Yin WC, Yu XH, Zhu ZG, Zhang JL, Chen GP (2016) The tomato floral homeotic protein FBP1-like gene, SlGLO1, plays key roles in petal and stamen development. Sci Rep. doi:10.1038/Srep20454
Hendelman A, Stav R, Zemach H, Arazi T (2013) The tomato NAC transcription factor SlNAM2 is involved in flower-boundary morphogenesis. J Exp Bot 64:5497–5507. doi:10.1093/jxb/ert324
Hileman LC, Sundstrom JF, Litt A, Chen M, Shumba T, Irish VF (2006) Molecular and phylogenetic analyses of the MADS-box gene family in tomato. Mol Biol Evolut 23:2245–2258. doi:10.1093/molbev/msl095
Hsu HF, Huang CH, Chou LT, Yang CH (2003) Ectopic expression of an orchid (Oncidium Gower Ramsey) AGL6-like gene promotes flowering by activating flowering time genes in Arabidopsis thaliana. Plant Cell Physiol 44:783–794. doi:10.1093/Pcp/Pcg099
Hsu WH, Yeh TJ, Huang KY, Li JY, Chen HY, Yang CH (2014) AGAMOUS-LIKE13, a putative ancestor for the E functional genes, specifies male and female gametophyte morphogenesis. Plant J 77:1–15. doi:10.1111/tpj.12363
Kim S et al (2005) Expression of floral MADS-box genes in basal angiosperms: implications for the evolution of floral regulators. Plant J 43:724–744. doi:10.1111/j.1365-313X.2005.02487.x
Kim S, Soltis PS, Soltis DE (2013) AGL6-like MADS-box genes are sister to AGL2-like MADS-box genes. J Plant Biol 56:315–325. doi:10.1007/s12374-013-0147-x
Klap C et al (2016) Tomato facultative parthenocarpy results from SlAGAMOUS-LIKE 6 loss of function. Plant Biotechnol J. doi:10.1111/pbi.12662
Koo SC et al (2010) Control of lateral organ development and flowering time by the Arabidopsis thaliana MADS-box Gene AGAMOUS-LIKE6. Plant J 62:807–816. doi:10.1111/j.1365-313X.2010.04192.x
Kramer EM, Dorit RL, Irish VF (1998) Molecular evolution of genes controlling petal and stamen development: duplication and divergence within the APETALA3 and PISTILLATA MADS-box gene lineages. Genetics 149:765–783
Li HF, Liang WQ, Jia RD, Yin CS, Zong J, Kong HZ, Zhang DB (2010) The AGL6-like gene OsMADS6 regulates floral organ and meristem identities in rice. Cell Res 20:299–313 doi. Doi:10.1038/Cr.2009.143
Li HF et al (2011) Rice MADS6 interacts with the floral homeotic genes SUPERWOMAN1, MADS3, MADS58, MADS13, and DROOPING LEAF in specifying floral organ identities and meristem fate. Plant Cell 23:2536–2552. doi:10.1105/tpc.111.087262
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25:402–408. doi:10.1006/meth.2001.1262
Mazzucato A, Olimpieri I, Siligato F, Picarella ME, Soressi GP (2008) Characterization of genes controlling stamen identity and development in a parthenocarpic tomato mutant indicates a role for the DEFICIENS ortholog in the control of fruit set. Physiol Plantarum 132:526–537. doi:10.1111/j.1399-3054.2007.01035.x
Ocarez N, Mejia N (2016) Suppression of the D-class MADS-box AGL11 gene triggers seedlessness in fleshy fruits. Plant Cell Rep 35:239–254. doi:10.1007/s00299-015-1882-x
Ohmori S et al (2009) MOSAIC FLORAL ORGANS1, an AGL6-like MADS box gene, regulates floral organ identity and meristem fate in rice. Plant Cell 21:3008–3025. doi:10.1105/tpc.109.068742
Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MF (2000) B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 405:200–203. doi:10.1038/35012103
Pelaz S, Tapia-Lopez R, Alvarez-Buylla ER, Yanofsky MF (2001) Conversion of leaves into petals in Arabidopsis. Curr Biol 11:182–184. doi:10.1016/S0960-9822(01)00024-0
Pichersky E, Tanksley SD, Piechulla B, Stayton MM, Dunsmuir P (1988) Nucleotide sequence and chromosomal location of Cab-7, the tomato gene encoding the type II chlorophyll a/b-binding polypeptide of photosystem I. Plant Mol Biol 11:69–71. doi:10.1007/BF00016015
Pnueli L, Hareven AD, Rounsley SD, Yanofsky MF, Lifschitz E (1994a) Isolation of the tomato agamous gene Tag1 and analysis of its homeotic role in transgenic plants. Plant Cell 6:163–173. doi:10.1105/Tpc.6.2.163
Pnueli L, Hareven D, Broday L, Hurwitz C, Lifschitz E (1994b) The TM5 MADS box gene mediates organ differentiation in the three inner whorls of tomato flowers. Plant Cell 6:175–186. doi:10.1105/tpc.6.2.175
Powell ALT et al (2012) Uniform ripening Encodes a Golden 2-like transcription factor regulating tomato fruit chloroplast development. Science 336:1711–1715. doi:10.1126/science.1222218
Quinet M et al (2014) Transcriptional and hormonal regulation of petal and stamen development by STAMENLESS, the tomato (Solanum lycopersicum L.) orthologue to the B-class APETALA3 gene. J Exp Bot 65:2243–2256. doi:10.1093/jxb/eru089
Rijpkema AS, Zethof J, Gerats T, Vandenbussche M (2009) The petunia AGL6 gene has a SEPALLATA-like function in floral patterning. Plant J 60:1–9 doi:10.1111/j.1365-313X.2009.03917.x
Schwarzsommer Z, Huijser P, Nacken W, Saedler H, Sommer H (1990) Genetic-control of flower development by homeotic genes in antirrhinum-majus. Science 250:931–936. doi:10.1126/science.250.4983.931
Smaczniak C, Immink RGH, Angenent GC, Kaufmann K (2012) Developmental and evolutionary diversity of plant MADS-domain factors: insights from recent studies. Development 139:3081–3098. doi:10.1242/dev.074674
Sugita M, Manzara T, Pichersky E, Cashmore A, Gruissem W (1987) Genomic organization, sequence analysis and expression of all five genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase from tomato. Mol Gen Genet 209:247–256
Sung SK, Yu GH, Nam J, Jeong DH, An G (2000) Developmentally regulated expression of two MADS-box genes, MdMADS3 and MdMADS4, in the morphogenesis of flower buds and fruits in apple. Planta 210:519–528
Theissen G (2001) Development of floral organ identity: stories from the MADS house. Curr Opin Plant Biol 4:75–85
Theissen G et al (2000) A short history of MADS-box genes in plants. Plant Mol Biol 42:115–149
Thompson BE, Bartling L, Whipple C, Hall DH, Sakai H, Schmidt R, Hake S (2009) bearded-ear encodes a MADS box transcription factor critical for maize floral development. Plant Cell 21:2578–2590. doi:10.1105/tpc.109.067751
Vandenbussche M et al (2003) Toward the analysis of the petunia MADS box gene family by reverse and forward transposon insertion mutagenesis approaches: B, C, and D floral organ identity functions require SEPALLATA-like MADS box genes in petunia. Plant Cell 15:2680–2693 doi. Doi:10.1105/Tpc.017376
Viaene T, Vekemans D, Becker A, Melzer S, Geuten K (2010) Expression divergence of the AGL6 MADS domain transcription factor lineage after a core eudicot duplication suggests functional diversification Bmc. Plant Biol 10:148. doi:10.1186/1471-2229-10-148
Vrebalov J et al (2002) A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science 296:343–346. doi:10.1126/science.1068181
Wang BG et al (2011) The AGL6-like Gene CpAGL6, a potential regulator of floral time and organ identity in wintersweet (Chimonanthus praecox). J Plant Growth Regul 30:343–352 doi. DOI:10.1007/s00344-011-9196-x
Wang PP et al (2016) Flexibility in the structure of spiral flowers and its underlying mechanisms. Nat Plants. doi:10.1038/Nplants.2015.188
Wellburn AR (1994) The spectral determination of chlorophyll-a and chlorophhyll-B, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Yan WH, Chen DJ, Kaufmann K (2016) Molecular mechanisms of floral organ specification by MADS domain proteins. Curr Opin Plant Biol 29:154–162. doi:10.1016/j.pbi.2015.12.004
Yoo SK, Hong SM, Lee JS, Ahn JH (2011a) A genetic screen for leaf movement mutants identifies a potential role for AGAMOUS-LIKE 6 (AGL6) in circadian-clock control. Mol Cells 31:281–287. doi:10.1007/s10059-011-0035-5
Yoo SK, Wu XL, Lee JS, Ahn JH (2011b) AGAMOUS-LIKE 6 is a floral promoter that negatively regulates the FLC/MAF clade genes and positively regulates FT in Arabidopsis. Plant J 65:62–76. doi:10.1111/j.1365-313X.2010.04402.x
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (No. 106112015CDJZR235504), Chongqing University Postgraduates’ Innovation Project (CYB15027) and Technology System of National Bulk Vegetable Industry–Eggplant Breeding Position (CARS-25-A-06).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Ray J. Rose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
299_2017_2129_MOESM1_ESM.docx
Supplementary Table S1.Details of primers used in this study. Supplementary Table S2.The accession numbers of proteins contained in multiple sequence alignment and phylogenic analysis. Supplementary Table S3.Number of total sepal and normal dehiscent sepal. Observation and statistic of sepals were performed at 3 dpa when the flowers completely opened. (DOCX 22 KB)
299_2017_2129_MOESM2_ESM.tif
Supplementary Figure S1. Detection of SlAGL6 in flower and each floral organ of WT and transgenic lines. Floral organs were sampled at 2 dpa. Each value represents the mean± SE of three replicates. Asterisks indicate a significant difference (P<0.05) between WT and transgenic lines. (TIF 368 KB)
299_2017_2129_MOESM3_ESM.tif
Supplementary Figure S2. Expression analysis of SlNAM2 in sepals (a), MC (b) and TPI (c) in petals, TAG1, AGL11 in stamens and carpels, and two E-class genes in four floral organs (f, g). Expression profiles of SlNAM2 in sepals (a), MC (b) and TPI (c) in petals, TAG1 (d) and AGL11 (e) in stamens and carpels, TAGL2 (f) and TM5 (g) in four floral organs. Floral organs used in gene detection were sampled at 2 dpa. Each value represents the mean± SE of three replicates. Asterisks indicate a significant difference (P<0.05) between WT and transgenic lines. (TIF 407 KB)
Rights and permissions
About this article
Cite this article
Yu, X., Chen, G., Guo, X. et al. Silencing SlAGL6, a tomato AGAMOUS-LIKE6 lineage gene, generates fused sepal and green petal. Plant Cell Rep 36, 959–969 (2017). https://doi.org/10.1007/s00299-017-2129-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-017-2129-9