Abstract
Transgenic ‘Duncan’ grapefruit (Citrus paradisi Macf.) and ‘Valencia’ sweet orange (Citrus sinensis [L.] Osbeck) plants ectopically expressing C. sinensis (cv. Washington navel orange) APETALA1 (CsAP1) or LEAFY (CsLFY) genes under control of the Arabidopsis thaliana stress-inducible promoter AtRD29A flowered under non-inductive (warm temperature, well-watered) greenhouse conditions, whereas their wild-type (WT) counterparts did not. The transgenic plants that flowered exhibited no altered morphological features, except the lack of thorns characteristic of juvenile WT plants. The most precocious T0 line, ‘Duncan’ grapefruit (Dun134-3) expressing the CsAP1 gene, flowered and fruited when it was 4.5 years old and the T1 siblings from this line flowered and fruited when they were just over 18 months old. In contrast, T1 seedlings from three lines of ‘Duncan’ grapefruit expressing the CsLFY gene flowered within 3 months after germination, but were unable to support fruit development. Transcript levels of corresponding transgenes in leaves were not correlated with earliness of flowering. To further study the activity of AtRD29A, leaves from three ‘Carrizo’ citrange (C. sinensis × Poncirus trifoliata) rootstock seedlings transformed with the green fluorescent protein (GFP) gene under regulation of the AtRD29A promoter were subjected to drought stress or well-watered conditions. Expression of GFP was not stress-dependent, consistent with the observation of flowering of CsAP1 and CsLFY transgenic plants under non-inductive conditions. Taken together, the results suggest that AtRD29A is constitutively expressed in a citrus background. Despite the loss of control over flowering time, transgenic citrus lines ectopically expressing C. sinensis AP1 or LFY genes under control of the A. thaliana RD29A promoter exhibit precocious flowering, fruit development and viable transgenic seed formation. These transformed lines can be useful tools to reduce the time between generations to accelerate breeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Citrus spp., hybrids and relatives are evergreen woody perennials with a juvenile phase that ranges from 5 to 13 years (Davies and Albrigo 1994). Moreover, genetic control of the process of floral induction resulting in formation of floral meristems and final development of individual flowers to produce fruit is complex and protracted (Ma 1994). A long juvenile phase and complex floral developmental process impedes conventional breeding based on crossing. Thus, a major goal of commercial tree crop improvement is to reduce the juvenile phase and favor precocious flowering to shorten the time between generations (Pillitteri et al. 2004a). As the worldwide citrus industry fights to survive huanglongbing (HLB), citrus canker, and other lingering and emerging diseases and pests, as well as the negative effects of climate change, rapid cultivar improvement has become even more important. Genetic transformation offers the fastest and most direct method for introduction of desired traits into elite citrus cultivars, with newer technologies (e.g., horizontal gene transfer, constitutive expression of chimeric proteins, and gene stacking) showing promise (Salonia et al. 2020; Sinn et al. 2020). Having plants with a short juvenile phase would facilitate citrus crop improvement.
The “FasTrack” breeding system for plum (Prunus domestica) utilizes plants overexpressing Populus tremuloides FLOWERING LOCUS T (PtFT), a floral timing gene, to shorten the juvenile phase and accelerate flowering (Petri et al. 2018). In apple (Malus × domestica), overexpression of a birch (Betula pendula) floral meristem identity gene was used to produce early-flowering plants to introgress disease resistance (Flachowksy et al. 2007; Le Roux et al. 2012). For citrus, creation of early-flowering phenotypes, the first step required for application of “Fast Track” breeding, has been achieved through ectopic expression of A. thaliana AP1 (AtAP1) and LFY (AtLFY) in two citrus rootstock cultivars, ‘Carrizo’ citrange and trifoliate orange (Poncirus trifoliata) (Pena et al. 2001; Endo et al. 2005; Cervera et al. 2009) and the scion cultivar ‘Meiwa’ kumquat (Fortunella crassifolia) (Duan et al. 2010); and also with the expression of C. unshiu FT (CiFT) in trifoliate orange (Endo et al. 2005). In addition, the potential for gene-stacking of selected traits was demonstrated with ‘Carrizo’ citrange rootstocks overexpressing the AtAP1 transgene (Cervera et al. 2009). However, to date, there is no early-flowering phenotype of a scion cultivar of commercial importance to the global citrus industry for use in “Fast Track” breeding.
Comparative studies suggest the genetic network regulating the flowering process is largely conserved among plant species (Benlloch et al. 2007; Jack 2004). The following genes, which are homologous to those found in A. thaliana, have been cloned from Citrus spp.: TERMINAL FLOWER1 (TFL1), FT, SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1), LFY, and AP1 (Endo et al. 2005; Samach 2012; Pillitteri et al. 2004a, b; Tan and Swain 2007). When ectopically expressed in A. thaliana, CsTFL1 delayed flowering (Pillitteri et al. 2004a), whereas the citrus homologs of FT, SOC1, LFY and AP1 resulted in precocious flowering (Endo et al. 2005; Pillitteri et al. 2004b; Tan and Swain 2007). In addition, overexpression of CsAP1 or CsLFY complemented respective delayed flowering-time mutants of A. thaliana (Pillitteri et al. 2004b). Thus, in A. thaliana, ectopic expression of either CsAP1, or CsLFY was sufficient to promote early flowering and convert the vegetative shoot apical meristem (SAM) to an inflorescence and the terminal bud to a flower (Pillitteri et al. 2004b). In Citrus spp., expression of CsLFY has been documented to regulate floral timing through the integration of floral induction pathways, and both CsLFY and CsAP1 have roles in floral meristem determinacy and subsequent downstream floral organogenesis (Nishikawa 2013). CsAP1 also controls the response of the SAM to factors that promote or inhibit flowering (Goldberg-Moeller et al. 2013; Tang and Lovatt 2019).
The research reported herein was undertaken to produce transgenic Citrus spp. (C. paradisi and C. sinensis) important to the global citrus industry that have an early-flowering, early-fruiting phenotype with improved control over flowering time than is typically attained with the use of a constitutive promoter, such as 35S CaMV (Behnman et al. 2006; Bihmidine et al. 2012; Qiu et al. 2012). Thus, in the current research, the stress-inducible AtRD29A promoter was used to control the expression of the CsAP1 and CsLFY genes and precocious flowering. Since the AtRD29A promoter is activated by low temperature, drought and salinity stress (Behnman et al. 2006; Bihmidine et al. 2012; Msanne et al. 2011; Qiu et al. 2012; Yamaguchi-Shinozaki and Shinozaki 2005), in theory flowering should occur in successfully transformed lines only in response to stress and its alleviation, making it possible to upregulate transgene expression and the floral development process when the transgenic plants had reached a size able to support the full development of fruit. The goal of the research was to produce transgenic lines of commercially important citrus scion cultivars with fast flowering and fruit bearing capabilities that might allow researchers to more rapidly examine phenotypic changes associated with commercially important traits related to fruit quality, disease and pest resistance, abiotic stress tolerance, and others through the use of gene stacking technology.
To meet this goal, the first objective of the current research was to create constructs that placed the expression of CsAP1 and CsLFY under control of the A. thaliana stress-inducible AtRD29A promoter and use these constructs to produce transgenic lines of ‘Duncan’ grapefruit (C. paradisi) and ‘Valencia’ sweet orange (C. sinensis). The second objective was to use low temperature stress or drought to control levels of transgene expression to regulate the floral development process and induce precocious flowering. Despite the outcome that the AtRD29A promoter was not induced by stress, but constitutively expressed in a citrus background, transgenic lines of ‘Duncan’ grapefruit and ‘Valencia’ sweet orange ectopically expressing C. sinensis AP1 or LFY exhibited precocious flowering, fruit development, and formation of viable transgenic seeds, more notably in the T1 generation. These transformed lines can be useful tools to reduce the time between generations to accelerate breeding.
Materials and methods
Plant material and growth conditions for transgenic plants
‘Duncan’ grapefruit (Citrus paradisi Macf.) and ‘Valencia’ sweet orange (Citrus sinensis (L.) Osbeck) scion cultivars, and ‘Carrizo’ citrange rootstock (Citrus sinensis [L.] Osbeck × Poncirus trifoliata [L.] Raf.) were selected for the research. All three cultivars are commercially important to the worldwide citrus industry. In addition, the high frequency of nucellar embryony in ‘Duncan’ grapefruit (Holland et al. 1996) and ‘Valencia’ orange (Koltunow et al. 1995) increased the probability that T1 and T2 generation plants would be derived from nucellar tissue and be genetically identical (clones) to the mother plant. Seeds from fruit collected from each cultivar were peeled, surface-sterilized and planted in tubes with MS medium (Murashige and Skoog 1962). Thirty days after seed germination, the seedlings were transferred to white light (60 μmol m−2 s−1) for 3 days to etiolate before being cut into explants used in genetic transformation.
Construction of T-DNA regions of binary vectors
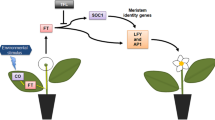
All three binary vectors were derivatives of pCAMBIA2301 (Fig. 1). The A. thaliana RD29A gene promoter fragment (750 bp) was amplified and cloned in the BamHI/PstI sites of pGEM-T Easy vector (Promega) for sequencing. Once the sequence was verified, the AtRD29A promoter was digested with BamHI/Pst, and ligated at the 5′-end of the CsLFY cDNA (Pillitteri et al. 2004b) cloned in pBS-SK. The NOS terminator (300 bp) was amplified from the Agrobacterium binary vector pCAMBIA 2301 and ligated at the 3′-end of the CsLFY cDNA (Pillitteri et al. 2004b) in pBS-SK. The AtRD29A-CsLEAFY-NOS gene cassette was finally digested out of pBS-SK with BamHI/SalI and cloned into pCAMBIA 2301 for plant transformation. The same strategy was applied for construction of the gene cassette containing the AtRD29A promoter, CsAP1 cDNA (Pillitteri et al. 2004b) and the NOS terminator. This gene cassette was also inserted into BamHI/SalI sites of pCAMBIA 2301. For the production of the AtRD29A-eGFP-NOS gene cassette, eGFP was amplified from the pLMNC95 plasmid (Mankin and Thompson 2001). After completion, the cassette was inserted XbaI/HindIII sites of pCAMBIA 2301.
Schematic presentation of T-DNA regions of binary vectors AtRD29A+CsAP1, AtRD29A+CsLFY, and AtRD29A+GFP used for transformation of citrus plants. All these vectors were derivatives of pCAMBIA2301. LB-left border, RB-right border, CsAP1-C. sinensis APETALA1 gene, CsLFY-C. sinensis LEAFY gene, GFP-green fluorescent protein gene, GUS-beta glucuronidase gene, KanR-nptII kanamycin resistance gene, 35S-Cauliflower mosaic virus promoter, RD29A-AtRD29 gene promoter, NOS-nopaline synthase gene terminator, CaMV polyA- Cauliflower mosaic virus polyadenylation sequence
Genetic transformation
For production of transgenic plants, we followed a protocol routinely used for citrus transformation (Orbović and Grosser 2006). Etiolated seedlings were used as the starting material. The stems of seedlings were cut into 15 to 20 mm-long explants that were placed into liquid co-cultivation medium (CCM) (Orbović and Grosser 2006) for 2 to 3 h prior to co-cultivation with Agrobacterium tumefaciens. Explants were soaked in freshly prepared Agrobacterium suspension for 3 min, then incubated on plates with solid CCM medium for 2 days. Explants were then transferred to regeneration medium (RM) (Orbović and Grosser 2006) with the antibiotic Cefotaxime (330 mg L−1) to eliminate Agrobacterium and Kanamycin (70 mg L−1) to suppress the growth of non-transformed shoots. Control wild type shoots were regenerated from non-inoculated explants on RM medium without antibiotics. Thin cross-sections of shoots that sprouted from explants, which had been co-incubated with Agrobacterium strains carrying different binary vectors, were used for GUS histochemical assay to reconfirm the presence of the ß-glucuronidase reporter gene present in the T-DNA of all pCAMBIA 2301 binary vector constructs. Pieces of leaves from transgenic plants were also tested by GUS assay (for details on GUS assay see Orbović and Grosser 2006).
Transgenic T0 shoots were micro-grafted on clonal ‘Carrizo’ citrange seedling rootstocks. The newly-obtained transgenic plants were kept in the greenhouse where they were watered and fertilized on a regular basis. In the period between mid-March and mid-November, these plants were fertilized two times a week and watered once a week. During the rest of the year, plants were fertilized once a week and watered once a week. The temperature in the greenhouse was never below 15.5 °C for longer than few minutes, i.e., the time needed for the thermostat to detect the low temperature threshold and turn on the heaters.
Analysis of CsAP1 and CsLFY genes expression levels
Transcript levels of CsAP1 and CsLFY in transgenic plants were estimated with qRT-PCR. Total RNA was extracted from leaves of transgenic ‘Duncan’ grapefruit and ‘Valencia’ orange plants with the RNeasy plant mini kit (Qiagen) and subjected to RT-qPCR analysis according to the manufacturer's instructions. cDNA was synthesized from 1 μg of RNA using the iScript cDNA Synthesis kit (Bio-Rad Labs). C. sinensis primers were used to amplify the Actin housekeeping gene fragment as a reference for normalization of transcripts of the CsAP1 and CsLFY target genes (Table 1). The qPCR validations were carried out using the iTaq Universal SYBR Green mix (Bio-Rad Labs) under the following conditions: 95 °C for 2 min denaturation, 42 cycles at 95 °C for 5 s, 57 °C for 10 s and 72 °C for 15 s. Amplification specificity was verified by melt curve analysis from 55 to 95 °C. The CsAP1 and CsLFY transcript levels in transgenic plants relative to WT plants were calculated using the 2−ΔΔCt method (Livak and Schmittgen 2001).
Low temperature stress treatment to induce flowering
Six-month-old seedlings of the T2 generation from the Dun134-3 CsAP1 transgenic line and WT seedlings of the same age growing in 200-mL cone-tainers (3.8 × 25 cm) of potting mix were subjected to 8 weeks of low temperature floral-induction conditions (11 ± 1 °C continuously during an 8-h day/16-h night) or non-inductive conditions (25 ± 1.5 °C continuously during an 8-h day/16-h night) in walk-in growth chambers. At the end of 8 weeks of treatment, all plants were maintained at 25 ± 1.5 °C with an 8-h day/16-h night. Leaf tissue samples were collected after 4 and 8 weeks of treatment to estimate CsAP1 transcript accumulation.
Drought stress treatment to induce GFP expression
Whole leaves were cut from three lines of 3-year-old ‘Carrizo’ citrange plants transformed with the GFP gene under control of the AtRD29A promoter and left in uncovered Petri dishes for 48 h at 26 ± 3.0 °C. At the end of this period, wilting, a symptom of drought stress, was visually obvious for all leaves. At that time, explants were cut from the drought-stressed leaves and examined for GFP fluorescence under a microscope equipped with a blue (λ 450–490 nm) light source. Control leaves were cut from well-watered WT plants of the same age just before they were inspected for the presence of GFP. Leaves of similar size and age were used for this comparison.
Results
CsAP1 transgenic lines
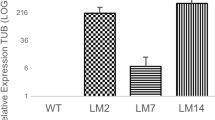
Two transgenic lines of citrus plants expressing CsAP1 were evaluated: ‘Duncan’ grapefruit line Dun134-3 and ‘Valencia’ sweet orange line Val190-2 (Table 2). The transgenic line Dun134-3 was the most precocious line of all transgenic plants produced. The T0 Dun134-3 plant flowered after just 4.5 years, with 56% of its T1 siblings (17/30) flowering after 18 months. Both the T0 plant and the T1 generation plants of the Dun134-3 CsAP1 line produced fruit from self-pollinated flowers. The harvested T1 fruit had 20 to 49 fertile seeds, from which the 6-month-old T2 generation plants discussed below were derived. The ‘Valencia’ orange CsAP1 expressing line, Val190-2, flowered after nine years. Although the Dun134-3 line flowered much earlier than Val190-2, the Val190-2 line expressed the CsAP1 transgene at a much greater level (a leaf relative expression of 240 for Val190-2 vs. 100 for Dun134-3) (Table 2). Growth of the Val190-2 tree was slowed due to a Chilli thrips (Scirtothrips dorsalis) infestation in the greenhouse. When the sixth fruit produced by this tree reached 4–5 cm in diameter, we collected all fruit and their underdeveloped seeds and recovered T1 plants under in vitro conditions (Shen et al. 2011).
CsLFY transgenic lines
Six T0 plants expressing the CsLFY gene were evaluated, five ‘Duncan’ grapefruit lines and one ‘Valencia’ orange line (Table 2). Four of the five CsLFY transgenic ‘Duncan’ grapefruit lines flowered in the greenhouse after 8 years, but the ‘Valencia’ orange CsLFY line did not flower. Relative expression levels of CsLFY gene in leaves from the transgenic plants varied from almost 70 to over 240 (Table 2). There was no clear relationship between the levels of CsLFY expression in leaves and the ability of the T0 transgenic plants to flower under non-inductive conditions (Table 2). For example, the Dun120-13 and Dun120-21C lines had relatively similar low levels of CsLFY expression (Table 2); Dun120-21C flowered and Dun120-13 did not. The Val178-1 line, which expressed CsLFY at a level 2.7 times greater than the flowering Dun120-21C line never flowered. Interestingly, for the T1 siblings, a variable percentage (given in parentheses) of the Dun120-21C (8%), Dun120-25 (51%), Dun124-11 (57%) and Dun124-13C (2%) transgenic lines expressing the CsLFY gene flowered within 3–8 months after germination under non-inductive conditions in the greenhouse (Table 2; Fig. 2). However, all flowers of these seedlings abscised before setting fruit.
Low temperature stress treatment to induce flowering
Six-month-old T2 generation seedlings of the Dun134-3 CsAP1 transgenic line and WT control plants were either treated for 8 weeks with low temperature (11 °C, 8-h day/16-h night) to induce flowering or maintained under non-inductive (control) conditions (25 °C, 8-h day/16-h night). At week 0, relative leaf transcript levels of CsAP1 were significantly greater in the T2 generation transgenic plants than WT plants (Fig. 3). After 4 weeks, leaf CsAP1 transcripts increased further in T2 Dun134-3 plants in both the low and warm temperature treatments compared to WT plants. Surprisingly, during the following 4 weeks, expression of CsAP1 decreased in leaves of the T2 generation transgenic plants in both treatments, but remained significantly greater than CsAP1 expression in leaves of WT plants (Fig. 3). None of the WT plants or the T2 generation plants of the Dun134-3 CsAP1 line flowered under either the low or warm temperature conditions. Although the T2 generation plants of the Dun134-3 CsAP1 line are now 27 months old, they have not flowered yet. In contrast, T1 generation plants of the Dun134-3 CsAP1 line are now 5 years old and have flowered multiple times under non-inductive (warm, well-watered) conditions (Fig. 4).
Relative expression levels of the APETALA1 (CsAP1) transgene under control of the A. thaliana RD29A promoter in leaves of the T2 generation ‘Duncan’ grapefruit Dun134-3 CsAP1 line and wild-type (WT) plants exposed to low (11 °C) and warm (25 °C) temperature treatments for 0, 4 and 8 weeks. In each treatment WT plants served as the control (leaf relative expression equals 1, which cannot be seen on the graph). Data are presented as means + SE (n = 5–20 plants). Means labeled with different capital letters are significantly different by the Duncan Multiple Range Test at P < 0.05
Expression of the β-glucuronidase (GUS) gene in transgenic lines
Histochemical analysis of GUS reporter gene expression was conducted on leaves of transgenic T0, T1 and T2 generation lines. All the transgenic plants analyzed showed high constitutive GUS expression in leaves (Table 2); WT plants used as the negative control did not show any GUS expression (data not shown).
Drought stress treatment to induce GFP expression
The placement of the GFP gene under control of the AtRD29A promoter resulted in constitutive, rather than stress-induced, expression of GFP in leaves from three transformed lines of 3-year-old Carrizo’ citrange rootstocks. GFP fluorescence observed in wilted leaves after exposure to drought stress for 48 h was equal to that of leaves collected from well-watered plants immediately before visual inspection for GFP (Fig. 5). The presence of GFP was not detected in drought stressed or well-watered leaves from WT plants.
Photographs of leaf explants from three ‘Carrizo’ citrange rootstock lines (TL1, TL2, and TL3) transformed with the A. thaliana RD29A inducible promoter + the green fluorescent protein gene (AtRD29A+GFP) binary vector and wild-type (WT) plants. The photographs were taken with the camera attached to microscope equipped with a blue light (λ 450–490 nm) source to induce GFP fluorescence. GFP fluorescence was present in the leaves of all three transgenic ‘Carrizo’ citrange lines independent of the treatment imposed. There was no GFP fluorescence in leaves of WT plants
Discussion
Eight transgenic lines of citrus plants expressing CsAP1 or CsLFY genes were evaluated in this research. The presence and expression of transgenes in selected, kanamycin-resistant T0 plants and T1 and T2 siblings were reconfirmed by RT-PCR gene expression analyses of the CsAP1 and CsLFY genes, and also by histochemical assays for GUS reporter gene expression. Despite the fact that each gene was under control of the AtRD29A stress-inducible promoter, in all cases, flowering occurred under non-inductive (warm temperature, well-watered) greenhouse conditions. Further, flowering occurred throughout the year and was not associated with potential seasonal changes in photoperiod or temperature in the greenhouse. The transgenic plants did not exhibit any morphological features that were different from their WT counterparts of the same age. Transgenic plants that flowered stopped producing thorns, a characteristic of juvenility in Citrus spp., whereas plants that did not flower continued to develop thorns.
Unexpectedly, the AtRD29A promoter appeared to be constitutively expressed in the Citrus species and hybrids used in this research. Three lines of evidence support this conclusion. First, all eight T0 CsAP1 and CsLFY transgenic ‘Duncan’ grapefruit and ‘Valencia’ orange plants expressed significantly greater levels of the respective transgene compared to WT plants under non-inductive greenhouse conditions (Table 2). Of these eight T0 plants, all flowered under the same non-inductive conditions, except one ‘Duncan’ grapefruit line and one ‘Valencia’ line expressing the CsLFY transgene, and the WT plants (Table 2). Second, exposing T2 generation Dun134-3 seedlings to either low (11 °C) or warm (25 °C) temperatures for 4 weeks resulted in significant similar increases in leaf expression of the CsAP1 transgene in plants in both treatments compared to week 0 and WT plants (Fig. 3). In addition, despite an additional 4 weeks of low and warm temperature treatment, respectively, by week 8 leaf CsAP1 expression in the T2 generation Dun134-3 line decreased to week 0 levels for plants at both temperatures. The scientific basis for these changes in leaf CsAP1 expression in transgenic plants under both temperature treatments is unknown. Low temperature stress is divided into chilling stress (< 20 °C) and cold stress (< 0 °C) (Ritonga and Chen 2020). The AtRD29A promoter is known to be upregulated at both chilling (≤ 19 °C) and cold stress temperatures (≥ − 5 °C) (Ishitani et al. 1998). Taken together, the results of these experiments provided no evidence to support the induction of AtRD29A at 11 °C. In contrast, low (chilling stress) temperatures between 10 and 15 °C are known to induce flowering in adult WT Citrus spp., independent of photoperiod (Chica and Albrigo 2013). A third line of evidence supporting constitutive expression of AtRD29A is that similar levels of GFP fluorescence were observed in both wilted drought-stressed and turgid well-watered leaves of transgenic AtRD29A-GFP ‘Carrizo’ citrange rootstocks; no GFP fluorescence was observed in WT plants (Fig. 5). Visible leaf wilting in response to drought stress is sufficient to upregulate AtRD29A in other plant species (Xiao et al. 2015). Although varying degrees of constitutive expression (“leakiness”) of target genes regulated by AtRD29A in the absence of stress have been documented (Bihmidine et al. 2012; Estrada-Melo et al. 2015; Qiu et al. 2012), the results presented here are the first to provide evidence of this phenomenon in Citrus species and hybrids. Altering the orientation and positioning of the gene cassette within the T-DNA region have been proposed as strategies to reduce “leakiness” of the target gene when AtRD29A is used as the promoter (Bihmidine et al. 2012). These factors may underlie the seemingly exclusive constitutive expression of AtRD29A and the target genes in the transgenic lines reported here. Other than the loss of control over flowering time, constitutive expression of AtRD29A and the CsAP1 and CsLFY target genes resulted in plants with an early-flowering phenotype and none of negative effects on plant morphology or health that have been reported with the use of the 35S CaMV promoter (Behnman et al. 2006; Bihmidine et al. 2012; Qiu et al. 2012; Xiao et al. 2015).
It is of interest that time to flowering of T1 generation siblings of four ‘Duncan’ grapefruit primary transformants (i.e., from selfed T0 scions) expressing the CsLFY gene, and one ‘Duncan’ grapefruit T1 scion expressing the CsAP1 gene was significantly reduced compared with the flowering time of the T0 scions (Table 2). Flowering time in the T0 plants could have been influenced by genetic, epigenetic, hormonal, and/or metabolic factors released from the rootstock into the scions (Prassinos et al. 2009; Jensen et al. 2010; Liu et al. 2017). Similarly, epigenetic reprograming and genome structural variations are known to occur during tissue culture and may explain the segregation of flowering time among T1 and T2 siblings (Stroud et al. 2013; Stelpflug et al. 2014; Fossi et al. 2019). Precocious flowering of a trifoliate orange spontaneous mutant has been related to changes in the DNA methylation status of genes controlling the flowering process (Zhang et al. 2014). Only about 20% of the trifoliate orange mutant seedlings showed a short juvenile phase, flowering within 1 and 2 years after germination (Zhang et al. 2014), like the segregation of the early flowering phenotype in T1 and T2 progeny of CsAP1 and CsLFY transgenic lines observed in this work. In contrast, GUS gene expression did not segregate in the T1 generation ‘Duncan’ grapefruit and ‘Valencia’ sweet orange lines expressing the CsAP1 gene, nor in the four T1 ‘Duncan’ grapefruit lines expressing the CsLFY gene (Table 2). Nucellar polyembryony is found in many Citrus groups (Davies and Albrigo 1994) and common in both ‘Duncan’ grapefruit (Holland et al. 1996) and ‘Valencia’ orange (Koltunow et al. 1995). The absence of segregation of GUS gene expression in T1 seedlings (Table 2) confirms that embryos developing from the ‘Duncan’ grapefruit and ‘Valencia’ orange seeds were not the products of sexual hybridization, but originated from nucellar tissue, making the T1 plants genetically identical to the mother plant.
The variable levels of transgene expression observed in leaves of the transformed lines of ‘Duncan’ grapefruit and ‘Valencia’ sweet orange may be related to changes in DNA methylation patterns during tissue culture, transgene copy number, T-DNA rearrangements, position effects and/or integration into the heterochromatin (Weinhold et al. 2013; Stroud et al. 2013; Stelpflug et al. 2014; Jupe et al. 2019). Some of these factors could lead to transgene silencing (Weinhold et al. 2013; Jupe et al. 2019). Transgene silencing results when transcription fails due to DNA methylation leading to chromatin compaction, or RNA is degraded post-transcription. Widely reported epigenetic transgene silencing by RNA-directed DNA methylation, which can be seen as a heritable decrease in transgene expression (Weinhold et al. 2013) was not observed in the transgenic ‘Duncan’ grapefruit line Dun134-3 expressing the CsAP1 gene under control of the AtRD29A the promoter. Relative expression levels in leaves of 6-month-old T2 siblings were 4- to sixfold greater (Fig. 3) than that of the T0 Dun134-3 transgenic plant (leaf relative expression 99.31) (Table 2). For Citrus, the goal of transformed plant lines with a stable heritable phenotype is likely facilitated by the frequency of nucellar embryony and parental cloning, but also complicated by the group’s genetics. Grapefruit (C. paradisi) originated as a hybrid of sweet orange (C. sinensis) × pummelo (C. maxima) and ‘Valencia’ orange (C. sinensis) as a hybrid of pummelo (C. maxima) and common mandarin (C. reticluata) (Li et al. 2010). Diploid differences in heterozygosity at loci also exist (Pillitteri et al. 2004a). In addition, spontaneous autotetraploids are found to varying degrees among polyembryonic citrus seeds, including those of ‘Duncan’ grapefruit (Aleza et al. 2008). Increased ploidy number would impact the factors associated with transgene copy number, expression and silencing.
The lack of correlation between leaf CsAP1 and CsLFY transcript levels and the earliness of flowering in the transgenic lines might also be anticipated due to the disparate floral developmental events that occur in the bud. Flower development requires multiple steps subsequent to successful induction and transition of the vegetative SAM to a floral meristem, including initiation of floral organ primordia, floral organ specification and development of individual flowers (Benlloch et al. 2007; Ma 1994). Overexpression of CsAP1 or CsLFY transgenes in a juvenile plant may not always be sufficient to successfully downregulate various inhibitors of flowering and upregulate critical flowering pathway genes downstream from LFY and AP1 (i.e., AP1 and/or APETALA2 [AP2], respectively). Upregulation of AP1 and AP2 is essential for floral meristem determinacy (irreversible commitment to flowering) in Citrus and subsequent activation of the downstream floral organ identity genes necessary for formation of individual flowers (Tang and Lovatt 2019).
Taken together, the results of this research provide evidence of early-flowering phenotypes in transgenic lines of two Citrus scion species, C. paradisi and C. sinensis, of commercial importance to the global citrus industry. Lines of ‘Duncan’ grapefruit and ‘Valencia’ sweet orange expressing the CsAP1 or CsLFY transgene flowered precociously, bore fruit and produced viable seeds through the T1 and T2 generations. Despite the loss of ability to control flowering time using low (chilling) temperature or drought stress due to the constitutive expression of CsAP1 and CsLFY transgenes under regulation of the AtRD29A promoter, the transgenic lines exhibit none of the negative effects on plant morphology or health associated with use of the constitutive 35S CaMV promoter. The precocious flowering capabilities of these lines might allow researchers to more rapidly examine phenotypic changes associated with commercially important traits related to fruit quality, disease and pest resistance, abiotic stress tolerance, and others through the use of gene stacking technology.
References
Aleza P, Jimirez J, Ollitrault P, Navarro L (2008) Selection of spontaneous autotetraploid plants from Citrus polyembryonic cultivars. In: Prohens J, Badenes ML (eds) Modern variety breeding for present and future needs. Universidad Politécnica de Valencia, Valencia
Behnman B, Kikuchi A, Celebi-Toprak F, Yamanaka S, Kasuga M, Yamaguchi-Shinozaki K, Watanabe KN (2006) The Arabidopsis DREB1A gene driven by the stress-inducible rd29A promoter increases salt-stress tolerance in proportion to its copy number in tetrasonic tetraploid potato (Solanum tuberosum). Plant Biotechnol 23:169–177
Benlloch R, Berbel A, Serrano-Mislata A, Madueno F (2007) Floral initiation and inflorescence architecture: a comparative view. Ann Bot 100:659–676
Bihmidine S, Lin J, Stone JM, Awada T, Specht JE, Clemente TE (2012) Activity of the Arabidopsis RD29A and RD29B promoter elements in soybean under water stress. Planta 237:55–64. https://doi.org/10.1007/s00425-012-1740-9
Cervera M, Navarro L, Pena L (2009) Gene stacking in 1-year cycling APETALA1 citrus plants for a rapid evaluation of transgenic traits in reproductive tissues. J Biotechnol 140:278–282
Chica EJ, Albrigo LG (2013) Changes in CsFT transcript abundance at the onset of low-temperature floral induction in sweet orange. J Am Soc Hortic Sci 138:184–189
Davies FS, Albrigo LG (1994) Citrus. CAB International, Wallingford
Duan YX, Fen J, Guo WW (2010) Regeneration and characterization of transgenic kumquat plants containing the Arabidopsis APETALA1 gene. Plant Cell Tissue Organ Cult 100:273–281. https://doi.org/10.1007/s11240-009-9646-3
Endo T, Shimada T, Fujii H, Kobayashi Y, Araki T, Omura M (2005) Ectopic expression of an FT homolog from Citrus confers an early flowering phenotype on trifoliate orange (Poncirus trifoliata L. Raf.). Transgenic Res 14:703–712
Estrada-Melo AC, Ma C, Reid MS, Jiang C-Z (2015) Overexpression of an ABA biosynthesis gene using a stress-inducible promoter enhances drought resistance in petunia. Hortic Res. https://doi.org/10.1038/hortres.2015.13
Flachowksy H, Peil A, Sopanen T, Elo A, Hanke V (2007) Overexpression of BpMADS4 from silver birch (Betula pendula Roth.) induces early-flowering in apple (Malus × domestica Borkh.). Plant Breed 126:137–145
Fossi M, Amundson K, Kuppu S, Britt A, Comai L (2019) Regeneration of Solanum tuberosum plants from protoplasts induces widespread genome instability. Plant Physiol 180(1):78–86. https://doi.org/10.1104/pp.18.00906
Goldberg-Moeller R, Shaloma L, Shlizermana L, Samuelsa S, Zura N, Ophira R, Blumwald E, Sadka A (2013) Effects of gibberellin treatment during flowering induction period on global gene expression and the transcription of flowering-control genes in Citrus buds. Plant Sci 198:46–57
Holland N, Saad S, Perl A, Holland D (1996) Tentoxin sensitivity of citrus: cotyledon-dependency of growth inhibition and reversibility of chlorosis. J Exp Bot 47(299):837–842
Ishitani M, Xiong L, Lee H, Stevenson B, Zhu J-K (1998) HOS1, a genetic locus involved in cold-responsive gene expression in Arabidopsis. Plant Cell 10:1151–1161
Jack T (2004) Molecular and genetic mechanisms of floral control. Plant Cell 16(Suppl 1):S1–S17
Jensen PJ, Makalowska I, Altman N, Fazio G, Praul C, Maximova SN, Crassweller RM, Travis JW, McNellis TW (2010) Rootstock-regulated gene expression patterns in apple tree scions. Tree Genet Genomes 6:57–72
Jupe F, Rivkin AC, Michael TP, Zander M, Motley ST, Sandoval JP, Slotkin RK, Chen H, Castanon R, Nery JR, Ecker JR (2019) The complex architecture and epigenomic impact of plant T-DNA insertions. PLoS Genet 15(1):e1007819. https://doi.org/10.1371/journal.pgen.1007819
Koltunow AM, Soltys K, Nito N, McClure S (1995) Anther, ovule, seed, and nucellar embryo development in Citrus sinensis cv. Valencia. Can J Bot 73:1567–1582
Le Roux P, Flachowsky H, Hanke M, Gessler C, Patocchi A (2012) Use of transgenic early flowering approach in apple (Malus × domestica Borkh.) to introgress fire blight resistance from cultivar Evereste. Mol Breed 30:857–874. https://doi.org/10.1007/s11032-011-9669-4
Li X, Xie R, Lu Z, Zhou Z (2010) The origin of cultivated citrus as inferred from internal transcribed spacer and chloroplast DNA sequence and amplified fragment length polymorphism fingerprints. J Am Soc Hortic Sci 135:341–350
Liu X-Y, Li J, Liu M-M, Yao Q, Chen JZ (2017) Transcriptome profiling to understand the effect of citrus rootstocks on the growth of ‘Shatangju’ mandarin. PLoS ONE 12(1):e0169897. https://doi.org/10.1371/journal.pone.0169897
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408
Ma H (1994) The unfolding drama of flower development: recent results from genetic and molecular analyses. Genes Dev 8:745–756
Mankin SL, Thompson WF (2001) New green fluorescent protein genes for plant transformation: intron-containing, ER-localized, and soluble-modified. Plant Mol Biol Rep 19:13–26
Msanne J, Lin J, Stone JM, Awada T (2011) Characterization of abiotic stress-responsive Arabidopsis thaliana RD29A and RD29B genes and evaluation of transgenes. Planta 234:97–107
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nishikawa F (2013) Review of floral induction in citrus. Jpn Soc Hortic Sci 82(4):283–292
Orbović V, Grosser W (2006) Citrus: sweet orange (Citrus sinensis L. Osbeck ‘Valencia’) and Carrizo citrange [Citrus sinensis (L.) Osbeck × Poncirus trifoliata (L.) Raf.]. In: Wang K (ed) Agrobacterium protocols. Methods in molecular biology. Humana Press Inc., Totowa, pp 177–189
Pena L, Martin-Trillo M, Juarez J, Pina JA, Navarro L, Martinez-Zapater JM (2001) Constitutive expression of Arabidopsis LEAFY or APETALA1 genes in citrus reduces their generation time. Nat Biotechnol 19:263–267
Petri C, Alburquerque N, Faize M, Scorza R, Dardick C (2018) Current achievements and future directions in genetic engineering of European plum (Prunus domestica L.). Transgenic Res 27:225–240
Pillitteri LJ, Lovatt CJ, Walling LL (2004a) Isolation and characterization of a TERMINAL FLOWER homolog and its correlation with juvenility in Citrus. Plant Physiol 135:1540–1551
Pillitteri LJ, Lovatt CJ, Walling LL (2004b) Isolation and characterization of LEAFY and APETALA1 homologues from Citrus sinensis L Osbeck ‘Washington.’ J Am Soc Hortic Sci 129:846–856
Prassinos C, Ko JH, Lang G, Iezzoni AF, Han KH (2009) Rootstock-induced dwarfing in cherries is caused by differential cessation of terminal meristem growth and is triggered by rootstock-specific gene regulation. Tree Physiol 29:927–936
Qiu W, Liu M, Qiao G, Jiang J, Xie L, Zhuo R (2012) An isopentyl transferase gene driven by the stress-inducible rd29A promoter improves salinity stress tolerance in transgenic tobacco. Plant Mol Biol Rep 30:519–528
Ritonga FN, Chen S (2020) Physiological and molecular mechanism involved in cold stress tolerance in plants. Plants 9(590):1–13. https://doi.org/10.3390/plants9050560
Salonia F, Ciacciuli A, Poles L, Pappalardo HD, La Malfa S, Licciardello C (2020) New plant breeding techniques in citrus for the improvement of important agronomic traits. A review. Front Plant Sci 11:1234. https://doi.org/10.3389/fpls.2020.01234
Samach A (2012) Congratulations, you have been carefully chosen to represent an important developmental regulator! Ann Bot 111:329–333
Shen X, Gmitter FG Jr, Grosser JW (2011) Immature embryo rescue and culture. In: Thorpe T, Yeung E (eds) Plant embryo culture. Methods in molecular biology, vol 710. Humana Press, Totowa, pp 75–92. https://doi.org/10.1007/978-1-61737-988-8_7
Sinn JP, Held J, Vosburg C, Klee SM, Orbovic V, Taylor E, Gottwald T, Stover E, Moore G, McNellis TW (2020) Flowering Locus T chimeric protein induces floral precocity in edible citrus. Plant Biotechnol J. https://doi.org/10.1111/pbi.13463
Stelpflug SC, Eichten SR, Hermanson PJ, Springer NM, Kaeppler SM (2014) Consistent and heritable alterations of DNA methylation are induced by tissue culture in maize. Genetics 198(1):209–218. https://doi.org/10.1534/genetics.114.165480
Stroud H, Ding B, Simon SA, Feng S, Bellizzi M, Pellegrini M, Wang G-L, Meyers BC, Jacobsen SE (2013) Plants regenerated from tissue culture contain stable epigenome changes in rice. eLife 2:e00354. https://doi.org/10.7554/eLife.00354
Tan F-C, Swain SM (2007) Functional characterization of AP3, SOC1 and WUS homologues from citrus (Citrus sinensis). Physiol Plant 131:481–495
Tang L, Lovatt CJ (2019) Effects of low temperature and gibberellic acid on floral gene expression and floral determinacy in ‘Washington’ navel orange (Citrus sinensis L. Osbeck). Sci Hortic 243:92–100
Weinhold A, Kallenbach M, Baldwin IT (2013) Progressive 35S promoter methylation increases rapidly during vegetative development in transgenic Nicotiana attenuata plants. BMC Plant Biol 13:99. https://doi.org/10.1186/1471-2229-13-99
Xiao H-J, Liu K-K, Li D-W, Arisha MH, Chai W-G, Gong Z-H (2015) Cloning and characterization of the pepper CaPAO gene for defense responses to salt-induced leaf senescence. BMC Biotechnol 15(100):1–12. https://doi.org/10.1186/s12896-015-0213-1
Yamaguchi-Shinozaki K, Shinozaki K (2005) Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci 10:88–94
Zhang J-Z, Mei L, Liu R, Khan MRG, Hu C-G (2014) Possible involvement of locus-specific methylation on expression regulation of LEAFY homologous gene (CiLFY) during precocious trifoliate orange phase change process. PLoS ONE 9(2):e88558. https://doi.org/10.1371/journal.pone.0088558
Acknowledgements
The authors thank Dr. Lynn Pillitteri for the full-length gene sequences for Citrus sinensis APETALA1 and LEAFY and Dr. Martha L. Orozco, Director of the Plant Transformation Research Center, University of California, Riverside, for providing the pCAMBIA2301 plasmid and for helping with the preparation of the transformation vectors containing the CsAP1 and CsLFY genes.
Author information
Authors and Affiliations
Contributions
CJL, VO, and YA designed the experiments, JN and YA constructed binary vectors, VO, SAR, BM, and YA preformed the experiments, all authors wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Orbović, V., Ravanfar, S.A., Acanda, Y. et al. Stress-inducible Arabidopsis thaliana RD29A promoter constitutively drives Citrus sinensis APETALA1 and LEAFY expression and precocious flowering in transgenic Citrus spp.. Transgenic Res 30, 687–699 (2021). https://doi.org/10.1007/s11248-021-00260-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-021-00260-z