Abstract
Key message
The AtOR gene enhances carotenoid levels in corn by promoting the formation of plastoglobuli when the carotenoid pool is limited, but has no further effect when carotenoids are already abundant.
Abstract
The cauliflower orange (or) gene mutation influences carotenoid accumulation in plants by promoting the transition of proplastids into chromoplasts, thus creating intracellular storage compartments that act as metabolic sink. We overexpressed the Arabidopsis OR gene under the control of the endosperm-specific wheat LMW glutenin promoter in a white corn variety that normally accumulates only trace amounts of carotenoids. The total endosperm carotenoid content in the best-performing AtOR transgenic corn line was 32-fold higher than wild-type controls (~25 µg/g DW at 30 days after pollination) but the principal carotenoids remained the same, suggesting that AtOR increases the abundance of existing carotenoids without changing the metabolic composition. We analyzed the expression of endogenous genes representing the carotenoid biosynthesis and MEP pathways, as well as the plastid fusion/translocation factor required for chromoplast formation, but only the DXS1 gene was upregulated in the transgenic corn plants. The line expressing AtOR at the highest level was crossed with four transgenic corn lines expressing different carotenogenic genes and accumulating different carotenoids. The introgression of AtOR increased the carotenoid content of the hybrids when there was a limited carotenoid pool in the parental line, but had no effect when carotenoids were already abundant in the parent. The AtOR gene therefore appears to enhance carotenoid levels by promoting the formation of carotenoid-sequestering plastoglobuli when the carotenoid pool is limited, but has no further effect when carotenoids are already abundant because high levels of carotenoids can induce the formation of carotenoid-sequestering plastoglobuli even in the absence of AtOR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carotenoids are natural pigments synthesized in the plastids of plants and algae, and in some non-photosynthetic organisms such as bacteria and fungi (Zhu et al. 2010). Several different strategies have been used to engineer carotenoid metabolism in plants (Fig. 1). Upstream 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway precursors are rate limiting in, e.g., tomato, potato, and rice (Enfissi et al. 2005; Morris et al. 2006; Bai et al. 2014, 2016), so increasing the precursor supply can direct additional flux into the pathway thus increasing total carotenoid levels. This strategy was used in tomato, where the overexpression of Escherichia coli 1-deoxy-D-xylulose-5-phosphate synthase gene (DXS) resulted in a 1.6-fold increase in total fruit carotenoids (Enfissi et al. 2005). A second strategy involves the overexpression of carotenogenic genes in order to relieve bottlenecks in the pathway, thus increasing carotenoid levels and/or changing the composition. For example, the overexpression of bacterial phytoene synthase gene (CRTB) or corn phytoene synthase 1 gene (PSY1) (phytoene synthase, the first committed step in carotenoid biosynthesis) achieved a 50-fold increase in total carotenoid levels in Golden canola and a 156-fold increase in high-carotenoid corn (Shewmaker et al. 1999; Zhu et al. 2008). Carotenogenic gene expression can also be suppressed to block specific pathway branches, or to prevent the conversion of desirable compounds into downstream metabolites. For example, the downregulation of lycopene ε-cyclase gene (LYCE) in rapeseed blocked the carotenoid α-branch and diverted flux into the β-branch, increasing the seed carotenoid content by 45-fold (Yu et al. 2008). It is also possible to extend the pathway to produce novel compounds that are not usually synthesized in plants, e.g., the accumulation of 91 µg/g fresh weight (FW) of the ketocarotenoid astaxanthin in carrot taproots was achieved by expressing the Haematococcus pluvialis β-carotene ketolase gene (BKT) (Jayaraj et al. 2008).
Strategies to modulate carotenoid levels in plants. IPP isopentenyl diphosphate, DMAPP dimethylallyl diphosphate, GGPP geranylgeranyl diphosphate, GGPPS, GGPP synthase, PSY phytoene synthase, PDS phytoene desaturase, ZISO ζ-carotene isomerase, ZDS ζ-carotene desaturase, CRTISO carotenoid isomerase, LYCB lycopene β-cyclase, LYCE lycopene ε-cyclase, CYP97C carotene ε-ring hydroxylase, HYDB β-carotene hydroxylase, CRTE bacterial GGPP synthase, CRTB bacterial phytoene synthase, CRTI bacterial phytoene desaturase/isomerase, CRTY bacterial lycopene β-cyclase, CRTZ bacterial hydroxylase, BKT β-carotene ketolase, CRTW bacterial β-carotene ketolase (adapted from Berman et al. 2015; light microscopy from Bai et al. 2014; scale bar 20 µm)
Carotenoid levels can also be modulated by creating a metabolic sink, i.e., promoting the formation of carotenoid-sequestering structures (Fig. 1). This process is induced by the orange (or) gene (Li et al. 2001; Lu et al. 2006; Paolillo et al. 2004) originally discovered as a spontaneous dominant mutation in cauliflower (Brassica oleracea var. botrytis) which resulted in orange curds (Crisp et al. 1975). In potato, expression of the dominant or allele of the cauliflower gene resulted in a tenfold increase in total carotenoids (Lopez et al. 2008). However, or did not influence metabolic flux through the cauliflower carotenoid pathway, nor did it affect the regulation of carotenogenic genes (Li et al. 2001). The plastid formation/translocation factor gene (PFTF) is upregulated in the or mutant, and the product of or is a plastid-associated protein with a DnaJ cysteine-rich domain, suggesting it may promote carotenoid accumulation by inducing the differentiation of proplastids into chromoplasts (Lu et al. 2006). In addition, the or mutation limits plastid replication so that the cell contains a single chromoplast, and orange crystalloid inclusions within this structure resemble the carotenoid inclusions found in carrot taproots (Paolillo et al. 2004).
The expression of cauliflower or in potato confirmed that the protein induces chromoplast differentiation and carotenoid sequestration, resulting in a sixfold increase in total carotenoids (Lopez et al. 2008). The only carotenoids present in wild-type tubers are violaxanthin and lutein, but the or transgenic tubers accumulated precursor carotenoids that are not detectable in wild-type tubers (such as phytoene, phytofluene, and ζ-carotene) as well as β-carotene, the latter representing 12–17% of the total carotenoids (Lopez et al. 2008). Carotenoids in the or transgenic lines remained stable for up to 6 months in cold storage leading to further accumulation, i.e., an 18-fold increase at 5 months and a 13-fold increase at 6 months (Li et al. 2012; Lopez et al. 2008). The co-expression of cauliflower or and Brevundimonas sp. SD212 CRTZ and CRTW increased the ketocarotenoid content of potato tubers (Campbell et al. 2015).
Orthologs of cauliflower wild-type OR have been identified in other plants and have been shown to influence carotenoid accumulation. For example, the sweet potato ortholog IbOR and the Arabidopsis thaliana ortholog AtOR can induce carotenoid accumulation in sweet potato callus and rice callus and plants (Kim et al. 2013; Bai et al. 2014, 2016). IbOR was shown to induce the expression of carotenoid biosynthesis genes as well as PFTF in transgenic sweet potato callus and the total carotenoid content increased by up to 12-fold (Kim et al. 2013). The total carotenoid content of rice callus expressing corn PSY1 and Pantoea ananatis CRTI increased by 2.2-fold when AtOR was also expressed, and the endosperm contained orange crystalloid inclusions similar to those observed in transgenic potato plants expressing cauliflower or (Bai et al. 2014). Interestingly, similar structures were also observed in transgenic callus co-expressing AtDXS, ZmPSY1, and PaCRTI, suggesting that orange crystalloid inclusions in rice may be triggered by the abundance of carotenoids (Fraser et al. 2007; Maass et al. 2009; Bai et al. 2014). Similarly, plastids containing plastoglobuli were observed in rice endosperm overexpressing AtOR but in this case the expression of the endogenous carotenogenic genes LYCE, LYCB, and BCH2 was also upregulated (Bai et al. 2016).
White corn (Zea mays cv. M37W) serves as a blank canvas for the analysis of carotenoid biosynthesis because the white endosperm contains only trace amounts of carotenoids due to the minimal expression of ZmPSY1 and ZmPSY2. We generated M37W corn lines expressing AtOR, resulting in a substantial increase in the total carotenoid content as shown by the yellow endosperm color, but the increase was not accompanied by a qualitative change in the profile of endosperm carotenoids. The best-performing AtOR transgenic line was crossed with four different transgenic corn lines expressing distinct sets of carotenogenic genes and accumulating different carotenoid profiles, including ketocarotenoids. The resulting hybrids were used to determine the impact of AtOR on carotenoid metabolism and accumulation at the transcriptional and metabolic levels.
Results
Corn lines expressing AtOR accumulate more carotenoids than wild-type plants but the expression of endogenous carotenogenic genes is mostly unchanged
Immature embryos from the white corn inbred M37W were transformed with a construct carrying the AtOR gene under the control of the endosperm-specific wheat low molecular-weight glutenin promoter and another construct carrying the selectable marker BAR under the control of the constitutive corn UBI-1 promoter. Two representative lines (OR1 and OR2) expressing AtOR at low and high levels, respectively, were selected for detailed analysis. Primary transformants were self-pollinated to homozygosity, and AtOR mRNA levels were measured by northern blot analysis in the endosperm of T2 homozygous plants 30 days after pollination (DAP) (Supplementary Fig. 1).
Wild-type M37W endosperm accumulated only traces of zeaxanthin, lutein, violaxanthin, and antheraxanthin, with a total carotenoid content of ~1 μg/g dry weight (DW). In contrast, the endosperm of line OR1 accumulated higher levels of zeaxanthin (~6 μg/g DW) and lutein (~2 μg/g DW), as well as traces of β-cryptoxanthin, antheraxanthin, and violaxanthin. Line OR2 accumulated much higher levels of zeaxanthin (~10 μg/g DW) as well as significant amounts of lutein (~2 μg/g DW), antheraxanthin (~2 μg/g DW), and β-cryptoxanthin (~1 μg/g DW), and traces of violaxanthin. The total carotenoid contents of the endosperm in lines OR1 and OR2 were ~9 and ~17 μg/g DW, respectively (Fig. 2a). Line OR2 therefore achieved a 20-fold increase in carotenoids compared to wild-type plants under greenhouse conditions.
a Carotenoid content and composition in wild-type M37W and transgenic lines OR1 and OR2 (T2 plants) at 30 DAP (µg/g DW ± SE) (n = 3–5 seeds). Relative expression levels of endogenous carotenogenic genes (b) MEP pathway-related genes (c) and PFTF (d) in 30 DAP corn endosperm, normalized against ACTIN mRNA and presented as the mean of three biological replicates ± SE. ZmPSY1 phytoene synthase 1, ZmPSY2 phytoene synthase 2, ZmLYCE lycopene ε-cyclase, ZmLYCB lycopene β-cyclase, ZmBCH1 carotenoid β-hydroxylase 1, ZmBCH2 carotenoid β-hydroxylase 2, ZmCYP97A/B carotene β-hydroxylase, ZmCYP97C carotene ε-hydroxylase, ZmDXS1/2/3 1-deoxy-D-xylulose-5-phosphate synthase, ZmDXR 1-deoxy-D-xylulose-5-phosphate reductase, ZmHDR 4-hydroxy-3-methylbut-2-enyl diphosphate reductase, ZmPFTF plastid fusion/translocation factor
The transcript levels of endogenous carotenogenic genes, MEP pathway genes, and PFTF were compared in lines OR1, OR2, and wild-type M37W by quantitative RT-PCR. The following carotenogenic genes were analyzed: ZmPSY1 and ZmPSY2, ZmLYCB (lycopene β-cyclase), ZmLYCE, ZmBCH1, ZmBCH2, ZmCYP97A, and ZmCYP97B (carotene β-hydroxylase) and ZmCYP97C (carotenoid ε-hydroxylase). Most of the genes were expressed at similar levels in the transgenic and wild-type lines—the only exception was ZmLYCE, which was downregulated in both transgenic lines (Fig. 2b). The following MEP pathway genes were analyzed: ZmDXS1, ZmDXS2, and ZmDXS3 (1-deoxy-D-xylulose-5-phosphate synthase 1, 2 and 3), ZmDXR (1-deoxy-D-xylulose-5-phosphate reductase), and ZmHDR (4-hydroxy-3-methylbut-2-enyl diphosphate reductase). Most of the genes were expressed at similar levels in the transgenic and wild-type lines—the only exception was ZmDXS1, which was upregulated by ~two-fold in the transgenic lines (Fig. 2c). The expression of ZmPFTF did not differ significantly among the three lines (Fig. 2d).
Introgression of AtOR increases the carotenoid content of hybrids with low carotenoid parents
The influence of AtOR on carotenoid accumulation was investigated in detail by crossing the best-performing AtOR line (OR2) with four transgenic lines characterized by different genetic backgrounds and different carotenoid profiles. Two of the lines were selected because they accumulate moderate (CARO1) or high (CARO2) levels of carotenoids and another two were selected because they accumulate low (KETO1) or high (KETO2) levels of ketocarotenoids. CARO1 (ZmPSY1) and KETO1 (sBrCRTZ, sCrBKT, and sBrCRTW) were produced via the same procedure used to generate the AtOR lines, whereas CARO2 (ZmPSY1, PaCRTI, and GlLYCB) and KETO2 (ZmPSY1, sCrBKT, sBrCRTZ, and LYCE-RNAi) were described previously (Zhu et al. 2008; Farré et al. 2016). Line OR2 was used as a pollen donor to generate hybrids ORxCARO1, ORxCARO2, ORxKETO1, and ORxKETO2 (Fig. 3).
Phenotype of wild-type (A) and transgenic seeds with different carotenoid and ketocarotenoid profiles: OR2 (B), CARO1 (C), CARO2 (E), KETO1 (G), and KETO2 (I), and the resulting seeds from the cross with OR2: ORxCARO1 (D), ORxCARO2 (F), ORxKETO1 (H), and ORxKETO2 (J). A substantial change in seed color phenotype resulted when AtOR was expressed in the M37W background (white to yellow) and in low-ketocarotenoid (KETO1) background (light pink to reddish-pink) suggesting that AtOR plays an important role in carotenoid and ketocarotenoid accumulation when the amounts of carotenoids are low in the original (parent) line (KETO1). No changes in color phenotype were observed when the amounts of carotenoids are already high in the parents (CARO1, CARO2, and KETO2)
The carotenoid content and composition of wild-type M37W, the five transgenic parental lines (OR2, CARO1, CARO2, KETO1, and KETO2), and the four hybrids (ORxCARO1, ORxCARO2, ORxKETO1, and ORxKETO2) were evaluated at 30 and 60 DAP to identify any changes in carotenoid content and composition at two different developmental stages, i.e., mid grain filling and mature seeds. Transgene expression in the parental lines and hybrids was measured by quantitative RT-PCR (Supplementary Fig. 2). As expected, the wild-type endosperm did not express any of the transgenes, and OR2 expressed AtOR alone. CARO1 expressed only ZmPSY1; CARO2 expressed ZmPYS1, PaCRTI, and GlLYCB; KETO1 expressed sBrCRTZ, sCrBKT, and sBrCRTW; and KETO2 expressed ZmPSY1, LYCE-RNAi, sCrBKT, and sBrCRTZ. The hybrid lines expressed AtOR as well as the appropriate transgene complement from the other parent (Supplementary Fig. 2).
Wild-type M37W at 30 DAP (Fig. 3A) accumulated low levels of zeaxanthin (~0.4 µg/g DW), lutein (~0.2 µg/g DW), and violaxanthin (0.1 µg/g DW) as well as traces of antheraxanthin. At 60 DAP, we detected zeaxanthin (~0.3 µg/g DW) and lutein (~0.2 µg/g DW) but no violaxanthin. When AtOR was expressed in this background, the endosperm turned yellow (Fig. 3B). The carotenoid profile in the endosperm of line OR2 remained the same as M37W but the total carotenoid content increased dramatically at 30 DAP from <1 µg/g DW in M37W to ~25 µg/g DW (a 32-fold increase). The levels of individual carotenoids increased to different degrees compared to the wild-type plants: zeaxanthin (~12 µg/g DW, 30-fold increase), antheraxanthin (~7 µg/g DW, 170-fold increase), lutein (~5 µg/g DW, 23-fold increase), and violaxanthin (~2 µg/g DW, 15-fold increase). At 60 DAP, the total carotenoid content was 20 µg/g DW. The downstream compounds violaxanthin and antheraxanthin could no longer be detected, but β-cryptoxanthin was detectable (~1 µg/g DW) and the principal carotenoids were zeaxanthin (~11 µg/g DW, 38-fold increase over wild type) and lutein (~8 µg/g DW, 39-fold increase over wild type) (Supplementary Table 1).
The endosperm of parental line CARO1 was orange (Fig. 3C). At 30 DAP, this accumulated ~66 µg/g DW in total carotenoids (94-fold increase over wild type) and at 60 DAP this increased to 91 µg/g DW (181-fold increase over wild type). At 30 DAP, the principal carotenoids were zeaxanthin (~23 µg/g DW; 58-fold increase over wild type), antheraxanthin (~14 µg/g DW; 350-fold increase over wild type), lutein (~9 µg/g DW; 47-fold increase over wild type), and phytoene (~8 µg/g DW), followed by violaxanthin (~4 µg/g DW; 39-fold increase over wild type), β-carotene (~3 µg/g DW), lycopene (~3 µg/g DW), and β-cryptoxanthin (~1 µg/g DW). By 60 DAP, the carotenoid composition had shifted upstream, with a larger increase in phytoene (~61 µg/g DW). The other principal carotenoids were zeaxanthin (~12 µg/g DW; 39-fold increase over wild type), lutein (~6 µg/g DW; 32-fold increase over wild type), and antheraxanthin (~6 µg/g DW), followed by β-carotene (~2 µg/g DW), violaxanthin (~2 µg/g DW), β-cryptoxanthin (~1 µg/g DW), and lycopene (~1 µg/g DW) (Supplementary Table 1). The endosperm of the ORxCARO1 hybrid was similar in color to the CARO1 parent (Fig. 3D). The carotenoid content and composition was also similar to CARO1, with a total carotenoid content at 30 DAP of ~68 µg/g DW. Adverse weather prevented the analysis of ORxCARO1 at 60 DAP (Supplementary Table 1).
The endosperm of parental line CARO2 was also orange (Fig. 3E) but it had a higher carotenoid content than CARO1, i.e., ~79 µg/g DW at 30 DAP (113-fold increase over wild type) and ~155 µg/g DW at 60 DAP (311-fold increase over wild type). At 30 DAP, the principal carotenoids were zeaxanthin (~23 µg/g DW; 58-fold increase over wild type), phytoene (~18 µg/g DW), antheraxanthin (~13 µg/g DW; 330-fold increase over wild type), β-carotene (~11 µg/g DW), and lutein (~9 µg/g DW; 47-fold increase over wild type), followed by violaxanthin (~2 µg/g DW; 24-fold increase over wild type) and β-cryptoxanthin (~2 µg/g DW). As discussed above for CARO1, the phytoene content had increased (~117 µg/g DW) at the expense of downstream carotenoids by 60 DAP. The principal carotenoids were zeaxanthin (~18 µg/g DW; 60-fold increase over wild type), lutein (~6 µg/g DW; 44-fold increase over wild type), and β-carotene (~8 µg/g DW), followed by antheraxanthin (~2 µg/g DW) and β-cryptoxanthin (~2 µg/g DW) (Supplementary Table 1). The endosperm of the ORxCARO2 hybrid was orange, similar to the CARO2 parent (Fig. 3F). The carotenoid content and composition in the hybrid was similar to CARO2, with a total carotenoid content of ~79 µg/g DW at 30 DAP and ~135 µg/g DW at 60 DAP (Supplementary Table 1).
The endosperm of the parental line KETO1 was pale pink (Fig. 3G) reflecting the accumulation of relatively low levels of the ketocarotenoid astaxanthin (~0.4 µg/g DW at 30 DAP and ~0.6 µg/g DW at 60 DAP) with undetectable levels of other carotenoids. The minimal carotenoid pool in the wild-type M37W endosperm was therefore converted almost entirely into ketocarotenoids by expressing the heterologous carotenoid β-hydroxylase (sBrCRTZ) and β-carotene ketolases (sCrBKT and sBrCRTW) (Supplementary Table 2). The endosperm of the ORxKETO1 hybrid was deeper pink (Fig. 3H) reflecting the accumulation of more ketocarotenoids than the KETO1 parent. However, the profile was the same, i.e., the total carotenoid content of ~9 µg/g DW at 30 DAP (22-fold increase over KETO1) and ~8 µg/g DW at 60 DAP (14-fold increase over KETO1) was almost entirely due to the accumulation of astaxanthin (Supplementary Tables 1 and 2).
The endosperm of the parental line KETO2 was red (Fig. 3I) reflecting the higher levels of ketocarotenoids compared to KETO1, i.e., ~32 µg/g DW at 30 DAP (80-fold increase over KETO1) and ~26 µg/g DW at 60 DAP (17-fold increase over KETO1). KETO2 also accumulated other (non-keto) carotenoids (~33 µg/g DW at 30 DAP and ~79 µg/g DW at 60 DAP). At 30 DAP, the principal carotenoid was astaxanthin (~22 µg/g DW), accompanied by lower amounts of the other ketocarotenoids adonirubin (~3 µg/g DW), 3-OH-echinenone (~3 µg/g DW), canthaxanthin (~2 µg/g DW), and adonixanthin (~2 µg/g DW). The KETO2 endosperm at 30 DAP also accumulated lutein (~1 µg/g DW; sixfold increase over wild type), zeaxanthin (~8 µg/g DW; 19-fold increase over wild type), β-carotene (~7 µg/g DW), antheraxanthin (~5 µg/g DW; 113-fold increase over wild type), β-cryptoxanthin (~3 µg/g DW), and violaxanthin (~1 µg/g DW; eightfold over wild type), as well as the upstream, precursors lycopene (~5 µg/g DW) and phytoene (~4 µg/g DW). At 60 DAP, the endosperm of line KETO2 followed the trend observed in CARO1 and CARO2 by accumulating higher amounts of the upstream precursor phytoene (~54 µg/g DW) at the expense of downstream carotenoids. Astaxanthin was still the predominant ketocarotenoid at 60 DAP (~16 µg/g DW), followed by adonirubin (~4 µg/g DW), canthaxanthin (~3 µg/g DW), 3-OH-echinenone (~2 µg/g DW), and adonixanthin (~1 µg/g DW) (Supplementary Table 2). The KETO2 endosperm at 60 DAP also accumulated β-carotene (~12 µg/g DW), zeaxanthin (~6 µg/g DW; 21-fold increase over wild type), and ~1 µg/g DW lutein, the latter much less abundant than in CARO1 and CARO2 (Supplementary Table 1). The hybrid ORxKETO2 had a similar red endosperm color to the KETO2 parent (Fig. 3j) and the carotenoid profile was also similar. ORxKETO2 accumulated ~30 µg/g DW total carotenoids (28 µg/g DW ketocarotenoids) at 30 DAP and ~74 µg/g DW total carotenoids (16 µg/g DW ketocarotenoids) at 60 DAP (Supplementary Tables 1 and 2).
Endogenous carotenoid pathway, MEP pathway, and PFTF gene expression is similar in the hybrids and their parents
The expression of endogenous carotenogenic genes, MEP pathway genes, and PFTF was compared in lines CARO2, KETO2, the corresponding hybrids, and wild-type M37W plants by quantitative RT-PCR (Table 1).
In line CARO2, most of the endogenous genes were expressed at the same level as wild-type line. Four genes were expressed at higher levels in the transgenic plants: ZmCYP97A (twofold), ZmBCH1 (threefold), and ZmLYCB (sixfold), representing the carotenoid pathway, and ZmDXS2 (11-fold) representing the MEP pathway. Furthermore, the PSY1 transcript was ~10,000-fold more abundant in line CARO2 because this was the transgene product. In hybrid ORxCARO2, ZmCYP97A expression was similar to wild-type levels, thus lower than CARO2, and ZmBCH1 expression was similar to CARO2, thus threefold higher than wild type. Two further genes were expressed at higher levels than the wild-type plants but at lower levels than CARO2 (ZmLYCB, ~twofold higher than wild type, and ZmDXS2, sevenfold higher than wild type). Two genes were expressed at higher levels than in either of the parental lines (ZmBCH2, ~threefold higher than wild type, and ZmDXR, ~twofold higher than wild type). As in line CARO2, the ZmPSY1 transcript was much more abundant than in wild-type plants (~5600-fold increase) due to transgene expression.
In KETO2, most of the endogenous genes were expressed at the same level as wild type but six genes were expressed at higher levels in the transgenic plants: ZmLYCB, ZmBCH1, ZmCYP97A, ZmCYP97B, ZmCYP97C, and ZmDXS2 (all ~fourfold). As above, the ZmPSY1 transcript was more abundant (~1000-fold) in the transgenic line due to transgene expression. In hybrid ORxKETO2, ZmLYCE, ZmBCH2, ZmDXS1, ZmDXS3, ZmDXR, and ZmPFTF were expressed at wild-type levels. The ZmCYP97A and ZmCYP97B transcripts were wild-type levels and were therefore less abundant than in the KETO2 parent, whereas the ZmPSY1, ZmPYS2, ZmLYCB, ZmBCH2, ZmHDR, and ZmDXS2 transcripts were present at similar levels to the KETO2 parent and were therefore more abundant than in wild-type plants. The ZmCYP97C transcript was present at similar levels in KETO2, ORxKETO2, and wild-type plants.
In KETO1, most of the endogenous genes were expressed at the same level as wild type but ZmLYCE and ZmBCH1 were downregulated by up to threefold in the transgenic line and similar profiles were observed for most genes in the hybrid ORxKETO1. There were two exceptions: ZmDXS1 mRNA accumulated at significantly higher levels in the hybrid line compared to wild-type plants, and ZmDXR levels remained similar to wild-type levels but were significantly higher compared to KETO1 plants (Table 1).
Higher carotenoid levels in diverse genetic backgrounds promote the creation of a metabolic sink
Transmission electron microscopy (TEM) was used to analyze of the first layer of endosperm cells under the epithelium of wild type, OR2, CARO1, CARO2, KETO1, KETO2, and ORxKETO1 plants at 30 DAP. This revealed electron-dense plastoglobuli inside the plastids. These were scarce in the wild-type and KETO1 lines, which accumulate only low levels of total carotenoids, but much more abundant in the CARO1, CARO2, and KETO2 lines, which have a high-carotenoid content. There were also more plastoglobuli in the OR2 and ORxKETO1 lines compared to the wild-type and KETO1 lines, respectively (Fig. 4).
Micrographs of 30 DAP endosperm from wild-type (WT) and transgenic corn lines OR2, CARO1, CARO2, KETO1, KETO2, and ORxKETO1. A Light micrograph of WT endosperm; arrows indicate aleurone cell layer (B–H). Transmission electron micrographs of WT (B), OR2 (C), CARO1 (D), CARO2 (E), KETO2 (F), KETO1 (G), and ORxKETO1 (H). Arrows indicate plastoglobuli inside plastids m mitochondria, cw cell wall, N nucleus. Scale bar 30 µm (A) and 0.7 μm (B–H)
Discussion
The overexpression of AtOR enhances total carotenoid levels in hybrids of parents with a restricted carotenoid pool
A splicing mutation in the cauliflower orange (or) gene yields a dominant allele (or) that increases carotenoid (particularly β-carotene) accumulation by promoting chromoplast differentiation and creating a metabolic sink (Li et al. 2001). The function is transferable to other plants, e.g., transgenic potato (cv. Desiree) tubers expressing cauliflower or also accumulated β-carotene and other carotenoids, with progressive accumulation during cold storage (Lopez et al. 2008; Li et al. 2012). Interestingly, when the same approach was tested in a different potato variety (cv. Phureja), there was no increase in the carotenoid content during cold storage and the principal carotenoids that accumulated were zeaxanthin, antheraxanthin, violaxanthin, and lutein (Campbell et al. 2015). The distinct behavior of different potato cultivars suggests that endogenous carotenoid profiles may influence the manner in which or gene expression influences carotenoid accumulation in other species.
In rice, the overexpression of AtOR alone had no impact on carotenoid accumulation, but carotenoids accumulated to higher levels in callus (Bai et al. 2014) and seeds (Bai et al. 2016) when AtOR was co-expressed with ZmPSY1 and PaCRTI. This suggested that chromoplast differentiation can be triggered by carotenoid accumulation above a certain threshold and that the presence of the OR protein may augment or potentiate this process but is not sufficient on its own to induce the formation of a metabolic sink (Bai et al. 2014). The sweet potato ortholog IbOR not only increased the β-carotene content of transgenic sweet potato callus, but also increased the levels of α-carotene, lutein, β-crytoxanthin, and zeaxanthin, suggesting that IbOR can influence carotenogenic gene expression. The transgenic IbOR callus also possessed higher antioxidant activity and was more tolerant to salt stress, probably reflecting the increase in carotenoid levels (Kim et al. 2013). The overexpression of IbOR in sweet potato plants increased the total carotenoid content by ~threefold although the composition was not affected, i.e., the wild-type distribution of carotenoids was maintained but the abundance of each carotenoid increased (Goo et al. 2015). The same phenomenon was observed in our OR1 and OR2 lines, but in potato the expression of cauliflower or changed both the content and the composition of carotenoids (Lopez et al. 2008), indicating that the function of or may be context-specific, i.e., in some backgrounds it may influence gene expression and carotenoid composition, but in others it may only induce a sink for existing carotenoids thereby increasing the quantity without affecting composition. In corn hybrids, the impact of AtOR introgression depended on the nature of the carotenoid pool in the carotenogenic parental line. If the parental carotenoid pool was restricted, as was the case for line KETO1, the introgression of AtOR increased the total carotenoid content by up to 22-fold at 30 DAP in the hybrid, again without affecting the carotenoid profile (Supplementary Table 1). This suggests that AtOR enhances carotenoid accumulation in corn but does not alter the underlying metabolic fluxes by modulating the expression of carotenogenic genes in a substantial manner. The carotenoid composition of line KETO1 did not change substantially between 30 and 60 DAP and the ORxKETO1 hybrid behaved in a similar manner, indicating that carotenoid levels remain relatively constant throughout seed development in this line (Fig. 5A). In contrast, when the carotenoid pool in the parent was abundant (i.e., above 70 µg/g DW in the case of CARO1, CARO2, and KETO2), AtOR had no influence on either the carotenoid content or the carotenoid profile at 30 or 60 DAP in the corresponding hybrids. The carotenoid composition of lines CARO1, CARO2, and KETO2 changed between 30 and 60 DAP mainly because of the increase in phytoene levels at the expense of downstream carotenoids such as lutein, zeaxanthin, and antheraxanthin. The behavior of the hybrids was similar to the carotenogenic parents (Fig. 5B). ORs from Arabidopsis (Arabidopsis thaliana), sweet potato (Ipomoea batatas), and melon (Cucumis melo) have been reported to stabilize PSY protein (Zhou et al. 2015; Park et al. 2016; Chayut et al. 2017). In white M37W maize, limited amounts of carotenoids accumulate in the endosperm due to low ZmPSY1 and ZmPSY2 mRNA accumulation. The interaction of AtOR with ZmPSY1 and/or ZmPSY2 might increase endogenous ZmPSY1 and/or ZmPSY2 protein levels via stabilization of PSY protein, resulting in increased carotenoid content in the endosperm of ORxKETO1 compared to KETO1. However, since ZmPSY1 was overexpressed in the endosperm of ORxCARO1, ORxCARO2, and ORxKETO2 hybrids at levels similar in CARO1, CARO2, and KETO2 parental lines, respectively, any post-transcriptional regulation of ZmPSY1 and/or ZmPSY2 protein levels does not appear to cause substantial differences in carotenoid accumulation in the endosperm of CARO1 and ORxCARO1, CARO2 and ORxCARO2, or KETO2 and ORxKETO2.
A Total carotenoid content and composition in wild-type M37W, transgenic lines OR2 and KETO1, and hybrid ORxKETO1 in the T1 generation at 30 DAP (*) and 60 DAP (**) (n = 3–5 seeds). B Carotenoid content and composition in CARO1, CARO2, and KETO2, and hybrids ORxCARO1, ORxCARO2, and ORxKETO2 in the T1 generation at 30 DAP (*) and 60 DAP (**) (n = 3–5 seeds)
AtOR has little impact on endogenous carotenoid and MEP pathway genes and PFTF despite the higher total carotenoid levels
The spontaneous cauliflower mutation or does not induce changes in endogenous carotenogenic gene expression (Li et al. 2001, 2006; Lu et al. 2006). The cauliflower OR protein has been shown to stabilize the enzyme phytoene synthase, enhancing its activity and allowing the continuous biosynthesis of carotenoids during storage (Zhou et al. 2015). The cauliflower OR protein was suggested to function in association with the molecular chaperone system to promote protein folding (Li et al. 2012). More recently, the AtOR protein has been shown to function as a major post-transcriptional regulator of phytoene synthase activity and can modulate carotenoid biosynthesis in this manner (Zhou et al. 2015). In contrast, the overexpression of IbOR in sweet potato callus increased carotenoid accumulation by inducing the endogenous carotenoid biosynthesis genes IbPSY1, IbCRTISO, IbLYCB, and IbBCH (Kim et al. 2013). Similarly, the overexpression of IbOR in an anthocyanin-rich purple-fleshed cultivar induced the carotenogenic genes IbPDS, IbZDS, IbLYCB, IbBCH, and IbZEP. Interestingly, IbLYCE expression was suppressed, whereas the genes encoding carotenoid cleavage dioxygenases (IbCCD1 and IbCCD4) and 9-cis-epoxycarotenoid dioxygenases (NCED) were induced (Goo et al. 2015). The overexpression of AtOR in rice endosperm induced the endogenous carotenogenic genes OsLYCE, OsLYCB, and OsBCH2 (Bai et al. 2016). These results suggested that AtOR may increase carotenoid levels in some backgrounds but not others by boosting endogenous carotenoid biosynthesis at the transcriptional level.
In our corn plants, the detailed analysis of carotenogenic gene expression revealed no dramatic changes in transcript levels between wild-type and AtOR transgenic lines, except the ~twofold downregulation of ZmLYCE, which correlates with the high zeaxanthin levels in the transgenic lines reflecting the redirection of flux from the α-branch to the β-branch of the pathway (Fig. 2). The downregulation of ZmLYCE was also observed in KETO1 (threefold decrease compared to wild type), suggesting that extending the carotenoid pathway to ketocarotenoids directs flux to the β-branch of the pathway. ZmLYCE was also downregulated when AtOR was introgressed into a more complex corn line expressing a carotenoid β-hydroxylase (sBrCRTZ) and two β-carotene ketolases (sCrBKT and sBrCRTW). ZmLYCE expression in the ORxKETO1 hybrid was similar to the OR2 and KETO1 parents (Table 1) but remained similar to wild-type levels in the CARO1, CARO2, and KETO2 lines and their corresponding hybrids (ORxCARO1, ORxCARO2, and ORxKETO2), suggesting that AtOR does not affect endogenous carotenogenic gene expression in these lines. Furthermore, ZmLYCB was induced in the transgenic lines overexpressing carotenogenic genes (CARO1, CARO2, and KETO2) and their corresponding hybrids (ORxCARO1, ORxCARO2, and ORxKETO2) suggesting that the overexpression of carotenogenic genes in CARO1 (ZmPSY1), CARO2 (ZmPSY1, PaCRTI, and GlLYCB) and KETO2 (ZmPSY1, sCRBKT, and sBRCRTZ) directs flux through the β-branch of the pathway in the M37W background.
The influence of AtOR on early steps in the carotenoid pathway was investigated by analyzing the expression of endogenous MEP pathway genes (ZmDXS1, ZmDXS2, ZmDXS3, ZmDXR, and ZmHDR). No changes were detected in line OR2 except a ~twofold upregulation of ZmDXS1, suggesting that flux towards the carotenoid pathway increases slightly due to AtOR, thus promoting the biosynthesis of all carotenoids (Fig. 2). Similar results were observed when AtOR was introgressed into the hybrid ORxKETO1, but ZmDXS1 transcript levels remained at wild-type levels in transgenic lines overexpressing carotenogenic genes (CARO1, CARO2 and KETO2) and their corresponding hybrids (Table 1). However, ZmDXS2 transcript levels increased in the same three lines and their hybrids, suggesting that the overexpression of carotenogenic genes causes positive feedback regulation and increases metabolic flux through the carotenoid pathway in the M37W background. In A. thaliana callus, AtOR marginally increased the expression of AtDXS (~1.2-fold) and AtDXR (~1.3-fold) compared to wild-type callus (Yuan et al. 2015).
The plastid fusion/translocation factor (PFTF) is required for chromoplast differentiation in red pepper (Hugueney et al. 1995). The overexpression of IbOR increased PFTF transcript levels in sweet potato callus and tubers, suggesting that IbOR can trigger chromoplast formation via PFTF (Kim et al. 2013; Goo et al. 2015). However, PFTF transcript levels were not affected in our corn hybrids expressing AtOR suggesting that AtOR does not trigger chromoplast formation by itself in corn (Fig. 5).
High levels of nascent carotenoids rather than AtOR gene expression trigger the formation of carotenoid-rich plastoglobuli in corn endosperm
Cauliflower or is the only known gene that acts as a bona fide molecular switch to trigger the differentiation of non-colored plastids into chromoplasts (Li et al. 2006, 2012; Lu et al. 2006). Experimental evidence to support the role of cauliflower or was provided by the formation of chromoplast-containing carotenoid-sequestering structures in potato tubers expressing the dominant allele or, whereas chromoplasts were not formed in potato cultivars accumulating high levels of carotenoids in the absence of or expression (Lopez et al. 2008). The replacement of a single amino acid in the wild-type AtOR sequence greatly enhanced its ability to promote carotenoid accumulation by triggering the biogenesis of membranous chromoplasts in A. thaliana callus without altering carotenogenic gene expression (Yuan et al. 2015). In contrast, morphological changes in plastids have been observed in tomato fruits and canola endosperm expressing phytoene synthase (Shewmaker et al. 1999; Fraser et al. 2007; Nogueira et al. 2013). These observations suggest that plastids are modified to accommodate higher levels of carotenoids. The overexpression of phytoene synthase can also induce crystalloid carotenoid-sequestering structures in A. thaliana callus, suggesting that the chromoplast differentiation program may respond to the accumulation of carotenoids above a certain threshold, unless differentiation is triggered by AtOR before this threshold is reached (Maass et al. 2009). In transgenic rice, AtOR expression might influence carotenoid levels directly as well as indirectly. Chromoplast differentiation is primarily triggered by carotenoid accumulation above a certain threshold and the presence of AtOR protein may augment and potentiate this process. However, the expression of AtOR alone in rice is not sufficient to trigger chromoplast differentiation (Bai et al. 2014). Storage organs in many species synthesize and deposit carotenoids primarily in the membranes of amyloplasts (Li et al. 2012), which sequester β-carotene in a crystalloid metabolic sink (Cao et al. 2012). These structures have two roles: first, they sequester excess carotenoids and keep them away from plastid membranes, thus stimulating continuous carotenoid biosynthesis, and second, they provide a stable metabolic sink that protects carotenoids from degradation (Li et al. 2012). Amyloplasts in corn contain carotenoids (Wurtzel 2004). In our experiments, we observed amyloplasts and other plastids with electron-dense plastoglobuli that might contain residual levels of carotenoids in a low-carotenoid genetic background (M37W and KETO1). However, more plastoglobuli were observed in high-carotenoid genetic backgrounds (CARO1, CARO2, and KETO2). Furthermore, there were fewer plastids containing plastoglobuli, as well as fewer plastoglobuli inside the plastids, in the OR2 and KETO1 lines with limited carotenoid pools, suggesting that plastoglobuli in corn are formed due to higher carotenoid levels. AtOR expression in A. thaliana callus does not change plastid morphology per se but a single amino acid substitution in AtOR yielded plastids with larger and more electron-dense plastoglobuli. The mutant AtOR triggered biogenesis of membranous chromoplasts in the Arabidopsis calli, which shared structures similar to chromoplasts found in the curd of the orange cauliflower (Brassica oleracea) mutant (Yuan et al. 2015).
In summary, the overexpression of AtOR in the white-endosperm inbred corn line M37W increased the carotenoid content of the endosperm without affecting the expression of endogenous carotenogenic or MEP pathway genes, except for the induction of ZmDXS1. Crossing the best-performing AtOR line with four different transgenic parental lines accumulating different levels of carotenoids and/or ketocarotenoids yielded hybrids with the same qualitative carotenoid profiles as their parents. The total carotenoid levels in the hybrid increased when the carotenoid pool in the parent was restricted but there was no significant change when carotenoids were already abundant. The overexpression of ZmPSY1 alone or in combination with other carotenogenic genes induced the formation of plastoglobuli that provide a metabolic sink for carotenoids inside the plastids, and similar structures were observed in the original AtOR line. However, the abundance of the plastids and the plastoglobuli within them appeared to be dependent on the carotenoid content. It therefore appears that in corn, as previously shown in rice, unlike the cauliflower orange (or) gene mutant version, the AtOR gene is not sufficient on its own to induce the differentiation of proplastids into chromoplasts en masse.
Experimental procedures
Gene cloning and vector construction
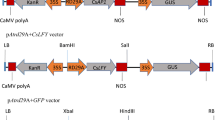
Vectors carrying the A. thaliana ORANGE (AtOR) and Chlamydomonas reinhardtii bkt (sCrBKT) genes were previously described by Bai et al. (2014). Vectors carrying the Brevundimonas sp. SD212 (MBIC 03018) β-carotene hydroxylase gene (sBrCRTZ) and the RNAi construct to block lycopene ε-cyclase expression were previously described by Farré et al. (2016). Vectors carrying the ZmPSY1, GlLYCB, and PaCRTI genes were previously described by Zhu et al. (2008). The vector carrying the Brevundimonas sp. SD212 β-carotene ketolase (sBrCRTW) was previously described by Breitenbach et al. (2014). The vectors are shown in Supplementary Fig. 3.
Corn transformation and plant growth
Transgenic corn plants expressing AtOR (lines OR1 and OR2); ZmPSY1 (line CARO1), ZmPSY1, PaCRTI, and GlLYCB (line CARO2); sBrCRTZ, sBrCRTW, and sCrBKT (line KETO1); and ZmPSY1, LYCE-RNAi, sCrBKT, and sBrCRTZ (line KETO2) were generated as previously described (Zhu et al. 2008; Farré et al. 2016). The best-performing line expressing each transgene combination was selected by RNA blot analysis as previously described (Zhu et al. 2008; Farré et al. 2016). Each line was self-pollinated to homozygosity and used as a parental line for hybridization. The CARO1, CARO2, KETO1, and KETO2 lines were outcrossed with an OR pollen donor to obtain hybrids ORxCARO1, ORxCARO2, ORxKETO1, and ORxKETO2. For further analysis, endosperm samples were taken from immature seeds at 30 and 60 DAP, frozen in liquid nitrogen, and stored at −80 °C.
Synthesis of cDNA and real-time quantitative RT-PCR
The synthesis of cDNA and real-time quantitative RT-PCR were carried out as reported by Naqvi et al. (2011) using the primers listed in Supplementary Table 3.
Carotenoid extraction and analysis
Corn endosperm was excised by removing the seed coat and embryo. Samples were freeze-dried before extraction, and 3–5 seeds per sample were ground to a fine powder. Samples of 50–100 mg were extracted in 15 ml methanol:ethyl acetate (6:4 v/v) at 58 °C for 20 min. The mixture was filtered, transferred to a funnel, and 15 ml hexane:diethyl ether (9:1 v/v) was added followed by gentle agitation for 1 min. The organic phase was washed twice with saturated NaCl, and the aqueous phase was removed. The samples were dried under N2 and stored at −80 °C.
The extracts were dissolved in 210–600 μl an injection solvent comprising three volumes of acetonitrile/methanol (7:3 v/v) and two volumes of acetone. The extracted carotenoids were separated by ultra-high performance liquid chromatography (UHPLC) at SCT-DATCEM (University of Lleida, Spain) using an ACQUITY Ultra Performance LC system linked to a PDA 2996 detector (Waters, Milford, USA). Mass detection was carried out using an ACQUITY TQD tandem-quadrupole MS equipped with a Z-spray electrospray interface (Waters). MassLynx software v4.1 (Waters) was used to control the instruments and also for data acquisition and processing. UHPLC separations were performed on a reversed-phase ACQUITY UPLC C18 BEH 130 Å, 1.7 μm, 2.1 × 150 mm column (Waters). The mobile phase consisted of solvent A (acetonitrile:methanol, 7:3 v/v) and solvent B (100% water). Carotenoids were quantified using a PDA detector and the external standard method (Rivera et al. 2013). MS analysis was conducted by atmospheric pressure chemical ionization (APCI) as described by Rivera et al. (2011). The following authentic standards were used for quantification: β-carotene, lutein, β-cryptoxanthin, and astaxanthin (Sigma, St Louis, MO, USA); zeaxanthin (Fluka, Buchs SG, Switzerland); phytoene and antheraxanthin (Carotenature, Lupsingen, Switzerland).
Transmission electron microscopy
Corn endosperm at 30 DAP (0.5 × 2.0 mm pieces) was processed as described by Bai et al. (2014).
References
Bai C, Rivera SM, Medina V, Alves R, Vilaprinyo E, Sorribas A et al (2014) An in vitro system for the rapid functional characterization of genes involved in carotenoid biosynthesis and accumulation. Plant J 77:464–475
Bai C, Capell T, Berman J, Medina V, Sandmann G, Christou P et al (2016) Bottlenecks in carotenoid biosynthesis and accumulation in rice endosperm are influenced by the precursor-product balance. Plant Biotechnol J 14:195–205
Berman J, Zorrilla-Lopez U, Farré G, Zhu C, Sandmann G, Twyman RM, Capell T, Christou P (2015) Nutritionally important carotenoids as consumer products. Phytochem Rev 14:727–743
Breitenbach J, Bai C, Rivera SM, Canela R, Capell T, Christou P, Zhu C, Sandmann G (2014) A novel carotenoid, 4-keto-α-carotene, as an unexpected by-product during genetic engineering of carotenogenesis in rice callus. Phytochemistry 98:85–91
Campbell R, Morris WL, Mortimer CL, Misawa N, Ducreux, LJM, Morris JA et al (2015) Optimising ketocarotenoid production in potato tubers: effect of genetic background, transgene combinations and environment. Plant Sci 234:27–37
Cao H, Zhang J, Xu J, Ye J, Yun Z, Xu Q et al (2012) Comprehending crystalline β-carotene accumulation by comparing engineered cell models and the natural carotenoid-rich system of citrus. J Exp Bot 63:4403–4417
Chayut N, Yuan H, Ohali S, Meir A, Sa’ar U, Tzuri G, Zheng Y, Mazourek M Gepstein S, Zhou X, Portnoy V, Lewinsohn E, Schaffer AA, Katzir N, Fei Z, Welsch R, Li L, Burger J, Tadmor Y (2017) Distinct mechanisms of the ORANGE protein in controlling carotenoid flux. Plant Physiol 173:376–389
Crisp P, Walkey DGA, Bellman E, Roberts E (1975) A mutation affecting curd colour in cauliflower (Brassica oleracea L var. Botrytis DC). Euphytica 24:173–176
Enfissi, EMA, Fraser PD, Lois LM, Boronat A, Schuch W, Bramley PM (2005) Metabolic engineering of the mevalonate and non-mevalonate isopentenyl diphosphate-forming pathways for the production of health-promoting isoprenoids in tomato. Plant Biotechnol J 3:17–27
Farré G, Perez-Fons L, Decourcelle M, Breitenbach J, Hem S, Zhu C, Capell T, Christou P, Fraser PD, Sandmann G (2016) Metabolic engineering of astaxanthin biosynthesis in maize endosperm and characterization of a prototype high oil hybrid. Transgenic Res 25:477–489
Fraser PD, Enfissi, EMA, Halket JM, Truesdale MR, Yu D, Gerrish C et al (2007) Manipulation of phytoene levels in tomato fruit: effects on isoprenoids, plastids, and intermediary metabolism. Plant Cell 19:3194–3211
Goo YM, Han EH, Jeong JC, Kwak SS, Yu J, Kim YH et al (2015) Overexpression of the sweet potato IbOr gene results in the increased accumulation of carotenoid and confers tolerance to environmental stresses in transgenic potato. C R Biol 338:12–20
Hugueney P, Bouvier F, Badillo A, D’Harlingue A, Kuntz M, Camara B (1995) Identification of a plastid protein involved in vesicle fusion and/or membrane protein translocation. Proc Natl Acad Sci USA 92:5630–5634
Jayaraj J, Devlin R, Punja Z (2008) Metabolic engineering of novel ketocarotenoid production in carrot plants. Transgenic Res 17:489–501
Kim SH, Ahn YO, Ahn MJ, Jeong JC, Lee HS, Kwak SS (2013) Cloning and characterization of an Orange gene that increases carotenoid accumulation and salt stress tolerance in transgenic sweetpotato cultures. Plant Physiol Biochem 70:445–554
Li L, Paolillo DJ, Parthasarathy MV, Dimuzio EM, Garvin DF, Plant US et al (2001) A novel gene mutation that confers abnormal patterns of β-carotene accumulation in cauliflower (Brassica oleracea var. botrytis). Plant J 26:59–67
Li L, Shan L, Cosman KM, Earle ED, Garvin DF, O’Neill J (2006) β-Carotene accumulation induced by the cauliflower Or gene is not due to an increased capacity of biosynthesis. Phytochemistry 67:1177–1184
Li L, Yang Y, Xu Q, Owsiany K, Welsch R, Chitchumroonchokchai C et al (2012) The or gene enhances carotenoid accumulation and stability during post-harvest storage of potato tubers. Mol Plant 5:339–352.
Lopez AB, Van Eck J, Conlin BJ, Paolillo DJ, O’Neill, JLL (2008) Effect of the cauliflower Or transgene on carotenoid accumulation and chromoplast formation in transgenic potato tubers. J Exp Bot 59:213–223
Lu S, Van Eck J, Zhou X, Lopez AB, O’Halloran DM, Cosman KM et al (2006) The cauliflower Or gene encodes a DnaJ cysteine-rich domain-containing protein that mediates high levels of beta-carotene accumulation. Plant Cell 18:3594–3605
Maass D, Arango J, Wüst F, Beyer P, Welsch R (2009) Carotenoid crystal formation in Arabidopsis and carrot roots caused by increased phytoene synthase protein levels. PLoS ONE 4:e6373
Morris WL, Ducreux, LJM, Hedden P, Millam S, Taylor MA (2006) Overexpression of a bacterial 1–deoxy-D–xylulose 5–phosphate synthase gene in potato tubers perturbs the isoprenoid metabolic network: implications for the control of the tuber life cycle. J Exp Bot 57:3007–3018
Naqvi S, Zhu C, Farre´ G et al (2011) Synergistic metabolism in hybrid corn indicates bottlenecks in the carotenoid path- way and leads to the accumulation of extraordinary levels of the nutritionally important carotenoid zeaxanthin. Plant Biotechnol J 9:384–393
Nogueira M, Mora L, En, EMA, Bramley PM, Fraser PD (2013) Subchromoplast sequestration of carotenoids affects regulatory mechanisms in tomato lines expressing different carotenoid gene combinations. Plant Cell 25:1–20
Paolillo DJ, Garvin DF, Parthasarathy MV (2004) The chromoplasts of Or mutants of cauliflower (Brassica oleracea L var. botrytis). Protoplasma 224:245–253
Park, S, Kim HS, Jung Y, Kim SH, Ji CY, Wang Z, Jeong JC, Lee HS, Lee SY, Kwak SS (2016) Orange protein has a role in phytoene synthase stabilization in sweetpotato. Sci Rep 16:33563. doi:10.1038/srep33563
Rivera S, Vilaró F, Canela R (2011) Determination of carotenoids by liquid chromatography/mass spectrometry: effect of several dopants. Anal Bioanal Chem 400:1339–1346
Rivera SM, Vilaró F, Zhu C, Bai C, Farré G, Christou P, Canela-Garayoa R (2013) Fast quantitative method for the analysis of carotenoids in transgenic maize. J Agric Food Chem 61:5279–5285
Shewmaker CK, Sheehy JA, Daley M, Colburn S, Ke DY (1999) Seed-specific overexpression of phytoene synthase: Increase in carotenoids and other metabolic effects. Plant J 20:401–412
Wurtzel ET (2004) Chapter five genomics, genetics, and biochemistry of maize carotenoid biosynthesis. Recent Adv Phytochem 38:85–110
Yu D, Lydiate DJ, Young LW et al (2008) Enhancing the carot enoid content of Brassica napus seeds by downregulating lycopene epsilon cyclase. Transgenic Res 17:573–585
Yuan H, Owsiany K, Sheeja TE, Zhou X, Rodriguez C, Li Y et al (2015) A Single amino acid substitution in an ORANGE protein promotes carotenoid overaccumulation in arabidopsis. Plant Physiol 169:421–431
Zhou X, Welsch R, Yang Y, Álvarez D, Riediger M, Yuan H et al (2015) Arabidopsis OR proteins are the major posttranscriptional regulators of phytoene synthase in controlling carotenoid biosynthesis. Proc Natl Acad Sci USA 112:3558–3563
Zhu C, Naqvi S, Breitenbach J, Sandmann G, Christou P, Capell T (2008) Combinatorial genetic transformation generates a library of metabolic phenotypes for the carotenoid pathway in maize. Proc Natl Acad Sci USA 105:18232–18237
Zhu C, Bai C, Sanahuja G et al (2010) The regulation of carotenoid pigmentation in flowers. Arch Biochem Biophys 504:132–141
Acknowledgements
Research at the Universitat de Lleida is supported by MINECO, Spain (BIO2014-54426-P; BIO2014-54441-P), by the Catalan Government (2014 SGR 1296 Agricultural Biotechnology Research Group), and by European Union Framework 7, European Research Council IDEAS Advanced Grant BIOFORCE and POC Grant (to PC). G. Farré is supported by a J de la C fellowship.
Author’s contribution
CZ, GS, TC, and PC designed research; JB, UZ, and GF performed research, analyzed data, and wrote the article; VM did TEM and analyzed these data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Salim Al-Babili.
Electronic supplementary material
Below is the link to the electronic supplementary material.
299_2017_2126_MOESM1_ESM.docx
Supplementary material 1 Supplementary Figure 1—RNA blot analysis of AtOR mRNA in wild type (M37W) and two different transgenic lines (OR1 and OR2) transformed with the AtOR gene driven by the wheat low-molecular-weight glutelin promoter. Each lane was loaded with 25 μg total RNA isolated from endosperm tissue Ribosomal RNA stained with ethidium bromide is shown as a loading control. Supplementary Figure 2—Transcript accumulation normalized against ACTIN mRNA in wild-type and transgenic lines presented as means of three technical replicates. Standard error bars were not included due to the use of technical replicates rather than biological replicates (the aim was to confirm transgene expression rather than to compare different transcript profiles). Supplementary Figure 3—Schematic representation of the transgenes expressed in our transgenic plants lines and hybrids. Supplementary Table 1—Carotenoid content and composition in wild-type M37W, transgenic lines OR2, CARO1, CARO2, KETO1 and KETO2, and hybrids ORxCARO1, ORxCARO2, ORxKETO1 and ORxKETO2 (T1 plants) at 30 (*) and 60 DAP (**) (µg/g DW±SE) (n = 3–5 seeds). Abbreviations: Phyt, phytoene; Lyco, lycopene; βcryp, β-cryptoxanthin; βcaro, β-carotene; Lut, lutein; Zea, zeaxanthin; Anthe, antheraxanthin; Viola, violaxanthin; CAROT, carotenoids. Supplementary Table 2—Ketocarotenoid content and composition of wild-type M37W, transgenic lines OR2, CARO1, CARO2, KETO1 and KETO2, and hybrids ORxCARO1, ORxCARO2, ORxKETO1 and ORxKETO2 T1 at 30 (*) and 60 DAP (**) (µg/g DW±SE) (n = 3–5 seeds). Abbreviations: Asta, astaxanthin; Cantha, canthaxanthin; Adonir, adonirubin; Adonix, adonixanthin; 3OHechi, 3-OH-echinenone; KETO, ketocarotenoids. Supplementary Table 3—Oligonucleotide sequences of corn ACTIN, endogenous carotenogenic genes and transgenes for real-time quantitative RT-PCR analysis. (DOCX 114 KB)
Rights and permissions
About this article
Cite this article
Berman, J., Zorrilla-López, U., Medina, V. et al. The Arabidopsis ORANGE (AtOR) gene promotes carotenoid accumulation in transgenic corn hybrids derived from parental lines with limited carotenoid pools. Plant Cell Rep 36, 933–945 (2017). https://doi.org/10.1007/s00299-017-2126-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-017-2126-z